Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
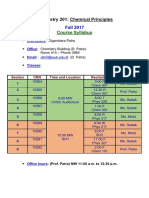
Chemistry 133/134 - Fall 2016 Syllabus: (All Readings and Assignments Are in Hein and Arena)
Caricato da
klaus danjolliTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Chemistry 133/134 - Fall 2016 Syllabus: (All Readings and Assignments Are in Hein and Arena)
Caricato da
klaus danjolliCopyright:
Formati disponibili
CHEMISTRY 133/134 - FALL 2016 SYLLABUS
(All readings and assignments are in Hein and Arena)
Exact pace of topics and associated problems subject to change, as determined in lecture
LEC #
DATE
M 10/17
W 10/19
REC
M 10/24
W 10/26
2.1 2.5
2.6 2.8
4.3
5.1 5.9
Th 10/27 or F 10/28
M 10/31
W 11/2
REC
1.1 1.4
3.1 3.4
4.1 4.2
10.1 10.4
10.4 10.5
6.2
Th 11/3 or F 11/4
M 11/7
11.1 11.5
W 11/9
11.6
11.11
REC
Th 11/10 or F 11/11
M 11/14
MIDTERM
10
W 11/16
6.2 6.6
REC
11
M 11/ 21
7.1 7.5
12
M11/28
8.1 8.3
9.1 9.4
13
W 11/30
9.4 9.5
15
REC
Dimensional analysis: a problem-solving method, density and percentage
Problem solving strategies: page 68
2: 20, 22, 24, 26, 28, 30, 32, 33, 34, 36, 40, 46, 52, 54, 59, 60, 61, 66, 72, 75, 78, 81, 87, 89
Early atomic theory and structure, isotopes, atomic mass
5: Review questions: All; 5: 16, 18, 20, 22, 24, 32, 34, 37, 38
QUIZ 1 (Lec 1, 2, 3)
Modern atomic theory, the Bohr atom, energy levels of electrons, electron configurations
10: Review questions: All; 10: 2, 4, 6, 8, 10, 12, 14, 16, 18, 22, 26, 28, 32, 46, 56, 57, 71
Electron configurations and the Periodic Table, elements and ions
10: 40, 42, 44, 48, 59, 65, 66, 67
QUIZ 2 (Lec 4, 5)
Chemical bonds, formation of compounds from atoms, periodic trends in atomic properties, Lewis
structure of atoms, ionization energy, ionic bond, covalent bond, predicting formula of ionic
compounds
11: Review questions: All; 11: 1, 2, 4, 6, 8, 10, 12, 14, 16, 18, 26, 28, 56
Electronegativity, Lewis structures of compounds, complex Lewis structures, VSEPR and molecular
shape
11: 24, 30, 32, 33, 34, 40, 51, 60, 72
QUIZ 3 (Lec 6, 7, 8)
No lecture on exam day. Exam at 9:40-11:00 pm. Exam covers Lec 1 8
Ions, Formulas and name of ionic and molecular compounds, polyatomic ions, acids and bases
6: 2, 4, 6, 8, 12, 14, 10, 16, 18, 19, 20, 22, 24
Th 11/17 or F 11/18
W 11/23 Friday classes
REC
14
TOPIC and ASSIGNMENT
Course introduction, the nature of chemistry, classification of matter, properties of matter, chemical
and physical change, elements and compounds, introduction to the Periodic Table, compounds and
formulas
1: 2, 4, 6, 9, 12
3: Review questions: All; 3: 2, 6, 8, 10, 12, 14, 16, 23, 26, 29, 30, 34, 36, 39, 44
4: Review questions: All; 4: 1, 2, 7, 8; Math Sheet
Standards for measurement, significant figures, SI units, unit conversions, dimensional analysis
2: Review questions: All; 2: 2, 4, 6, 8, 10,12, 14, 18
Th 10/20 or F 10/21
REC
READING
Th 12/1 or F 12/2
M 12/5
12.1 12.5
W 12/7
12.6 12.9
Th 12/8 or F 12/9
Quantitative composition of compounds, mole, molar mass, empirical and molecular formula
7: Review questions: All; 7: 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 26, 30, 32, 34, 40, 47, 64
No recitation 11/22 and 11/23
