Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
1 s2.0 S073170850600166X Main
Caricato da
Nur Atiqah AhmadTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
1 s2.0 S073170850600166X Main
Caricato da
Nur Atiqah AhmadCopyright:
Formati disponibili
Journal of Pharmaceutical and Biomedical Analysis 41 (2006) 714719
Simultaneous determination of catechin, rutin, quercetin
kaempferol and isorhamnetin in the extract of sea buckthorn
(Hippophae rhamnoides L.) leaves by RP-HPLC with DAD
Yuangang Zu , Chunying Li, Yujie Fu, Chunjian Zhao
Key Laboratory of Forest Plant Ecology, Ministry of Education, Northeast Forestry University, Harbin 150040, PR China
Received 19 October 2004; received in revised form 24 April 2005; accepted 25 April 2005
Available online 7 March 2006
Abstract
A rapid and specific reversed-phase high performance liquid chromatography (RP-HPLC) method with diode array detection (DAD) at room
temperature was used and validated for the simultaneous determination of five flavonoids (catechin, CA; rutin, RU; quercetin, QU; kaempferol,
KA; isorhamnetin, IS) in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves. The sample pretreatment process involved ultrasonic
extraction with 85% ethanol under the frequency of 80 kHz, at a temperature of 45 C for 30 min and with the ratio of liquor to material of 15 mL g1 ,
followed by separation on HIQ SIL C18V column with methanolacetonitrilewater (40:15:45, v/v/v) containing 1.0% acetic acid as a mobile
phase. The extract was detected by DAD at the wavelength of 279 nm for CA, 257 nm for RU, 368 nm for QU, KA and IS. Calibration curves were
found to be linear with the ranges of 0.0110.520 mg ml1 (CA), 0.0070.500 mg ml1 (RU), 0.0190.280 mg ml1 (QU), 0.0100.440 mg ml1
(KA) and 0.0080.400 mg ml1 (IS). The correlation coefficients of linear regression analysis and detection limits were between 0.99630.9999
and 0.000790.00290 mg ml1 . The contents of CA, RU, QU, KA and IS in sea buckthorn leaves were successfully determined with 3.8, 5.2, 7.3,
10.9 and 11.9 min with satisfactory reproducibility and recovery. Recoveries of the five flavonoids were between 97.27 and 99.98%. The method
was applied to the determination of flavonoids in sea buckthorn leaves and was found to be simple, rapid and efficient.
2006 Elsevier B.V. All rights reserved.
Keywords: Sea buckthorn (Hippophae Rhamnoides L.) leaves; RP-HPLC; Catechin; Rutin; Quercetin; Kaempferol; Isorhamnetin
1. Introduction
Sea buckthorn (Hippophae rhamnoides L.) is a thorny nitrogen fixing deciduous shrub, naturally distributed throughout
Asia and Europe [1]. In China sea buckthorn, grown widely
in the northern and southwestern regions, is a phytoprotective
agent to human health. All parts of the plant are considered to
be a good source of a large number of bioactive substances. The
bioactive substances are reputed to have considerable medicinal value and are frequently used for cure cough, skin wounds,
cardiovascular diseases, improving blood circulation and have
antioxidant activity [24]. The chemical compositions of sea
buckthorn have attracted considerable attentions in the world.
The flavonoids in sea buckthorn are bioactive constituents,
which are reported to suppress platelet aggregation, relieve
Corresponding author. Tel.: +86 451 82191517; fax: +86 451 82102082.
E-mail address: zygorl@vip.hl.cn (Y. Zu).
0731-7085/$ see front matter 2006 Elsevier B.V. All rights reserved.
doi:10.1016/j.jpba.2005.04.052
cardiac disease and have antioxidants, hepato-protective and
immunomodulatory properties [59]. CA, RU, QU, KA and
IS are five major flavonoids in the extract of sea buckthorn
leaves [10], which have similar molecular structures as shown in
Fig. 1.
