Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
C6 PDF
Caricato da
Suryansh SrivastavaTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
C6 PDF
Caricato da
Suryansh SrivastavaCopyright:
Formati disponibili
NARAYANA IIT ACADEMY
INDIA
CHEMISTRY-ASSIGNMENT-6
CHEMICAL EQUILIBRIUM & THERMODYNAMICS
Sec: Sr.IIT-IZ & NIZ-COSPARK
PONGAL WORKSHEET
MULTIPLE CHOICE QUESTIONS
1.
For the reaction AB2 (g) AB (g)+B(g)
If is negligiable w.r.t. 1 then degree of dissociation ( ) of AB2 is proportional to :
a).
2.
1
p
b).
1
v
c).
1
p
d).
Consider the reaction 2CO(g)+O 2 (g) 2CO 2 (g)+Heat under what conditions shift
is undeterminable ?
a). Addition of O 2 and decrease in volume
3.
4.
5.
b). Addition of CO and removal of CO 2 at constant volume
c). Increase in temperature and decrease in volume
d). Addition of CO and increase in temperature at constant volume
What will be the effect of constant addition of catalyst at constant temperature ?
a). The equilibrium constant will remain constant
b). H of the reaction will remain constant
c). K f and K b will increase up to same extent
d). equilibrium composition will change
Increase in the pressure for the following equilibrium : H 2O (l ) H 2O ( g ) result in
the :
a). formation of more H 2O (l )
b). formation of more H 2O ( g )
c). increase in b.p. of H 2O (l )
d). decrease in b.p. of H 2O (l )
N 2 ( g ) and H 2 (g) are allowed to each in a closed vessel at given temp. and pressure
for the formation of NH 3 (g)[N 2 (g)+3H 2 (g) 2NH3 (g)+22.4 kcal] if He(g) is added
at equilibrium at constant pressure then which is/are correct?
a). Concentration of N 2 (g) , H 2 (g) and NH 3 (g) decrease
b). Moles of NH 3 (g) decrease.
c). The extent of cooling depends on amount of He(g) added.
d). Concentration of N 2 and H 2 increases and concentration of NH 3 decreases.
Narayana IIT Academy
6.
Sr.IIT-IZ & NIZ-COSPARK_PONGAL WORKSHEET
Select the correct statement(s):
a). The rate of reaction decreases with decrease in temperature
b). The rate of reaction is uniform in zero order reaction
c). The rate of reaction depends upon the surface area of the solid reactants
d). Average and instantaneous rate of reaction defined for micro and macro-scopic
time interval respectively
7.
Select the correct statements(s) :
a). The rate law of the elementary reaction ; 2 A B C , must be r=k[A]2
b). The rate law for the complex reaction
A+B C, might not be r k [ A][ B ]
c). If the partial orders differ from the coefficients in the balanced reaction, the
reaction must be complex
d). If the partial orders are equal to corresponding coefficients in the balanced
reaction, the reaction must be elementary
8.
Select the correct statement(s):
a). Every substance that appears in the rate law of reaction must be a reactant or
product in that reaction
b). If we know the rate law of a reaction; we can deduce its mechanism must be
c). If the reaction has rate r k[ A][ B]3/2 then reaction may be elementary
d). A zero order reaction must be a complex reaction
9.
Consider a reaction A B C , in which both reaction are in the same phase may be
a). unimolecular elementary reaction
b). Exothermic
c). Heterogeneous
d). Photochemical
10.
Identify the true statement(s)
a). A catalyst is chemically unchanged at the end of a reaction
b). A catalyst may appear in the kinetic rate equation of the reaction
c). A catalyst will not affect the composition of an equilibrium mixture
d). A catalyst cannot cause a non-spontaneous (G 0) reaction to proceed
CHEMISTRY ASSIGNMENT-6
Page 2
Narayana IIT Academy
Sr.IIT-IZ & NIZ-COSPARK_PONGAL WORKSHEET
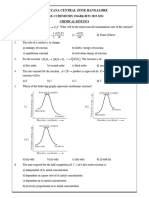
K1 sec
11.
K 2 sec 1
For the reaction
following graph is given,
k1 =4 x 10-2 sec-1. Which is /are correct statement (s) (ln 2=0.7, ln 8/7=0.14)
a). Equilibrium constant is 4.0
b). Time taken for the completion of 50% of equilibrium conc. B is 14 sec.
c). Time taken for the completion of 10% of initial conc. Of A is 2.8sec.
d). Rate constant of backward reaction is 102 sec1
12.