Chemical equations, writing and balancing chemical equations, type of chemical equations,
calculations from chemical equations, mole-mole, mole-mass, mass-mass calculations
8: Review questions: All; 8: 4, 8, 10, 12, 16
9: Review questions: All; 9: 2, 4, 8, 10, 12, 16, 18
Limiting reactants and yield calculations
9: 24, 26, 28, 30, 32, 48, 50, 58, 59
QUIZ 4 (Lec 10, 11, 12)
Gaseous state of matter, pressure, gas laws, combined gas laws
12: Review questions: All; 12: 2, 4, 6, 8, 9, 10, 12, 14, 16, 57, 72, 90
Ideal gas equation, gas mixtures, partial pressure, density of gas; stoichiometry involving gas
12: 76, 79, 81, 82, 83
12: 46, 48, 84, 85, 86, 87, 88
QUIZ 5 (Lec 13, 14, 15)
Energy, heat, quantitative measurement of heat
4: 16, 18, 20, 21, 22, 27, 32, 33, 35
16
M 12/12
4.4-4.5
17
W 12/14
F 12/16
Review
FINAL EXAM 12:00 3:00 PM
Potrebbero piacerti anche
- Regents Chemistry--Physical Setting Power Pack Revised EditionDa EverandRegents Chemistry--Physical Setting Power Pack Revised EditionNessuna valutazione finora
- Chemistry 201: Chemical Principles: Course SyllabusDocumento8 pagineChemistry 201: Chemical Principles: Course SyllabushjuhjNessuna valutazione finora
- ENGINEERING 3014 Engineering ChemistrySummerDocumento2 pagineENGINEERING 3014 Engineering ChemistrySummerKevin HuangNessuna valutazione finora
- Coslet Ap Chemistry: Analysis of Food Dyes in BevragesDocumento7 pagineCoslet Ap Chemistry: Analysis of Food Dyes in BevragesKostas KarageorgiouNessuna valutazione finora
- BIOC 301 Course Syllabus Fall 2013 - Keck Hall 100 - 11 To 11:55 AM MWFDocumento2 pagineBIOC 301 Course Syllabus Fall 2013 - Keck Hall 100 - 11 To 11:55 AM MWFasdadssadNessuna valutazione finora
- Che101 ChemistryDocumento9 pagineChe101 ChemistrySiddharth MohanNessuna valutazione finora
- ScheduleDocumento1 paginaSchedulejalalhNessuna valutazione finora
- Lovely Professional University, PunjabDocumento8 pagineLovely Professional University, PunjabSaurabh PandeyNessuna valutazione finora
- Course Information: Middle East Technical UniversityDocumento5 pagineCourse Information: Middle East Technical UniversityErgin ÖzdikicioğluNessuna valutazione finora
- Natural Science (Chemistry)Documento3 pagineNatural Science (Chemistry)Nabil AbdullahNessuna valutazione finora
- GUESS##03028924284788$$Documento2 pagineGUESS##03028924284788$$zada08755Nessuna valutazione finora
- Lovely Professional University, Phagwara: INSTRUCTIONAL PLAN (For Lectures)Documento13 pagineLovely Professional University, Phagwara: INSTRUCTIONAL PLAN (For Lectures)Er Vishal Vaibhav VermaNessuna valutazione finora
- Course Compact STC 111Documento6 pagineCourse Compact STC 111Benjamen FolarinNessuna valutazione finora
- Theory NUCLEUS CHAPTERSDocumento1 paginaTheory NUCLEUS CHAPTERSRamshaNessuna valutazione finora
- Subject: Chapter Name Correct in SUBJ / OBJ (6/6)Documento1 paginaSubject: Chapter Name Correct in SUBJ / OBJ (6/6)Prajwal SethNessuna valutazione finora
- 260 Schedule W12 v1Documento3 pagine260 Schedule W12 v1Kurt Supertramp KwiatkowskiNessuna valutazione finora
- IX Phy Ch10 Gravitation ChapterNotesDocumento8 pagineIX Phy Ch10 Gravitation ChapterNotesGKJK2530Nessuna valutazione finora