Several chromatographic methods have been documented for
the determination of chemical compositions present in sea buckthorn [1115]. All of the chromatographic methods, HPLC is
applied widely [1619]. CA, RU, QU, KA and IS are five major
flavonoids in the extract of sea buckthorn leaves. Therefore,
it is important for the simultaneous determination of the five
major flavonoids. So far, no report on simultaneous determination of the five major flavonoids in sea buckthorn leaves by
HPLC method.
In the study, HPLC with DAD was used for the simultaneous
determination of CA, RU, QU, KA and IS in the extract of sea
buckthorn leaves. The results indicate that this method is fast,
sensitive and suitable to quantitative assessment in the flavonoids
of sea buckthorn leaves.
Y. Zu et al. / Journal of Pharmaceutical and Biomedical Analysis 41 (2006) 714719
715
tered through 0.45 m membrane filter (Millipore), and injected
directly.
2.4. Preparation of sample solution
The fresh leaves were cleaned and dried at 60 C. The extraction was carried out using 2 g of powdered leaves with 30 ml of
85% ethanol under 80 kHz, 45 C in an ultrasonic extraction
device for 30 min, repeated for twice. The extract was collected
and filtered, the filtrate was dried at 50 C under reduced pressure in a rotary evaporator. The dried extract was dissolved in the
mobile phase. After filtering through a filter paper and a 0.45 m
membrane filter (Millipore), the extract was injected directly.
2.5. Chromatographic conditions
Fig. 1. Structures of five flavonoids in the extract of sea buckthorn leaves.
2. Experimental
2.1. Equipments
The chromatographic system consisted of Millennium32
system software(Waters company, USA), Waters 717 plus
Autosampler (Waters company, USA), Model Waters Delta 600
pump (Waters company, USA), and Model Waters 2996 Diode
Array Detector (Waters company, USA), HIQ SIL C18 V column( 4.6 mm 250 mm, KYA TECH Corporation, Japan).
2.2. Reagents and materials
2.2.1. Reagents and chemicals
Methanol, acetonitrile and acetic acid were of HPLC grade
(Tedia Company, USA). Ethanol was of analytical grade (Beijing Chemical Reagents Company, China). Deionized water was
prepared by a Milli-Q Water Purification system (Millipore, MA,
USA). CA, RU, QU, KA and IS standards were purchased from
Sigma company (USA).
Chromatographic analysis was carried out by HIQ SIL
C18V reversed-phase column ( 4.6 mm 250 mm) packed
with 5 m diameter particles, the mobile phase was methanol
acetonitrilewater (40:15:45, v/v/v) containing 1.0% acetic acid.
This mobile phase was filtered through a 0.45 m membrane filter (Millipore), then deaerated ultrasonically prior to use. CA,
RU, QU, KA and IS were quantified by DAD following RPHPLC separation at 279 nm for CA, 257 nm for RU, 368 nm for
QU, KA and IS. Flow rate and injection volume were 1.0 ml/min
and l0 l, respectively. The chromatographic peaks of the analytes were confirmed by comparing their retention time and UV
spectra with those of the reference standards. Quantification was
carried out by the integration of the peak using external standard method. All chromatographic operations were carried out
at ambient temperature.
3. Results and discussion
3.1. Optimization of sample preparation
Reflux, soxhlet and ultrasonication (all with 85% ethanol as
extractants, leaves powder dried under 60 C, 60 meshes sifter)
were, respectively, used to extract total flavonoids in the sea
buckthorn leaves, repeated twice. The extraction yield of total
flavonoids in sea buckthorn leaves was determined according to
the method of reference [20]. The results are shown in Fig. 2.
2.2.2. Plant material
Leaves of sea buckthorn were collected from the hilly Da He
Kou forestry centre of Sunwu county, Heilongjiang province.