Select the correct statement(s) :
a). In the reaction 92U 235 0 n1 56 Ba140 2 0n1 x , produced x is
b). In the reaction
11
36
Kr 94
Na 23 +z 12 Mg 23 +0n1 the bombarding particle z is deuteron
c). Very large amount of energy is produced during nuclear fission and nuclear fussion
d). In a fission reaction, a loss in mass occurs releasing a vast amount a energy
13.
Select the correct statement(s):
a). SI unit of radioactivity is Becquerel (Bq)
b). 1 ci=3.7 x 107 Bq
c). 3 Li7 +1H1 2 He4 is ( p, ) type reaction
d). The half-life of a particular radioactive isotope is a characteristics constant of that
isotope
14.
Select the correct statement(s) :
a). On bombarding 7 N14 unclei with - particle, the unclei of the product formed after
release of proton would be 8 O17
b). Decay constant does not depend upon tempareture
c). Nuclide and its decay product after - emission are called isodiaphers
d). Half-life of radium is 1580 years. Its average life will be 1097.22 years
CHEMISTRY ASSIGNMENT-6
Page 3
Narayana IIT Academy
15.
16.
Sr.IIT-IZ & NIZ-COSPARK_PONGAL WORKSHEET
Select the correct statement (s) for positron emission by unstable uncleus:
a). X-ray emission takes place
b). A neutron is formed
n
c). of daughter uncleus increases
d). A neutron is consumed
p
Select the correct statement (s):
a). Mass number remains constant when positron emission takes place
0
b). One neutron converts into proton in ( 1e) emission process
17.
18.
19.
20.
c). Activity of a radioactive substance double when temp. increase from 300 K
to
310 K
d). Isodiaphers formed when one alpha particle emitted and isotopes formed when 2
beta particles emitted.
Identify the intensive quantities from the following :
a). Enthalpy
b). Temperature
c). Pressure
d). Mass
Which of the following statements is/are correct as per IUPAC sign convention ?
a). The work done by the system on the surrounding is negative
b). The work done by the surrounding on the system is positive
c). The heat absorbed by the system from the surrounding is positive
d). The heat absorbed by the surrounding from the system is positive
In adiabatic process, the work involved during expansion or compression of an ideal
gas is given by :
T P -T P
v
c). -nRPext 2 1 1 2 d). -2.303 RT log 2
V1
P1P2
One mole of an ideal gas is subjected to a two step reversible process
a). nC v T b).
nR
(T2 -T1 )
-1
(A
C ) The pressure at A and C is same . mark the correct statement(S):
B and B
a). Work involved in the path AB is zero
b). In the path AB work will be done on the gas by the surrounding
c). Volume of gas at C=3 x volume of gas at A
d). Volume of gas at B is 16.42 litres
CHEMISTRY ASSIGNMENT-6
Page 4
Narayana IIT Academy
21.
Sr.IIT-IZ & NIZ-COSPARK_PONGAL WORKSHEET
Which of the following is/are correct?
a). H=U+(PV) when P and V both change
b). H=U+PV when pressure is constant
c). H=U+VP when volume is constant
d). H=U+PV+VP when P and V both changes
22.
23.
24.
For a process to be spontaneous :
a). (Gsystem )T,P =0
b). Ssystem +Ssurrounding >0
c). Ssystem +Ssurrounding <0
d). (Gsystem )T,P <0
When ice melts at 10C :
a). an increase in entropy
b). a decrease in enthalpy
c). a decrease in free energy
d). process is spontaneous
Which of the following statement(s) is /are false ?
a). All adiabatic processes are isoentropic (or isentropic) processes
b). When (G system )T,P <0 ; the reaction must be exothermic
c). dG=VdP-SdT is applicable for closed system, both PV and non- PV work
d). The heat vaporisation of water at 1000C is 40.6 KJ/mol. When 9 gm of water
vapour condenses to liquid at 1000C of 1 atm, then Ssystem =54.42 J/K
25.
From the following data, mark the option(s) where H is correctly written for the
given reaction
Given : H + aq +OH - (aq) H 2O(l);H=-57.3kJ
H solution of HA(g)=-70.7 kJ/mol
H solution of BOH(g)=20 kJ/mol
H ionization of HA=15kJ/mol and BOH is a strong base.