- Lovely Professional University, Punjab: Instruction PlanDocumento13 pagineLovely Professional University, Punjab: Instruction PlanGourav KumarNessuna valutazione finora
- Chemistry ScheduleDocumento4 pagineChemistry SchedulePrinceOfAsturiasNessuna valutazione finora
- H - M Guess Paper Chemistry 9TH 2024Documento2 pagineH - M Guess Paper Chemistry 9TH 2024zianali2005Nessuna valutazione finora
- Literature For Canvas Tests ChemistryDocumento24 pagineLiterature For Canvas Tests Chemistryrio kurniaNessuna valutazione finora
- Mastering Organic Chemistry and INORGANICDocumento11 pagineMastering Organic Chemistry and INORGANICsatyag24Nessuna valutazione finora
- 6040 Lab Ex 4 DrukDocumento16 pagine6040 Lab Ex 4 DrukДмитрий БарановNessuna valutazione finora
- Handout - 2021 - CHEM F111Documento2 pagineHandout - 2021 - CHEM F111vishnuNessuna valutazione finora
- Syllabus 1277333391Documento2 pagineSyllabus 1277333391Ivan Jorge Clemente BaptistaNessuna valutazione finora
- Instructors:: Chem 260 Chemical Principles Winter 2001Documento5 pagineInstructors:: Chem 260 Chemical Principles Winter 2001hiNessuna valutazione finora
- CHEM 101 - Principles of ChemistryDocumento4 pagineCHEM 101 - Principles of ChemistrySaad Abdul AleemNessuna valutazione finora
- RPT f5 Chemistry + PekaDocumento6 pagineRPT f5 Chemistry + Pekafizaali87Nessuna valutazione finora
- Lecture Plan - Chem - Spring 2022-23 - 17weekDocumento3 pagineLecture Plan - Chem - Spring 2022-23 - 17weekreduan sadikNessuna valutazione finora
- Chemistry Imp Topics ChapterwiseDocumento5 pagineChemistry Imp Topics ChapterwiseTayseer SaudiaNessuna valutazione finora
- Course OutlineDocumento7 pagineCourse OutlineZaid AweidaNessuna valutazione finora
- Department of Natural Science (Chemistry) : Lecture PlanDocumento3 pagineDepartment of Natural Science (Chemistry) : Lecture Planjahidul islamNessuna valutazione finora
- Topics For AP Chemistry ExamDocumento4 pagineTopics For AP Chemistry Examnoura ahajriNessuna valutazione finora
- Chemistry Text BookDocumento1.651 pagineChemistry Text Bookengr.okaforaugustineNessuna valutazione finora
- C 47 Cdecf 5010707033 Ead 928 e 1 FF 70 HJJBDDocumento8 pagineC 47 Cdecf 5010707033 Ead 928 e 1 FF 70 HJJBDMukul RishirajNessuna valutazione finora
- Class 12 Chemistry Support Material Part-ADocumento117 pagineClass 12 Chemistry Support Material Part-ANIRUPAMA VNessuna valutazione finora
- LP CHY Fall 2010-2011Documento4 pagineLP CHY Fall 2010-2011Mahmud ShaadNessuna valutazione finora
- ZUMDAHL 7th Ed - Table of ContentsDocumento5 pagineZUMDAHL 7th Ed - Table of ContentsHelen NguyenNessuna valutazione finora
- Relevant Textbook Sections Organic Chemistry, 5 Edition by Jones and FlemingDocumento3 pagineRelevant Textbook Sections Organic Chemistry, 5 Edition by Jones and FlemingQuagmilionNessuna valutazione finora
- Chem 171 - 001Documento5 pagineChem 171 - 001princegodsonmailNessuna valutazione finora
- Introduction To Inorganic ChemistryDocumento5 pagineIntroduction To Inorganic Chemistrymsc6Nessuna valutazione finora
- Chem1001 2016 Sem-1Documento4 pagineChem1001 2016 Sem-1DoonkieNessuna valutazione finora