2.3. Preparation of standard solutions
Standard stock solutions of five flavonoids were prepared in ethanol, at concentration of 0.520 mg ml1 for CA,
0.500 mg ml1 for RU, 0.280 mg ml1 for QU, 0.440 mg ml1
for KA and 0.400 mg ml1 for IS. All sample solutions were fil-
Fig. 2. Effect of different sample preparation methods on extraction yield of
total flavonoids in the sea buckthorn leaves.
716
Y. Zu et al. / Journal of Pharmaceutical and Biomedical Analysis 41 (2006) 714719
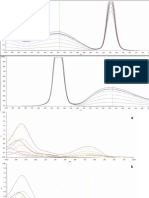
Fig. 3. Chromatogram of CA, RU, QU, KA extract, methanol, acetonitrile or
water is and IS standard mixture (adding no acetic acid to mobile phase), Peak
1 for CA, usually used as a component of the mobile Peak 2 for RU, Peak 3 for
QU, Peak 4 for KA, Peak 5 for IS.
Fig. 2 shows the extraction yield by reflux is obvious low,
extraction yields by soxhlet and ultrasonication are higher and
the results with different the extraction completeness by soxhlet depended to some extent on the extraction time, whereas
that by ultrasonication was almost independent on the extraction time. Moreover, ultrasonication for 30 min gave the almost
same result as soxhlet for 240 min did. Therefore, in this work,
ultrasonic extraction is considered a simpler and more effective
method for extraction of flavonoids in the sea buckthorn leaves
and consequentially used in the following tests.
3.2. Optimization of chromatographic conditions
3.2.1. Effect of mobile phase
The five compounds, CA, RU, QU, KA, and IS are polar
molecules. For polar phase. In the beginning, various proportions methanolwater or acetonitrilewater system was chosen as a mobile phase but separation was not satisfactory.
In succession, a mixture of methanol, acetonitrile and water
in different ratios was tested. Eventually, it was found that
methanolacetonitrilewater (40:15:45, v/v/v) system gave a
much better separation for CA, RU, QU, KA, and IS except
it couldnt baseline separate CA and RU. Under this system,
slight trailing peaks were observed in the chromatograph (see
Fig. 3.)
The presence of acid in a mobile phase system can improve
peak trailing of compounds and change pH value of the mobile
phase, having a significant effect on the resolution of compounds. Therefore, on the basis of methanolacetonitrilewater
(40:15:45, v/v/v) system, adding acetic acid to the mobile phase
was considered at this point. The results obtained are illustrated
in Fig. 4.
The concentration of acetic acid in the mobile phase was varied from 0.00 to 1.00%. The calculated capacity factor increases
as the concentration of acetic acid increases (see Fig. 4). The significant difference is in the capacity factor between CA, RU and
QU, KA, IS. The change in the capacity factor is very small
for CA and RU. It is necessary to choose a proper volume percent acetic acid that gives enough differences in the values of
the capacity factor for a good resolution in the RP-HPLC. In
addition, volume percent of acetic acid in a mobile phase is crucial for column lifetime. The higher concentration acid added
Fig. 4. Effect of acetic acid concentration on the values of the capacity factor.
in mobile phase may produce more better sample separation,
which in turn shorts the HPLC column lifetime. For above these
reasons, 1.0% of acetic acid was chosen.
As a result, a mixture system of methanolacetonitrilewater
(40:15:45, v/v/v) containing 1.0% acetic acid was confirmed
as the optimum mobile phase. Under this system, the chromatogram of CA, RU, QU, KA and IS standard mixture is shown
in Fig. 5.
It can be seen from Fig. 5 that a good separation can be
achieved within 15 min using the conditions described. Symmetrical, sharp and well-resolved peaks were observed for CA,
RU, QU, KA and IS. The retention time for CA, RU, QU, KA
and IS was 3.8, 5.2, 7.3, 10.9 and 1.9 min, respectively.
