Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Pset1 s05 PDF
Caricato da
Đạt LêDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Pset1 s05 PDF
Caricato da
Đạt LêCopyright:
Formati disponibili
Massachusetts Institute of Technology
5.12, Spring 2005
Problem Set #1
Due: February 10, 4:00 pm
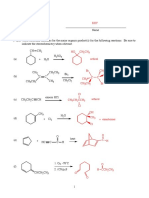
1. Assign formal charges to each atom below (a formal charge of zero is assumed if
no charge is indicated). Cross out the configurations that are not reasonable, and
provide an explanation (large charge - greater than +/- 1, incomplete octet, octet
exceeded).
Br
2. Reorient the molecule at the left to match the partially drawn perspective at the
right. Complete the drawing at the right by adding the two missing substituents at their
correct positions. Build a model if necessary.
a)
CH2CH3
H3 C
b)
CH3
CH2CH3
CH2CH3
Massachusetts Institute of Technology
5.12, Spring 2005
3. Provide Kekul structures for the following molecules, including all major resonance
contributors (no more than 2 formal charges, no formal charge greater than +/- 1).
a)
N2
b)
CH3CO2Na
c)
O3
4. Label all of the functional groups in amoxicillin, an antibiotic from the penicillin
family.
NH2
HO
H
N
CH3
CH3
O
HO
Massachusetts Institute of Technology
5.12, Spring 2005
5. Provide orbital drawings of the following molecules. (Dont forget to shade the p
orbitals appropriately!) Indicate the hybridization and bond angle at each nonhydrogen atom. Indicate the sigma and pi bonds and all lone pairs of electrons.
a) BeCl2
b)
H2C C O
Massachusetts Institute of Technology
5.12, Spring 2005
6. Circle the following pairs of structures that do not constitute resonance structures.
For the proper resonance pairs, draw curved arrows to convert the first structure to the
second. Draw in all lone pairs of electrons.
H3 C
CH3
H3 C
CH3
a)
b)
H3C C CH
H2C C CH2
c)
d)
H3 C
S
C
H
CH2
H3C
e)
H3 C
C
H
CH2
OH
CH3
H3C
CH2
Massachusetts Institute of Technology
5.12, Spring 2005
7.
Smith, Janice G. Organic Chemistry. 1st ed. New York, NY: McGraw-Hill,
2006, p. 77. ISBN: 0072397462.
8. When you ingest aspirin, it passes through your stomach, which has an acidic pH,
before traveling through the basic environment of your intestine. Provide the correct
structure of aspirin a) as it exists in the stomach and b) as it exists in the intestine.
CH3
aspirin
Massachusetts Institute of Technology
5.12, Spring 2005
9. Rank the following sets of molecules according to acidity (1= most acidic). Explain
your choices.
H Cl H
a) H C C C H
H Cl H
b)
H Cl H
H C C C H
H H H
N
H3C
c)
H3C
CH3
e)
N
H
CH3
H2O
HCl
d) H2C
H
N
H H H
Cl C C C H
H H H
H2 S
O
H2 C
H
N
O
O
H
C
H2C
C
H
10. Rank the following molecules according to basicity ( 1 = most basic). Explain.
H3 C C N
H2C N CH3
H3C N CH3
H
Massachusetts Institute of Technology
5.12, Spring 2005
11. Rank the molecules in order of acidity (1 = most acidic). Explain your answer by
drawing all resonance contributors of each conjugate base. Use the back of this page,
if necessary.
OH
OH
OH
O2N
NO2
Massachusetts Institute of Technology
5.12, Spring 2005
12. Rank the hydrogen atoms (Ha, Hb, Hc) in the following molecules according to
acidity.
Ha
H2C
Hb
H H Hc
CH3
Ha
Hc
Hb
___ > ___ > ___
___ > ___ > ___
13. Circle the most acidic H atom in ascorbic acid (vitamin C).
OH
HO
O
HO
O
OH
14.