Reaction
H r (kJ/mol)
a). HA(aq)+BOH(aq) BA(aq)+H 2O
-42.3
b). HA(g)+BOH(g) BA(aq)+H 2O
-93
c). HA(g) H+ (aq)+A- (aq)
-55.7
d). B+ (aq)+OH- (aq) BOH(aq)
-20
CHEMISTRY ASSIGNMENT-6
Page 5
Potrebbero piacerti anche
- O Level Biology Practice Questions And Answers EnzymesDa EverandO Level Biology Practice Questions And Answers EnzymesValutazione: 5 su 5 stelle5/5 (1)
- Chem 1120 - Chapter 14: Chemical Equilibrium Practice Quiz 1Documento10 pagineChem 1120 - Chapter 14: Chemical Equilibrium Practice Quiz 1Danielle Lois AbagNessuna valutazione finora
- A Modern Course in Statistical PhysicsDa EverandA Modern Course in Statistical PhysicsValutazione: 3.5 su 5 stelle3.5/5 (2)
- Test Chapter 13 2014-2015Documento6 pagineTest Chapter 13 2014-2015Youssef samehNessuna valutazione finora
- Xi Chem 13.01.24Documento2 pagineXi Chem 13.01.24faraazahmed70058Nessuna valutazione finora
- CH 017Documento31 pagineCH 017phdf5s2p5gNessuna valutazione finora
- SCH4U Practice Exam 07 08Documento18 pagineSCH4U Practice Exam 07 08Mahir AhmedNessuna valutazione finora
- Chapter-6 Chemical-Equilibrium ExercisesDocumento8 pagineChapter-6 Chemical-Equilibrium Exercisestran huyNessuna valutazione finora
- JRS PhyChemDocumento13 pagineJRS PhyChemsalazarjoelNessuna valutazione finora
- CHEM311 211 Major2 SolvedDocumento9 pagineCHEM311 211 Major2 SolvedhussainNessuna valutazione finora
- Chapter 5 Chemical Kinetics - ExercisesDocumento7 pagineChapter 5 Chemical Kinetics - Exercisestran huyNessuna valutazione finora
- Tutorial Kit (Chemistry-100 L) - Vol. 2Documento30 pagineTutorial Kit (Chemistry-100 L) - Vol. 2Terhemen AnjiraNessuna valutazione finora
- Chemical Kinetics TestDocumento5 pagineChemical Kinetics Testrajneesh kumarNessuna valutazione finora
- Revisin Test - Chemical KineticsDocumento4 pagineRevisin Test - Chemical KineticsSABIQNessuna valutazione finora
- CHEM 1212 202002 Exam 1 Form A KeyDocumento7 pagineCHEM 1212 202002 Exam 1 Form A KeyHamza AhmedNessuna valutazione finora
- @bohring - Bot CHEMICAL KINETICS ASSIGNMENT @HeyitsyashXDDocumento77 pagine@bohring - Bot CHEMICAL KINETICS ASSIGNMENT @HeyitsyashXDxkryxxzNessuna valutazione finora
- CRE - Diagnostic Exam (USA)Documento2 pagineCRE - Diagnostic Exam (USA)Kuo SarongNessuna valutazione finora
- Chem Final Exa, MDocumento14 pagineChem Final Exa, MOMARNessuna valutazione finora
- Chemical Kinetics FinalDocumento7 pagineChemical Kinetics Finalaxiliya6Nessuna valutazione finora
- XI Chemistry Pre-Annual 02.02.2022Documento5 pagineXI Chemistry Pre-Annual 02.02.2022Ankit TanwarNessuna valutazione finora
- CHEM2 Long Quiz 2Documento4 pagineCHEM2 Long Quiz 2Maria Leonora PaltaoNessuna valutazione finora
- Exam I Review QuestionsDocumento9 pagineExam I Review QuestionsRylan SmolikNessuna valutazione finora
- CHEM 178 Stauffer Chelsea Practice Multiple Choice Exam 1 - KeyDocumento5 pagineCHEM 178 Stauffer Chelsea Practice Multiple Choice Exam 1 - KeyWillie SalimNessuna valutazione finora
- CY2301Documento11 pagineCY2301Prarabdha SharmaNessuna valutazione finora
- GATE 2017 Chemical Engineering Question PaperDocumento15 pagineGATE 2017 Chemical Engineering Question PaperGurunath EpiliNessuna valutazione finora
- CHEM110 Practice Exam 4 KeyDocumento4 pagineCHEM110 Practice Exam 4 KeyZama MtnNessuna valutazione finora
- Equilibrium Review Packet - KEYDocumento12 pagineEquilibrium Review Packet - KEYRené A. BarreraNessuna valutazione finora
- CHEM 178 Stauffer Chelsea Practice Multiple Choice Exam 1 - KeyDocumento4 pagineCHEM 178 Stauffer Chelsea Practice Multiple Choice Exam 1 - KeyTristan PereyNessuna valutazione finora
- R - CH 15 Practice TestDocumento4 pagineR - CH 15 Practice TestRodel RemolanaNessuna valutazione finora
- CH-4 Kinetics MaterialDocumento18 pagineCH-4 Kinetics MaterialBishal MishraNessuna valutazione finora
- Thermodynamics 1 0df4f738 588a 4bdf A3ea 851471138ddeDocumento91 pagineThermodynamics 1 0df4f738 588a 4bdf A3ea 851471138ddeAnkit SinghNessuna valutazione finora