3.2.2. Effect of detection wavelength
The choice of proper detection mode is crucial to ensure
that all the components are detected. With DAD, this problem can be overcome by using a multiple wavelength scanning
program which is capable of monitoring several wavelengths
simultaneously. It provides assurance that all the UVvis absorbing components are detected, if present in sufficient quantity.
DAD are used to record spectro-chromatograms of compounds
simultaneously. So DAD was used to optimize determination
wavelength in the work.
The UVvis spectrums of CA, RU, QU, KA and IS standards dissolved in the mobile phase were obtained by DAD.
Fig. 5. Chromatogram of CA, RU, QU, KA and IS standard mixture (adding
acetic acid to mobile phase ), Peak 1 for CA, Peak 2 for RU, Peak 3 for QU,
Peak 4 for KA, Peak 5 for IS.
Y. Zu et al. / Journal of Pharmaceutical and Biomedical Analysis 41 (2006) 714719
717
(see Fig. 6 (d) and IS (see Fig. 6(e)), also two peaks separately
appeared at two different wavelength. 368 nm was confirmed as
determination wavelength for QU, KA and IS according to their
corresponding UVvis absorbing value.
3.3. System suitability test
3.3.1. Reproducibility, linearity and detection limit
A standard mixture solution of 0.26 mg ml1 CA,
0.25 mg ml1 RU, 0.14 mg ml1 QU, 0.22 mg ml1 KA and
0.20 mg ml1 IS was analyzed five times to determine the reproducibility of the peak areas and retention time under the optimum
conditions in this experiment. The relative standard deviations
(R.S.D.) of the peak areas and retention time were 1.02 and
1.05% for CA, 1.46 and 1.58% for RU, 2.06 and 2.23% for QU,
1.73 and 2.04% for KA, 1.69 and 1.85% for IS.
A series of the standard mixture solutions of these five
flavonoids were tested to determine the linearity between the
standard mixture concentration and peak areas. The results of
regression analysis on calibration curves and detection limits are
presented in Table 1. The detection limits were evaluated on the
basis of a signal-to-noise ratio of 3 (S/N = 3), the detection limits
was between 0.00079 and 0.00290 mg ml1 for five compounds.
Fig. 6. UVvis absorbing spectrograms of a mixture of CA, RU, QU, KA and
IS standardard: (a) for CA; (b) for RU; (c) for QU; (d) for KA; (e) for IS.
UVvis absorbing spectrograms of the five flavonoids are shown
in Fig. 6.
From the spectrogram of CA (see Fig. 6(a)), we can see
only a peak appeared at 279 nm. Two hundred and seventynine nanometers was confirmed as determination wavelength
for CA. While from the spectrogram of RU (see Fig. 6(b)), we
can see two clear peaks appeared at 257 and 355 nm separately.
The peak at 257 nm is higher than the peak at 355 nm, that is
RUs UV-absorbing value at 257 nm is higher than the value at
355 nm. So 257 nm was confirmed as determination wavelength
for RU. Alike, on the spectrogram of QU (see Fig. 6(c), KA
3.3.2. Precision and stability
The intra-day and inter-day precisions (expressed as the relative standard deviation (R.S.D.) for retention time and peak area
were determined for all five flavonoids standards by repeated
analysis (n = 5) (see Table 2).
The results obtained from Table 2 show that intra-day and
inter-day relative standard deviations for retention time and for
peak area are both quite low and the precision is good.
For stability test, a sample solution (ethanol extract) was analyzed every 12 h in 2 days, and the sample solution was found
to be rather stable within 48 h (R.S.D. < 2.0%).
3.3.3. Sample analysis and recovery
Ethanol solutions in the extract of sea buckthorn leaves were
injected directly and separated under the optimum condition
mentioned earlier. The typical chromatograms in the extract of
sea buckthorn leaves were shown in Fig. 7.
The calculated contents of the five flavonoids were given in
Table 3.
