Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Review of Materials Revolution So Far
Caricato da
OCRChemistrySaltersDescrizione originale:
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Review of Materials Revolution So Far
Caricato da
OCRChemistrySaltersCopyright:
Formati disponibili
Review of Materials Revolution so far.
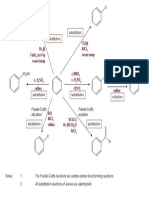
Reactions of Amines:
September 2009. Write an equation for the reaction of 1-
aminopropane with:
SL MR2 is about Making and Breaking polymers. The polymers are ___________________ polymers which mean that when they a. water and use this to explain why the
were made, many monomers joined together and small molecules such as water were also produced. Two types of polymer in the MR2 solution is alkaline and why the amine is
Storyline are poly _________ and poly _________. In Polymer revolution at AS, ___________________ polymers were studied. called a base
When the monomers join together, no small molecules are lost. This is typical of monomers which are _____________________.
Polyamides polyamides polyamide b. with hydrochloric acid and describe what
Polyesters polyesters polyesters polyesters effect this will have on the smell
Making a polyester: Making Nylon-6,5
Write an equation for the formation of a polyester from butanedioc acid Give the skeletal formulae of the two monomers used
and ethane-1,2-diol. Use full structural formulae. in industry.
c. ethanoyl chloride and explain why this
reaction is sometimes called an acylation
reaction
Draw the repeating unit in the polymer.
Recrystallisation:
Breaking esters - hydrolysis: Why is the acyl chloride often used in the lab? You have some hexanedioic acid which may have the
Write the structural formulae of the products of hydrolysis of ethyl imputities 1,6-diaminohexane and nylon. You need to
methanoate purify the acid.
a. heat and reflux with dilute HCl What type of amide is the polymer? 1. You dissolve the hexanedioc acid in the
minimum quantity of hot water. Why use the
minimum quantity?
You decant or filter to remove any solid. Which
Breaking amides - hydrolysis:
b. heat and reflux with dilute NaOH impurity is removed? Explain why only this
This is bond ________________ involving
impurity is removed.
_________________.
Write the structural formulae of the products of
O 2. Which crystals will appear on cooling? Why
are these the ones produced?
CH3 C N CH3
hydrolysis of :
H
Breaking polyseters - hydrolysis: a. heat and reflux with moderately 3. The crystals are filtered and washed with
Give two examples from the Storylines of where the hydrolysis of concentrated HCl cold water. Why cold water rather than hot?
polyesters is utilized:
b. heat and reflux with moderately 4. The crystals are dried in an oven. Their
concentrated NaOH melting point is then determined. How do you
know that the crystals are pure from the
melting point??
Potrebbero piacerti anche
- Medicines by Design..: Revision of Reagents, Conditions and Reaction Types. Click To Go To A Particular SectionDocumento39 pagineMedicines by Design..: Revision of Reagents, Conditions and Reaction Types. Click To Go To A Particular SectionOCRChemistrySaltersNessuna valutazione finora
- MBD Toolkit BDocumento1 paginaMBD Toolkit BOCRChemistrySaltersNessuna valutazione finora
- Memory Map AromaticsDocumento1 paginaMemory Map AromaticsOCRChemistrySaltersNessuna valutazione finora
- Is This Your RoomDocumento25 pagineIs This Your RoomOCRChemistrySaltersNessuna valutazione finora
- MBD Toolkit ADocumento1 paginaMBD Toolkit AOCRChemistrySaltersNessuna valutazione finora
- Buffer Solutions: How Do Buffers Work?Documento2 pagineBuffer Solutions: How Do Buffers Work?OCRChemistrySaltersNessuna valutazione finora
- AromaticsDocumento1 paginaAromaticsOCRChemistrySaltersNessuna valutazione finora
- Energy Changes in SolutionsDocumento18 pagineEnergy Changes in SolutionsOCRChemistrySaltersNessuna valutazione finora
- Buffers: Calculating The PH of A Buffer SolutionDocumento8 pagineBuffers: Calculating The PH of A Buffer SolutionOCRChemistrySaltersNessuna valutazione finora
- Ions in Solids and Solutions.: CI 4.5 and Revision of 5.1Documento23 pagineIons in Solids and Solutions.: CI 4.5 and Revision of 5.1OCRChemistrySaltersNessuna valutazione finora
- CC Entropy 1Documento10 pagineCC Entropy 1OCRChemistrySaltersNessuna valutazione finora
- The Size of IonsDocumento20 pagineThe Size of IonsOCRChemistrySaltersNessuna valutazione finora
- X Marks The Spot SS1Documento1 paginaX Marks The Spot SS1OCRChemistrySaltersNessuna valutazione finora
- 13.6 Oils and FatsDocumento9 pagine13.6 Oils and FatsOCRChemistrySaltersNessuna valutazione finora
- JB Chemical Ideas 12point3arenesDocumento9 pagineJB Chemical Ideas 12point3arenesOCRChemistrySaltersNessuna valutazione finora