- Chemistry 201: Chemical Principles: Course SyllabusDocumento8 pagineChemistry 201: Chemical Principles: Course SyllabushjuhjNessuna valutazione finora
- Notes From The Chemistry Director 2023-2024Documento5 pagineNotes From The Chemistry Director 2023-2024gaminginsane372Nessuna valutazione finora
- General Chemistry 2 Learning CompetencieDocumento6 pagineGeneral Chemistry 2 Learning CompetencieJennifer ConchaNessuna valutazione finora
- Chema 103 Schedule Fa 15Documento3 pagineChema 103 Schedule Fa 15Cindy Hogan TrussellNessuna valutazione finora
- (Advanced Chemistry) Herbert August Laitinen, Walter E. Harris-Chemical Analysis - An Advanced Text and Reference-McGraw-Hill (1975) PDFDocumento627 pagine(Advanced Chemistry) Herbert August Laitinen, Walter E. Harris-Chemical Analysis - An Advanced Text and Reference-McGraw-Hill (1975) PDFarefNessuna valutazione finora
- MSBSHSE Class 11 Chemistry 2021 22 Deleted PortionsDocumento1 paginaMSBSHSE Class 11 Chemistry 2021 22 Deleted PortionsRafiqkhot KhotNessuna valutazione finora
- MATH 126 - Calculus II: Fall 2016 Laboratory ScheduleDocumento4 pagineMATH 126 - Calculus II: Fall 2016 Laboratory ScheduleZack RosenkransNessuna valutazione finora
- Chemistry Chapter List PDFDocumento1 paginaChemistry Chapter List PDFNitin JethwaNessuna valutazione finora
- Unit 2: Physics Unit 3: Biology Unit 1: Chemistry: KeywordsDocumento30 pagineUnit 2: Physics Unit 3: Biology Unit 1: Chemistry: Keywordsd_feeneyNessuna valutazione finora
- Maharashtra-HSC-d & F Paper-2 TargetDocumento39 pagineMaharashtra-HSC-d & F Paper-2 TargetkrritikksNessuna valutazione finora
- High School General Chemistry Science and Technology IIIDocumento6 pagineHigh School General Chemistry Science and Technology IIICarlo Joseph MoskitoNessuna valutazione finora
- Chemisty Unit PlanDocumento6 pagineChemisty Unit Planapi-266413007Nessuna valutazione finora
- As Support Pack ContentsDocumento2 pagineAs Support Pack Contents7182vvNessuna valutazione finora
- Sample Organic Chemistry Outline 1246Documento12 pagineSample Organic Chemistry Outline 1246hugo2008870% (1)
- FullDocumento681 pagineFullMaria Luisa BorjasNessuna valutazione finora
- Yearly Lesson Plan Chemistry 2015 Form 4Documento15 pagineYearly Lesson Plan Chemistry 2015 Form 4suzi0108Nessuna valutazione finora
- EML5526 Finite Element Analysis: Basic InformationDocumento2 pagineEML5526 Finite Element Analysis: Basic InformationsadiksnmNessuna valutazione finora
- Summarised Yearly Teaching Plan F4Documento14 pagineSummarised Yearly Teaching Plan F4FatimahHishamuddinNessuna valutazione finora
- 1.5 Atomic Structure and PeriodicityDocumento13 pagine1.5 Atomic Structure and PeriodicitychwalidNessuna valutazione finora
- Polarity in Covalent BondsDocumento15 paginePolarity in Covalent BondsMarcoNessuna valutazione finora
- STAAR Chemistry BookDocumento272 pagineSTAAR Chemistry BookNiloy Ghosh100% (1)
- Specification Points Covered: Ionic SubstancesDocumento11 pagineSpecification Points Covered: Ionic SubstancesIsabella ThomasNessuna valutazione finora
- CHEM Types of Solids POGILDocumento7 pagineCHEM Types of Solids POGILKosakenNessuna valutazione finora