Smith, Janice G. Organic Chemistry. 1st ed. New York, NY: McGraw-Hill,
2006, p. 77. ISBN: 0072397462.
Massachusetts Institute of Technology
5.12, Spring 2005
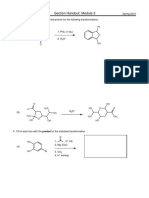
15. Draw the products of each reaction. Show all lone pairs in reactants and
products. Circle the side of the reaction that is favored at equilibrium.
a)
H C C H
Li
CH2CH3
b)
F 3C
OH
OCH2CH3
c)
CH3CH2NH2
CH3CH2S
d)
CH3CH2SH2
CH3CH2OH
NH3
OH
e)
Massachusetts Institute of Technology
5.12, Spring 2005
16. Draw in all lone pairs and provide the product of each reaction. Use curved arrow
notation to show the mechanism. Show all resonance contributors of reactants and
products, if applicable.
H2O
a)
b)
Cl
Cl
Al
Cl CH3
Cl
c)
OH
Cl
H3C
d)
HS
e)
CH3
N
CH3
H C C
H Cl
f)
H
Hint: find nucleophile (electron rich)
and electrophile (electron poor).
g)
H3C
OH
10
Potrebbero piacerti anche
- Camp's Biochemistry and Cell Biology by the NumbersDa EverandCamp's Biochemistry and Cell Biology by the NumbersNessuna valutazione finora
- Reactive Oxygen Species: Signaling Between Hierarchical Levels in PlantsDa EverandReactive Oxygen Species: Signaling Between Hierarchical Levels in PlantsFranz-Josef SchmittNessuna valutazione finora
- Pset1 s05Documento10 paginePset1 s05samuelifamilyNessuna valutazione finora
- Chem 112A F11 Homework 3Documento6 pagineChem 112A F11 Homework 3Shyam BhaktaNessuna valutazione finora
- OCW Exam 1Documento10 pagineOCW Exam 1iliketospam123Nessuna valutazione finora
- Pset2 s05 PDFDocumento8 paginePset2 s05 PDFĐạt LêNessuna valutazione finora
- CHM 1321 Assignment #2 - : AnswersDocumento11 pagineCHM 1321 Assignment #2 - : AnswersSara YuenNessuna valutazione finora
- Organic Chemistry Exam 1 (Practice) Chem 237Documento3 pagineOrganic Chemistry Exam 1 (Practice) Chem 237Ngoc Minh NgoNessuna valutazione finora
- 2,5-Dimethylheptane: Condensed Structural Formula (6 Points)Documento4 pagine2,5-Dimethylheptane: Condensed Structural Formula (6 Points)Andra AlNessuna valutazione finora
- Organic Chemistry Questions 3Documento12 pagineOrganic Chemistry Questions 3Ram KrishnaNessuna valutazione finora
- Sp2001 Final Organic II 200pts (Weighted As 300) : RNH C N H O O O R RDocumento25 pagineSp2001 Final Organic II 200pts (Weighted As 300) : RNH C N H O O O R RUmmi Khairani UrfaNessuna valutazione finora
- Chem 112A Homework D KeyDocumento13 pagineChem 112A Homework D KeyShyam Bhakta100% (1)
- Chem 112A Homework 1Documento4 pagineChem 112A Homework 1Shyam BhaktaNessuna valutazione finora
- O-Chem II Exam 2Documento17 pagineO-Chem II Exam 2NayeNessuna valutazione finora
- CL CL: Hex-1-En-4-Yne or 1-Hexen-4-YneDocumento4 pagineCL CL: Hex-1-En-4-Yne or 1-Hexen-4-YneSamuel Espinoza GarciaNessuna valutazione finora
- Exam 1 BDocumento6 pagineExam 1 BTouqueer AhmedNessuna valutazione finora
- Answer Key: Chemistry 206 First Hour ExaminationDocumento9 pagineAnswer Key: Chemistry 206 First Hour Examinationsudipta88Nessuna valutazione finora
- Orgo 1 QuzzesDocumento34 pagineOrgo 1 QuzzesDanny NguyenNessuna valutazione finora