- Acids and Bases TranslationDocumento1 paginaAcids and Bases TranslationOCRChemistrySaltersNessuna valutazione finora
- CimaDocumento11 pagineCimaOCRChemistrySaltersNessuna valutazione finora
- ChromatographyDocumento10 pagineChromatographyjayNessuna valutazione finora
- 6.9 Chemistry of ColourDocumento7 pagine6.9 Chemistry of ColourOCRChemistrySaltersNessuna valutazione finora
- N (G) + 3H (G) 2Nh (G) H - 92 KJ MolDocumento7 pagineN (G) + 3H (G) 2Nh (G) H - 92 KJ MolOCRChemistrySaltersNessuna valutazione finora
- ThedblockDocumento32 pagineThedblockOCRChemistrySaltersNessuna valutazione finora
- Starter For Lesson 11Documento5 pagineStarter For Lesson 11OCRChemistrySaltersNessuna valutazione finora
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- ELEMAGDocumento1 paginaELEMAGJasper BantulaNessuna valutazione finora
- ElectrolysisDocumento3 pagineElectrolysisRaymond ChanNessuna valutazione finora
- Introducing Small Basic-1 PDFDocumento69 pagineIntroducing Small Basic-1 PDFnilaNessuna valutazione finora
- Practical - Magnetic - Design (Fill Factor) PDFDocumento20 paginePractical - Magnetic - Design (Fill Factor) PDFAhtasham ChaudhryNessuna valutazione finora
- 8 Coil PWM Drivers PDFDocumento4 pagine8 Coil PWM Drivers PDFDuzng Hoang TriNessuna valutazione finora
- Mathematical TricksDocumento4 pagineMathematical Tricksapi-440622270Nessuna valutazione finora
- FP - ES - 28 - Rindu Grahabhakti Intani - PERMEABLE ENTRY CHARACTERIZATION AT DARAJAT FIELD, WEST JAVA PDFDocumento4 pagineFP - ES - 28 - Rindu Grahabhakti Intani - PERMEABLE ENTRY CHARACTERIZATION AT DARAJAT FIELD, WEST JAVA PDFrindu_intaniNessuna valutazione finora
- Robot Sensors and TransducersDocumento176 pagineRobot Sensors and TransducerssakthivelNessuna valutazione finora
- Mathematics GR 11 Paper 2Documento13 pagineMathematics GR 11 Paper 2ora mashaNessuna valutazione finora
- Detection of Repetitive Forex Chart PatternsDocumento8 pagineDetection of Repetitive Forex Chart PatternsDwight ThothNessuna valutazione finora
- HST TrainingDocumento11 pagineHST TrainingRamesh BabuNessuna valutazione finora
- EXCEL - How To Write Perfect VLOOKUP and INDEX and MATCH FormulasDocumento29 pagineEXCEL - How To Write Perfect VLOOKUP and INDEX and MATCH Formulasgerrydimayuga100% (1)
- Solution of Linear System Theory and Design 3ed For Chi Tsong ChenDocumento106 pagineSolution of Linear System Theory and Design 3ed For Chi Tsong ChensepehrNessuna valutazione finora
- Javascript Html5 CanavasDocumento13 pagineJavascript Html5 CanavasmihailuNessuna valutazione finora
- Integrals - Integral Calculus - Math - Khan Academy PDFDocumento7 pagineIntegrals - Integral Calculus - Math - Khan Academy PDFroberteleeroyNessuna valutazione finora
- Day 3 Polygons Lesson PlanDocumento6 pagineDay 3 Polygons Lesson PlanBA RTNessuna valutazione finora
- Tecumseh Parts List OHV 135Documento5 pagineTecumseh Parts List OHV 135M MNessuna valutazione finora
- Good 1983Documento352 pagineGood 1983ASDA75% (4)
- Automatic Pneumatic Bumper Mission: International Journal of Pure and Applied Mathematics No. 16 2017, 137-140Documento4 pagineAutomatic Pneumatic Bumper Mission: International Journal of Pure and Applied Mathematics No. 16 2017, 137-140VinayNessuna valutazione finora
- Knowage Suite Readthedocs Io en 7.4Documento536 pagineKnowage Suite Readthedocs Io en 7.4Sergio Daniel Marambio NuñezNessuna valutazione finora
- 00.concrete Mix Design-RailwayDocumento38 pagine00.concrete Mix Design-RailwaySoundar PachiappanNessuna valutazione finora
- 06 DoniaDocumento12 pagine06 DoniaOmar ZazaNessuna valutazione finora
- Creative Computing v06 n12 1980 DecemberDocumento232 pagineCreative Computing v06 n12 1980 Decemberdarkstar314Nessuna valutazione finora
- Cat Hammers C842898Documento8 pagineCat Hammers C842898maggioraNessuna valutazione finora
- JSF + JPA + JasperReports (Ireport) Part 2 - Ramki Java BlogDocumento7 pagineJSF + JPA + JasperReports (Ireport) Part 2 - Ramki Java BlogMartin MurciegoNessuna valutazione finora
- PR100 BrochureDocumento28 paginePR100 Brochuregus289Nessuna valutazione finora
- Hydrocarbon RecoveryDocumento29 pagineHydrocarbon RecoveryAlekhya BandaruNessuna valutazione finora
- Start Up and Commissioning of Chilled Water PumpsDocumento6 pagineStart Up and Commissioning of Chilled Water PumpsAlaa AnwerNessuna valutazione finora
- Auto-Tune Pid Temperature & Timer General Specifications: N L1 L2 L3Documento4 pagineAuto-Tune Pid Temperature & Timer General Specifications: N L1 L2 L3sharawany 20Nessuna valutazione finora
- Gregory, Robert Wayne Et Al. - 'The Role of Artificial Intelligence and Data Network Effects For Creating User Value'Documento18 pagineGregory, Robert Wayne Et Al. - 'The Role of Artificial Intelligence and Data Network Effects For Creating User Value'DylanOSullivanNessuna valutazione finora