The recovery experiments of the five flavonoids were performed by adding CA, RU, QU, KA and IS standards to the
extract of sea buckthorn leaves, which were treated according to
Table 1
Validation data from calibration curves of five flavonoids in the extract of sea buckthorn leaves
Compound
Regression equation
Correlation coefficient (R)
Linear range (mg ml1 )
Detection limit (mg ml1 )
CA
RU
QU
KA
IS
y = 3.09 l08 x 0.0261
y = 2.08 l08 x + 0.0629
y = 9.60 l09 x + 0.0145
y = 1.44 l08 x + 0.0088
y = 1.15 108 x + 0.0103
0.9963
0.9981
0.9995
0.9999
0.9993
0.0110.520
0.0070.500
0.0190.280
0.0100.440
0.0080.400
0.00092
0.00079
0.00290
0.00115
0.00233
x, peak area; y, concentration (mg ml1 ).
718
Y. Zu et al. / Journal of Pharmaceutical and Biomedical Analysis 41 (2006) 714719
Table 2
Precision (n = 5)
Compound
Intra-day R.S.D. for
retention time (%)
Intra-day R.S.D. for
peak area (%)
Inter-day R.S.D. for
retention time (%)
Inter-day R.S.D. for
peak area (%)
CA
RU
QU
KA
IS
1.12
1.04
1.36
1.26
1.51
1.20
1.32
1.36
1.19
1.46
1.93
1.73
1.82
1.60
1.72
1.53
1.68
1.42
1.22
1.56
Table 4
Recoveries of the five flavonoids in the extract of sea buckthorn leaves (n = 5)
Compound
Amount added (mg/ml)
Recovery (%)
R.S.D. (%)
CA
0.10
0.30
98.59
97.27
0.68
0.54
RU
0.10
0.30
99.57
98.04
0.58
0.24
QU
0.10
0.30
98.37
97.52
0.91
0.56
KA
0.10
0.30
99.98
97.93
0.32
0.25
IS
0.10
0.30
99.19
99.02
0.82
0.23
the procedure described in Section 2.4 for five times. The recoveries for the five flavonoids was between 97.27 and 99.98% (see
Table 4).
4. Conclusion
The RP-HPLC method mentioned here represented an excellent technique for simultaneous determination of CA, RU, QU,
KA and IS in the extract of sea buckthorn leaves, with good
sensitivity, precision and reproducibility. The method gives a
good resolution among CA, RU, QU, KA and IS with a short
analysis time (15 min). The sample preparation involving ultrasonic extraction is very simple. Furthermore, the method can be
used as quality control of flavonoids in sea buckthorn and will
play a reference role on the determination of flavonoids in other
medicinal plants or pharmaceutical preparations.
Fig. 7. Chromatograms of a sample under different detection wavelength.
256 nm for (a), 279 nm for (b), 368 nm for (c), Peak 1 for CA, Peak 2 for RU,
Peak 3 for QU, Peak 4 for KA, Peak 5 for IS.
Table 3
Contents of five flavonoids in the extract of sea buckthorn leaves (n = 5)
Component
Content (%)
R.S.D. (%)
CA
RU
QU
KA
IS
0.04
0.30
0.49
0.37
0.42
2.14
1.72
1.55
1.28
1.41
Acknowledgement
This work was supported by Cooperate Research Foundation between China and Germany government (grant no.
CHN03/028).
References
[1] A. Rousi, Ann. Bot. Fenn. 8 (1971) 177227.
[2] D. Rosch, A. Krumbein, L.W. Kroh, Eur. Food Res. Technol. 219 (2004)
605613.
[3] H. Kallio, B.R. Yang, P.J. Peippo, Agric. Food Chem. 50 (2002)
61366142.
[4] B. Yang, H. Kallio, Trends Food Sci. Technol. 13 (2002) 160167.