- CHEM 33 Extra Practice 05 AnswersDocumento7 pagineCHEM 33 Extra Practice 05 Answershuang.sundi3134Nessuna valutazione finora
- CH203 Fall 2014 Exam 1 Grading Key PDFDocumento8 pagineCH203 Fall 2014 Exam 1 Grading Key PDFBUCH203Nessuna valutazione finora
- NTU CB1117 Tutorial 1Documento3 pagineNTU CB1117 Tutorial 1cassidychandidiNessuna valutazione finora
- Quiz1 1ANSDocumento1 paginaQuiz1 1ANSabubakarabubakarbah563Nessuna valutazione finora
- CH 231 Old Exams 1 and 2Documento13 pagineCH 231 Old Exams 1 and 2Brian DaSilva100% (3)
- This Study Resource Was: Glance Through The Entire Questions and Solve The Easiest Problems FirstDocumento4 pagineThis Study Resource Was: Glance Through The Entire Questions and Solve The Easiest Problems FirstSandipan SahaNessuna valutazione finora
- Assignment 1 BLC F20Documento2 pagineAssignment 1 BLC F20Rémi MartineauNessuna valutazione finora
- H I HOH Tso H: Opposite StereochemistryDocumento4 pagineH I HOH Tso H: Opposite Stereochemistrylp_blackoutNessuna valutazione finora
- Sample Paper For M. Phil Admission PreparationDocumento11 pagineSample Paper For M. Phil Admission PreparationRabiaNessuna valutazione finora
- Glance Through The Entire Questions and Solve The Easiest Problems FirstDocumento4 pagineGlance Through The Entire Questions and Solve The Easiest Problems FirstRutul JainNessuna valutazione finora
- ReactionsDocumento5 pagineReactionsYeKwon Patrick HuhNessuna valutazione finora
- Assignment 2 BLC F20Documento3 pagineAssignment 2 BLC F20Rémi MartineauNessuna valutazione finora
- Sp2009 Final Organic II 200pts (Weighted As 300) : RNH C N H O O O R RDocumento26 pagineSp2009 Final Organic II 200pts (Weighted As 300) : RNH C N H O O O R RUmmi Khairani UrfaNessuna valutazione finora
- CHEM1280 2011 Midterm Exam PDFDocumento3 pagineCHEM1280 2011 Midterm Exam PDFLouisNessuna valutazione finora
- CHEM 135 Exam 2 F15 KeyDocumento7 pagineCHEM 135 Exam 2 F15 KeyMikeNessuna valutazione finora
- CH1O3 Questions PDFDocumento52 pagineCH1O3 Questions PDFPrince T MashandaNessuna valutazione finora
- 2019 Tests and KeysDocumento69 pagine2019 Tests and Keyslorianeallard7Nessuna valutazione finora
- Problems For Chapter 1 & 2 ANSWERS: 2xH 2 2xN 10 O 6Documento6 pagineProblems For Chapter 1 & 2 ANSWERS: 2xH 2 2xN 10 O 6JibrilAttawarahNessuna valutazione finora
- Organic Chemistry Structure and BondingDocumento13 pagineOrganic Chemistry Structure and BondingHossNessuna valutazione finora
- CHEM 2OA3 Fall 2013 Assignment 3Documento14 pagineCHEM 2OA3 Fall 2013 Assignment 3klibzyNessuna valutazione finora
- OCHEM Practice FinalDocumento24 pagineOCHEM Practice FinalNoleNessuna valutazione finora
- Chem 112A: First Midterm: October 6th, 2011Documento7 pagineChem 112A: First Midterm: October 6th, 2011Shyam BhaktaNessuna valutazione finora
- Calculation:: CHEM 4341/6371 Exam 4Documento9 pagineCalculation:: CHEM 4341/6371 Exam 4Vinh HoangNessuna valutazione finora
- Introduction To Organic Chemistry Ii CHEM 224: Answer KeyDocumento8 pagineIntroduction To Organic Chemistry Ii CHEM 224: Answer Keygautamtajesh1983Nessuna valutazione finora