- Ionic and Covalent Properties LabDocumento4 pagineIonic and Covalent Properties LabDustin MoenchNessuna valutazione finora
- Polymer Carrer 12Documento33 paginePolymer Carrer 12Ếch OrsonNessuna valutazione finora
- Redox and Equivalent Concepts (Stochiometry-Ii) PDFDocumento24 pagineRedox and Equivalent Concepts (Stochiometry-Ii) PDFaman Kumar Gupta100% (1)
- Dieletric ppt-1Documento18 pagineDieletric ppt-1YASH JAINNessuna valutazione finora
- F.Y.B.sc.-ChemistryDocumento15 pagineF.Y.B.sc.-ChemistryRakesh JamesNessuna valutazione finora
- (R D Shannon) Chemical Bonding in Solids (B-Ok - CC) PDFDocumento171 pagine(R D Shannon) Chemical Bonding in Solids (B-Ok - CC) PDFJaga ParamunitaNessuna valutazione finora
- Ch8 SQDocumento16 pagineCh8 SQhihiNessuna valutazione finora
- Book (Complete)Documento63 pagineBook (Complete)Aditi ShuklaNessuna valutazione finora
- Chemical Bonding Basic (Micro)Documento37 pagineChemical Bonding Basic (Micro)Anant JainNessuna valutazione finora
- Chemical Bonding I: Lewis TheoryDocumento33 pagineChemical Bonding I: Lewis TheoryBiruk BtNessuna valutazione finora
- Equilibrium Thermodynamics Predicts The Concentrations (Or, More Precisely, Activities) ofDocumento60 pagineEquilibrium Thermodynamics Predicts The Concentrations (Or, More Precisely, Activities) ofJulian Felipe Peña RamirezNessuna valutazione finora
- 11 Chemistry Notes ch11 The P Block Element PDFDocumento4 pagine11 Chemistry Notes ch11 The P Block Element PDFRangbaaz DA FIRENZENessuna valutazione finora
- Chemistry Chapter 2 NotesDocumento5 pagineChemistry Chapter 2 Notesvolleycrew818Nessuna valutazione finora
- Am. J - Med. and Hyg.,: Communications Enr VolDocumento3 pagineAm. J - Med. and Hyg.,: Communications Enr VolFELIPE DANIEL MONTERO BRUNINessuna valutazione finora
- Introduction To Organic Chemistry:: Atomic Orbitals and Molecular OrbitalsDocumento64 pagineIntroduction To Organic Chemistry:: Atomic Orbitals and Molecular OrbitalsChristine Mae VeaNessuna valutazione finora
- Ionic Bond NotesDocumento4 pagineIonic Bond Notesapi-197752333100% (1)
- CUP IBChemistry c03 It BondingDocumento59 pagineCUP IBChemistry c03 It BondingAdnan ChowdhuryNessuna valutazione finora
- Science 9 2nd QTR Exam With Answer KeyDocumento3 pagineScience 9 2nd QTR Exam With Answer KeyKatrina Lourdes SorianoNessuna valutazione finora
- 4 Intermolecular Forces (S)Documento15 pagine4 Intermolecular Forces (S)Mr TanNessuna valutazione finora
- Chap 1 Periodic Table ExerciseDocumento26 pagineChap 1 Periodic Table ExerciseAbhimanyu GuptaNessuna valutazione finora
- 5129 - Y10 - Sy (Combined Science Syllabus)Documento30 pagine5129 - Y10 - Sy (Combined Science Syllabus)Ahmed Kaleem Khan NiaziNessuna valutazione finora
- X - TS Important QPsDocumento8 pagineX - TS Important QPsAman PrasadNessuna valutazione finora
- Physical ScienceDocumento117 paginePhysical Sciencechi582552Nessuna valutazione finora
- ZIMSEC O' Level Chemistry HandoutDocumento120 pagineZIMSEC O' Level Chemistry Handoutjacob t Ngwenya100% (10)
- Chemical Bond Assig (Ans) 04 11 20Documento4 pagineChemical Bond Assig (Ans) 04 11 20Rushikesh ThoratNessuna valutazione finora