Y. Zu et al. / Journal of Pharmaceutical and Biomedical Analysis 41 (2006) 714719
[5] Z.R. Wang, L. Wang, H.H. Yin, F.J. Yang, Y.Q. Gao, Z.J. Zhang, Space
Med. Med. Eng. 13 (2000) 69.
[6] S. Geetha, M. Sai Ram, V. Singh, G. Ilavazhagan, R.C. Sawhney, J.
Ethnopharmacol. 79 (2002) 373378.
[7] B. Sun, P. Zhang, W.Q. Zhai, X.L. Zhang, X.Y. Zhuang, H.J. Yang, J.
Chin. Med. Mater. 26 (2003) 875877.
[8] J. Cheng, K. Kondo, Y. Suzuki, Y. Ikeda, X.S. Meng, K. Umemura, Life
Sci. 72 (2003) 22632271.
[9] S. Geetha, M. Sai Rama, S.S. Mongiaa, V. Singh, G. Ilavazhagan, R.C.
Sawhney, J. Ethnopharmacol. 87 (2003) 247251.
[10] X.Y. Ge, G.F. Shi, C.Y. Ma, Chin. Tradit. Herb. Drugs 17 (1986) 42
44.
[11] L.H. Zhao, Y. Tu, Z.Y. Zhao, G.J. Wang, Chem. Pharm. Bull. 52 (2004)
150152.
719
[12] N. Jeppsson, X.Q. Gao, Agric. Food Sci. Finland 9 (2000) 1722.
[13] R. Zadernowski, M. Naczk, R. Amarowicz, J. Am. Oil Chem. Soc. 80
(2003) 5558.
[14] V.B. Guliyev, M. Gul, A. Yildirim, J. Chromatogr. B 812 (2004)
291307.
[15] M. Vaher, M. Koel, J. Chromatogr. A 990 (2003) 225230.
[16] V.I. Deineka, L.A. Deineka, J. Anal. Chem. 59 (2004) 895898.
[17] L. Stralsjo, H. Ahlin, C.M. Witthoft, J. Jastrebova, Eur. Food Res. Technol. 216 (2003) 264269.
[18] S.H. Hakkinen, S.O. Karenlampi, I.M. Heinonen, H.M. Mykkanen, A.R.
Torronen, J. Agric. Food Chem. 47 (1999) 22742279.
[19] D. Rosch, M. Bergmann, D. Knorr, L.W. Kroh, J. Agric. Food Chem.
51 (2003) 42334239.
[20] Z.S. Jia, M.C. Tang, J.M. Wu, Food Chem. 64 (1999) 555559.
Potrebbero piacerti anche
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (890)
- 68rfe IntroductionDocumento71 pagine68rfe IntroductionThePokeOne83% (6)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Vedic Astrology OverviewDocumento1 paginaVedic Astrology Overviewhuman999100% (8)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- Aligning With New Digital Strategy A Dynamic CapabilitiesDocumento16 pagineAligning With New Digital Strategy A Dynamic Capabilitiesyasit10Nessuna valutazione finora
- VV016042 Service Manual OS4 PDFDocumento141 pagineVV016042 Service Manual OS4 PDFCamilo Andres Uribe Lopez100% (1)
- Japanese For Professionals 1Documento113 pagineJapanese For Professionals 1Kimi100% (1)
- Cbydp Draft SK BaracbacDocumento13 pagineCbydp Draft SK BaracbacLikey PromiseNessuna valutazione finora
- Telecomm SwitchingDocumento49 pagineTelecomm SwitchingTalha KhalidNessuna valutazione finora
- The Role of Historical Cultural Memory in Uzbek Documentary CinemaDocumento5 pagineThe Role of Historical Cultural Memory in Uzbek Documentary CinemaResearch ParkNessuna valutazione finora
- Chich The ChickenDocumento23 pagineChich The ChickenSil100% (4)
- Openstack Deployment Ops Guide PDFDocumento197 pagineOpenstack Deployment Ops Guide PDFBinank PatelNessuna valutazione finora
- Effect of Turmeric Powder To Estriol and Progesterone Hormone Profile of Laying Hens During One Cycle of OvulationDocumento6 pagineEffect of Turmeric Powder To Estriol and Progesterone Hormone Profile of Laying Hens During One Cycle of OvulationNur Atiqah AhmadNessuna valutazione finora