- Exam 2 2000 PDFDocumento14 pagineExam 2 2000 PDFaegaisNessuna valutazione finora
- Solutions For Biochemistry Unit Exam: H-C-C-H H H H HDocumento7 pagineSolutions For Biochemistry Unit Exam: H-C-C-H H H H HTim SilvaNessuna valutazione finora
- 08exam 1keyDocumento7 pagine08exam 1keySeyi Martins ObandoNessuna valutazione finora
- Organic Chemistry FHSC1124Documento64 pagineOrganic Chemistry FHSC1124Hema Jothy100% (1)
- CH 105Documento3 pagineCH 105Rutul JainNessuna valutazione finora
- Biology S6Documento10 pagineBiology S6AKAYEZU Body santiveNessuna valutazione finora
- ChemX6X MT 1Documento8 pagineChemX6X MT 1Phương Nail TócNessuna valutazione finora
- Clayden 2e - End of Chapter Questions - Ch2Documento3 pagineClayden 2e - End of Chapter Questions - Ch2kartikroy0116Nessuna valutazione finora
- Pentadienyl CationDocumento7 paginePentadienyl CationAbhishek SardaNessuna valutazione finora
- Exam 2 2002 Main PDFDocumento11 pagineExam 2 2002 Main PDFaegaisNessuna valutazione finora
- Exam2 Practice A PDFDocumento8 pagineExam2 Practice A PDFĐạt LêNessuna valutazione finora
- SCGS F.7 AL Chemistry Assignment 2 - HALOALKANESDocumento1 paginaSCGS F.7 AL Chemistry Assignment 2 - HALOALKANESsachinkurhekarNessuna valutazione finora
- Organic ChemistryDocumento3 pagineOrganic ChemistryMohammed AltahirNessuna valutazione finora
- DSE Chemistry - Paper 2 by Dr. Samuel ChongDocumento11 pagineDSE Chemistry - Paper 2 by Dr. Samuel Chonglht001023Nessuna valutazione finora
- 332 Practice Exam 4 AnswersDocumento7 pagine332 Practice Exam 4 AnswerskanilkadianNessuna valutazione finora
- The Elements from Neutron to Magnesium: Nuclear ReactionsDa EverandThe Elements from Neutron to Magnesium: Nuclear ReactionsNessuna valutazione finora
- O Level Biology Practice Questions And Answers EnzymesDa EverandO Level Biology Practice Questions And Answers EnzymesValutazione: 5 su 5 stelle5/5 (1)
- Selected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionDa EverandSelected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionNessuna valutazione finora
- 2016 Section Module 3Documento2 pagine2016 Section Module 3Đạt LêNessuna valutazione finora
- 2016 Section Module 2Documento3 pagine2016 Section Module 2Đạt LêNessuna valutazione finora
- Exam2 Practice A PDFDocumento8 pagineExam2 Practice A PDFĐạt LêNessuna valutazione finora
- Pratice 02Documento2 paginePratice 02Đạt LêNessuna valutazione finora
- Acidity & Basicity (Theory)Documento8 pagineAcidity & Basicity (Theory)Saif KhanNessuna valutazione finora
- Polymer Notes 2014 Parts 5-7Documento103 paginePolymer Notes 2014 Parts 5-7RadhiNessuna valutazione finora
- Antibiotic ProductionDocumento19 pagineAntibiotic ProductionBright OgwoNessuna valutazione finora
- CHEM 531-Advanced Organic Chemistry I-Irshad HussainDocumento4 pagineCHEM 531-Advanced Organic Chemistry I-Irshad HussainAnonymous sF8ZuiGNessuna valutazione finora
- Ceramic TechnologyDocumento5 pagineCeramic TechnologySrini VasanNessuna valutazione finora