- Problem Set 1Documento8 pagineProblem Set 1Bj LarracasNessuna valutazione finora
- tb13 PDFDocumento22 paginetb13 PDFMavis VermillionNessuna valutazione finora
- Class Xii Pre Board Question Paper ChemistryDocumento17 pagineClass Xii Pre Board Question Paper ChemistryJeremiah ShibuNessuna valutazione finora
- PCP Diag 2 Trial 1Documento4 paginePCP Diag 2 Trial 1Paulo Emmanuele BetitaNessuna valutazione finora
- Practice Test Chapter 12 and 13Documento9 paginePractice Test Chapter 12 and 13luis arauzNessuna valutazione finora
- Kinetics Ans Key Master FileDocumento10 pagineKinetics Ans Key Master FileJOANA RHEA SAGPAEYNessuna valutazione finora
- Exam 2 Chem 135 Blue - AnswersDocumento10 pagineExam 2 Chem 135 Blue - AnswersSerena GaskellNessuna valutazione finora
- Chem Class Xi-2022Documento7 pagineChem Class Xi-2022Gourav SwainNessuna valutazione finora
- 201B Work 1 KineticsDocumento9 pagine201B Work 1 Kineticsahraz93Nessuna valutazione finora
- Chapter 13 Chemical KineticsDocumento10 pagineChapter 13 Chemical KineticsJacob McPhersonNessuna valutazione finora
- Equilibrium Multiple Choice ReviewDocumento33 pagineEquilibrium Multiple Choice ReviewXUNessuna valutazione finora
- Section-I: IIT - JEE 2014 TW Test MARKS: 65 Time: 1Hr Topics:Chemical EquilibriumDocumento5 pagineSection-I: IIT - JEE 2014 TW Test MARKS: 65 Time: 1Hr Topics:Chemical EquilibriumAnshul JindalNessuna valutazione finora
- Chemistry CH 16 Whitten 10th EditionDocumento6 pagineChemistry CH 16 Whitten 10th EditionGabriel WilliamsNessuna valutazione finora
- Kinetics Mc1Documento6 pagineKinetics Mc1hylee102594Nessuna valutazione finora
- PLTL Ch. 16 AssignmentDocumento6 paginePLTL Ch. 16 AssignmentJules BrunoNessuna valutazione finora
- JEE - Chemistry - Chemical KineticsDocumento27 pagineJEE - Chemistry - Chemical Kineticsdaiwikchilukuri321Nessuna valutazione finora
- Physical Chemistry 2Documento10 paginePhysical Chemistry 2Clara MazangoNessuna valutazione finora
- Final Exam CRIM ADGEDocumento9 pagineFinal Exam CRIM ADGEBernard Agcanas FelipeNessuna valutazione finora
- Answer Key TEST-1 Paper 1 11th PCMB CHEMISTRY (19-01-2024)Documento10 pagineAnswer Key TEST-1 Paper 1 11th PCMB CHEMISTRY (19-01-2024)9C Jagmeet SinghNessuna valutazione finora
- XII ChemistryDocumento6 pagineXII ChemistrySaraswati maharanaNessuna valutazione finora
- 12th KCET Chemistry PaperDocumento8 pagine12th KCET Chemistry PaperGokul yadavNessuna valutazione finora
- Cbse+2 Chemistry 1mark Bits 2023-2024Documento41 pagineCbse+2 Chemistry 1mark Bits 2023-2024lama lamaNessuna valutazione finora
- Exam 2 ChemistryDocumento7 pagineExam 2 ChemistryEvelynNessuna valutazione finora
- Deodhar Classes Edited PDF 12Documento6 pagineDeodhar Classes Edited PDF 12Aditya MoreNessuna valutazione finora
- C CC CDocumento9 pagineC CC CAkhil KhannaNessuna valutazione finora
- Deodhar Classes PDF 2Documento6 pagineDeodhar Classes PDF 2Aditya MoreNessuna valutazione finora
- CH13 Practice ExamDocumento8 pagineCH13 Practice ExamAnonymous WI0nbsNessuna valutazione finora
- Multiple Choices Questions: K K K K (CO)Documento14 pagineMultiple Choices Questions: K K K K (CO)MutasimNessuna valutazione finora
- Answer: B: Selected/modified From Brown Et Al: Chemistry The Central Science, 10e, 12e, 13e TestbanksDocumento6 pagineAnswer: B: Selected/modified From Brown Et Al: Chemistry The Central Science, 10e, 12e, 13e Testbanksفاطمة كليبNessuna valutazione finora
- Periodic Properties Full Reading Resources - S2K17Documento41 paginePeriodic Properties Full Reading Resources - S2K17Suryansh SrivastavaNessuna valutazione finora
- AIiTS 5 (XII) - SET ADocumento16 pagineAIiTS 5 (XII) - SET ASuryansh SrivastavaNessuna valutazione finora
- TestBookLet (A)Documento30 pagineTestBookLet (A)Suryansh SrivastavaNessuna valutazione finora
- Speed Reading Tips: For Graduate StudentsDocumento18 pagineSpeed Reading Tips: For Graduate StudentsTinNessuna valutazione finora