- 15 Types of Titration-With Various Examples For AnalysisDocumento3 pagine15 Types of Titration-With Various Examples For AnalysisNur Atiqah AhmadNessuna valutazione finora
- IQ Test Answers - WritingDocumento5 pagineIQ Test Answers - WritingNur Atiqah AhmadNessuna valutazione finora
- Citric Acid Cycle PDFDocumento12 pagineCitric Acid Cycle PDFNur Atiqah AhmadNessuna valutazione finora
- 2017 Year Calendar Template Us Holidays 02Documento1 pagina2017 Year Calendar Template Us Holidays 02Nur Atiqah AhmadNessuna valutazione finora
- 7 Exercises To Get Rid of Bra Bulge - YouBeauty PDFDocumento12 pagine7 Exercises To Get Rid of Bra Bulge - YouBeauty PDFNur Atiqah AhmadNessuna valutazione finora
- Oleochemical - WikipediaDocumento3 pagineOleochemical - WikipediaNur Atiqah Ahmad100% (1)
- Fyp ContractDocumento2 pagineFyp ContractNur Atiqah AhmadNessuna valutazione finora
- Trig ReviewDocumento6 pagineTrig ReviewNur Atiqah AhmadNessuna valutazione finora
- Result and DiscussionDocumento10 pagineResult and DiscussionNur Atiqah AhmadNessuna valutazione finora
- MidaDocumento35 pagineMidaNur Atiqah AhmadNessuna valutazione finora
- Determination of Cholesterol inDocumento1 paginaDetermination of Cholesterol inNur Atiqah AhmadNessuna valutazione finora
- Lab Report - Determination of Accelerants in Fire Debris Using Solid Phase MicroextractionDocumento6 pagineLab Report - Determination of Accelerants in Fire Debris Using Solid Phase MicroextractionNur Atiqah AhmadNessuna valutazione finora
- Content of Heavy Metals in Forms: Gentiana Lutea L. Roots and GalenicDocumento6 pagineContent of Heavy Metals in Forms: Gentiana Lutea L. Roots and GalenicNur Atiqah AhmadNessuna valutazione finora
- Template FYP ReportDocumento4 pagineTemplate FYP ReportNur Atiqah AhmadNessuna valutazione finora
- Lipids Are A Class of Biological Molecules That Are Grouped Based On Their Similarities in SolubilityDocumento1 paginaLipids Are A Class of Biological Molecules That Are Grouped Based On Their Similarities in SolubilityNur Atiqah AhmadNessuna valutazione finora
- Experiment 9 - Analysis of Fibre Dyes by HPLCDocumento5 pagineExperiment 9 - Analysis of Fibre Dyes by HPLCNur Atiqah AhmadNessuna valutazione finora
- Frs 1Documento4 pagineFrs 1Nur Atiqah AhmadNessuna valutazione finora
- Full Report Project PhyDocumento26 pagineFull Report Project PhyNur Atiqah AhmadNessuna valutazione finora
- Presentation 1Documento8 paginePresentation 1Nur Atiqah AhmadNessuna valutazione finora
- IDENTIFY UNKNOWN SOLUTIONSDocumento11 pagineIDENTIFY UNKNOWN SOLUTIONSJCortes93Nessuna valutazione finora
- Enzyme Is A Protein That Functions As A Biological CatalystDocumento1 paginaEnzyme Is A Protein That Functions As A Biological CatalystNur Atiqah AhmadNessuna valutazione finora
- Frs 6Documento11 pagineFrs 6Nur Atiqah AhmadNessuna valutazione finora