- A 350 Tonne Per Day Phthalic Anhydride Plant: Presentation On Plant Design ForDocumento29 pagineA 350 Tonne Per Day Phthalic Anhydride Plant: Presentation On Plant Design Forbaniya is hereNessuna valutazione finora
- Nitoprime PrimerDocumento2 pagineNitoprime PrimerBalasubramanian AnanthNessuna valutazione finora
- Assignment 1Documento2 pagineAssignment 1Tony TroxNessuna valutazione finora
- 2020 O Level Chemsitry 6092 Paper 1 Suggested AnswersDocumento4 pagine2020 O Level Chemsitry 6092 Paper 1 Suggested Answerswi.nn.yNessuna valutazione finora
- CE 423 Mechanical Behaviour of Construction MaterialsDocumento20 pagineCE 423 Mechanical Behaviour of Construction MaterialsHüseyin xNessuna valutazione finora
- Properties and Characterization of A Clay Raw Material From Miličinica (Serbia) For Use in The Ceramic IndustryDocumento13 pagineProperties and Characterization of A Clay Raw Material From Miličinica (Serbia) For Use in The Ceramic IndustryFOUTOUNessuna valutazione finora
- Seminar Presentation Ce20214Documento13 pagineSeminar Presentation Ce20214Anji Reddy KommasaniNessuna valutazione finora
- Khalil M. K. KhaderDocumento8 pagineKhalil M. K. KhaderajmanNessuna valutazione finora
- Nanostructural Reorganization of Bacterial Cellulose by Ultrasonic TreatmentDocumento8 pagineNanostructural Reorganization of Bacterial Cellulose by Ultrasonic Treatmentdhy182Nessuna valutazione finora
- CN102807528-PREPARATION METHOD OF 10-Methoxy IminostilbeneDocumento4 pagineCN102807528-PREPARATION METHOD OF 10-Methoxy IminostilbeneDipti DodiyaNessuna valutazione finora
- Practical Pharmacognosy - Third YearDocumento43 paginePractical Pharmacognosy - Third YearraviomjNessuna valutazione finora
- Brake Pads Compendium CompressedDocumento230 pagineBrake Pads Compendium CompressedThillai RajanNessuna valutazione finora
- Mineralogi 1Documento90 pagineMineralogi 1baihaqiNessuna valutazione finora
- Topic 4 Periodic TableDocumento36 pagineTopic 4 Periodic TableadamskbdNessuna valutazione finora
- Aerobic 1Documento7 pagineAerobic 1Ariella AmandaNessuna valutazione finora
- Organic Compound Identification Using Infrared SpectrosDocumento34 pagineOrganic Compound Identification Using Infrared SpectrosRohit SinghNessuna valutazione finora
- AGM 301 - After Mid Semester MaterialsDocumento63 pagineAGM 301 - After Mid Semester Materialsshubham100% (2)
- Polymers 01 PDFDocumento86 paginePolymers 01 PDFUlisesCastilloNessuna valutazione finora
- AWWA Standard List 2014Documento3 pagineAWWA Standard List 2014VI King Pro100% (1)
- Preregistration SeminarDocumento34 paginePreregistration Seminarchirag chawareNessuna valutazione finora
- HCP PracticlesDocumento8 pagineHCP PracticlesDeepa PundirNessuna valutazione finora
- Periodic TrendsDocumento3 paginePeriodic TrendsJessica ShinNessuna valutazione finora
- Amount of Acetic Acid Present in Vinegar - ChemistryDocumento16 pagineAmount of Acetic Acid Present in Vinegar - ChemistryKgmaster100% (1)
- Operational Definition: Example 1Documento4 pagineOperational Definition: Example 1AishaizlNessuna valutazione finora
- PatilDocumento6 paginePatilNur Aini IktikhafsariNessuna valutazione finora