- Using The Linux Command LineDocumento10 pagineUsing The Linux Command LineSuryansh SrivastavaNessuna valutazione finora
- Narayana Iit Academy: Chemistry-Assignment-2Documento2 pagineNarayana Iit Academy: Chemistry-Assignment-2Suryansh SrivastavaNessuna valutazione finora
- Nuclear Chemistry: Unit 11Documento15 pagineNuclear Chemistry: Unit 11Suryansh SrivastavaNessuna valutazione finora
- Alkyl and Aryl Halides Test Level 1 Friday 29 April, 2016: SP SP SP SPDocumento10 pagineAlkyl and Aryl Halides Test Level 1 Friday 29 April, 2016: SP SP SP SPSuryansh SrivastavaNessuna valutazione finora
- BMC Int Jan22 2013 HatsAndLogicPuzzles PDFDocumento5 pagineBMC Int Jan22 2013 HatsAndLogicPuzzles PDFSuryansh SrivastavaNessuna valutazione finora
- Effect of Velocity On Orbital Motion: Starting Points For Orbital EquationsDocumento4 pagineEffect of Velocity On Orbital Motion: Starting Points For Orbital EquationsSuryansh SrivastavaNessuna valutazione finora
- ICH Quality Guidelines: An Implementation GuideDa EverandICH Quality Guidelines: An Implementation GuideAndrew TeasdaleNessuna valutazione finora
- Chemistry for Breakfast: The Amazing Science of Everyday LifeDa EverandChemistry for Breakfast: The Amazing Science of Everyday LifeValutazione: 4.5 su 5 stelle4.5/5 (14)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincDa EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincValutazione: 3.5 su 5 stelle3.5/5 (137)
- It's Elemental: The Hidden Chemistry in EverythingDa EverandIt's Elemental: The Hidden Chemistry in EverythingValutazione: 4 su 5 stelle4/5 (10)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsDa EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsValutazione: 5 su 5 stelle5/5 (3)
- Taste: Surprising Stories and Science About Why Food Tastes GoodDa EverandTaste: Surprising Stories and Science About Why Food Tastes GoodValutazione: 3 su 5 stelle3/5 (20)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactDa EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactValutazione: 5 su 5 stelle5/5 (5)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeDa EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeValutazione: 5 su 5 stelle5/5 (1)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactDa EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactValutazione: 5 su 5 stelle5/5 (1)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeDa EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeValutazione: 4 su 5 stelle4/5 (1)
- The Production of Volatile Oils and Perfumery Plants in the United StatesDa EverandThe Production of Volatile Oils and Perfumery Plants in the United StatesNessuna valutazione finora
- Guidelines for Defining Process Safety Competency RequirementsDa EverandGuidelines for Defining Process Safety Competency RequirementsValutazione: 3 su 5 stelle3/5 (1)
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeDa EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeNessuna valutazione finora
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideDa EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNessuna valutazione finora
- Chemistry for Breakfast: The Amazing Science of Everyday LifeDa EverandChemistry for Breakfast: The Amazing Science of Everyday LifeValutazione: 4.5 su 5 stelle4.5/5 (90)
- Essential Chemistry for Formulators of Semisolid and Liquid DosagesDa EverandEssential Chemistry for Formulators of Semisolid and Liquid DosagesValutazione: 5 su 5 stelle5/5 (2)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeDa EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeValutazione: 5 su 5 stelle5/5 (4)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsDa EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNessuna valutazione finora
- The Periodic Table: A Very Short IntroductionDa EverandThe Periodic Table: A Very Short IntroductionValutazione: 4.5 su 5 stelle4.5/5 (3)