- Lab Manual FRS581Documento5 pagineLab Manual FRS581Nur Atiqah AhmadNessuna valutazione finora
- Constant ValueDocumento1 paginaConstant ValueNur Atiqah AhmadNessuna valutazione finora
- Enzyme Is A Protein That Functions As A Biological CatalystDocumento1 paginaEnzyme Is A Protein That Functions As A Biological CatalystNur Atiqah AhmadNessuna valutazione finora
- Cambridge IGCSE: 0450/11 Business StudiesDocumento12 pagineCambridge IGCSE: 0450/11 Business StudiesGodfreyFrankMwakalingaNessuna valutazione finora
- Important instructions on judicial procedure from Narada SmritiDocumento6 pagineImportant instructions on judicial procedure from Narada SmritirohitNessuna valutazione finora
- ARTS 9 Q4 Week 1Documento3 pagineARTS 9 Q4 Week 1Elaissa MaglanqueNessuna valutazione finora
- Transactionreceipt Ethereum: Transaction IdentifierDocumento1 paginaTransactionreceipt Ethereum: Transaction IdentifierVALR INVESTMENTNessuna valutazione finora
- Macbeth Introduction0Documento40 pagineMacbeth Introduction0MohammedelamineNessuna valutazione finora
- Viscosity IA - CHEMDocumento4 pagineViscosity IA - CHEMMatthew Cole50% (2)
- Diwali - An Overview of The Festival of LightsDocumento3 pagineDiwali - An Overview of The Festival of LightsSumeetNessuna valutazione finora
- Linked ListDocumento83 pagineLinked Listshahida18Nessuna valutazione finora
- Oposa vs. Factoran 224 Scra 792Documento28 pagineOposa vs. Factoran 224 Scra 792albemartNessuna valutazione finora
- Gardiner 1979Documento16 pagineGardiner 1979Oswaldo Manuel Ramirez MarinNessuna valutazione finora
- Atomic Structure - One Shot by Sakshi Mam #BounceBackDocumento231 pagineAtomic Structure - One Shot by Sakshi Mam #BounceBackchansiray7870Nessuna valutazione finora
- Demo TeachingDocumento22 pagineDemo TeachingCrissy Alison NonNessuna valutazione finora
- Corporate Subsidies On A Massive ScaleDocumento2 pagineCorporate Subsidies On A Massive ScaleBurchell WilsonNessuna valutazione finora
- Restaurant Social Media GuideDocumento30 pagineRestaurant Social Media GuideHoàng gia NghiêmNessuna valutazione finora
- New Wordpad DocumentDocumento6 pagineNew Wordpad DocumentJonelle D'melloNessuna valutazione finora
- TG KPWKPDocumento8 pagineTG KPWKPDanmar CamilotNessuna valutazione finora
- PPPoE Packet Format - HCNADocumento6 paginePPPoE Packet Format - HCNARobert Sanchez OchochoqueNessuna valutazione finora
- UNDERSTANDING CULTURE SOCIETY & POLITICS12 - LAS - Week7Documento6 pagineUNDERSTANDING CULTURE SOCIETY & POLITICS12 - LAS - Week7Bergonsolutions AingelNessuna valutazione finora
- Image Formation in Plane Mirrors: Ray DiagramsDocumento3 pagineImage Formation in Plane Mirrors: Ray DiagramsSouvik BanerjeeNessuna valutazione finora
- Limit Switch 1LX7001-J AZBILDocumento8 pagineLimit Switch 1LX7001-J AZBILHoàng Sơn PhạmNessuna valutazione finora
- Hempathane Topcoat 55219 Base 5521967280 En-UsDocumento11 pagineHempathane Topcoat 55219 Base 5521967280 En-UsSantiago Rafael Galarza JacomeNessuna valutazione finora