Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Coronary Scaffold - REVA Medical, Inc
Caricato da
bezpomukeTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Coronary Scaffold - REVA Medical, Inc
Caricato da
bezpomukeCopyright:
Formati disponibili
5/20/2014
Coronary Scaffold / REVA Medical, Inc.
ABOUT US
PROD UC TS
INVE STORS
C ONTAC T US
CO RO NARY SCAFFO L D
The Next Generation
Coronary Artery Disease
Products
Coronary Scaffold
Information Center
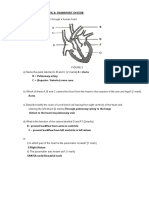
The coronary arteries, which supply blood to the heart
muscle, are susceptible to the buildup of plaque, which
can block or inhibit blood flow, a condition known as
coronary artery disease (CAD). If the coronary arteries
become too narrow, cardiac tissue can become starved
of nutrients and oxygen, resulting in severe chest pain
known as angina. As artery narrowing becomes more
severe, death of cardiac muscle downstream from the
blockage can occur due to the lack of oxygen. The sudden
death of cardiac muscle can result in a myocardial
An accumulation of plaque in
arteries supplying blood to
the heart causes constriction
of blood flow.
infarction, commonly known as a heart attack.
Conventional Coronary Stents
Today stents, which are small, tube-like devices permanently implanted into an artery after
angioplasty, are commonly used to treat CAD. They provide patients with minimally invasive
and effective treatment, avoiding the need for patients to undergo invasive bypass procedures.
All permanent stents are made of metal and help stabilize diseased coronary arteries by
propping them open and restoring blood flow. When a metal stent is placed inside the vessel, it
serves as a permanent scaffold. Although rare, placement of a permanent metal stent can
result in long-term complications.
ReZolve2 Sirolimus-Eluting Bioresorbable Coronary Scaffold
The ReZolve2 scaffold integrates a proprietary drug-eluting polymer and a novel design to
create a temporary scaffold with metal-like performance out of a polymer material. The
scaffold restores blood flow and supports the artery through healing, then completely dissolves
from the body, leaving the patient free of a permanent implant.
The ReZolve2 Bioresorbable Coronary Scaffold restores, remodels and resorbs
REVAs Proprietary Polymer Composition
The ReZolve2 scaffold incorporates a proprietary
desaminotyrosine polycarbonate polymer, which
was developed specifically for bioresorbable
scaffold performance and provides adequate
strength both initially and over time. Unlike
permanent metal alloys, the REVA polymer
dissolves from the body after the artery has healed,
providing additional treatment options in the future.
http://www.teamreva.com/coronary-stent.shtml
1/2
5/20/2014
Coronary Scaffold / REVA Medical, Inc.
Another unique feature of the polymer is that it is visible under x-ray, allowing the entire scaffold
to be visualized during the implant procedure and at follow up. Other bioresorbable polymer
scaffolds are invisible and require permanently attached radiopaque markers to enable their
limited visibility.
Optimal Drug Delivery
ReZolve2 is a drug-eluting scaffold coated with sirolimus, a therapeutic agent that inhibits
restenosis, or renarrowing of the artery, which can occur in the area of scaffold implantation.
The drug delivered by the scaffold is chosen from the family of anti-restenotic pharmaceuticals
most preferred by physicians and industry experts. The abluminal surface of the scaffold is
coated using a polymer solution containing sirolimus. The polymer used for the coating is the
same polymer used in the scaffold structure. There is a controlled release of the drug over 30
days, with the majority of the drug released within 90 days. This early and slow release
characteristic may help with the healing process.
Clinical Program
The RESTORE Trial (Pilot Study of the ReZolve Sirolimus-Eluting Bioresorbable Coronary
Scaffold) is evaluating the safety and performance of the first-generation ReZolve scaffold in 26
patients that were enrolled between December 2011 and July 2012 at multiple centers in Brazil
and Europe. The study's primary endpoint is freedom from symptomatic target lesion
revascularization (retreatment) at a six-month clinical evaluation. This will be followed by
imaging of the stented area at twelve months to measure various quantitative parameters such
as Late Loss and Restenosis Rate. Patients will be followed for five years.
The ReZolve2 scaffold, a lower profile and sheathless version of the original ReZolve scaffold,
is being evaluated clinically in the RESTORE II Trial. Up to 125 patients will be enrolled in the
trial at multiple centers in Australia, Brazil, Europe and New Zealand to provide the data needed
to apply for European CE Marking. The CE Mark allows for commercial sales in Europe and
other countries that recognize the mark. Patient enrollments with ReZolve2 began in March
2013.
REVA Medical, Inc.
http://www.teamreva.com/coronary-stent.shtml
About Us
| Products
| Investors
| Contact Us
2/2
Potrebbero piacerti anche
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Critical Care NursingDocumento10 pagineCritical Care Nursingianecunar100% (10)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Heart Catheterization & AngioplastyDocumento25 pagineHeart Catheterization & AngioplastyRifa'atul MahmudahNessuna valutazione finora
- Congenital Heart DiseasesDocumento6 pagineCongenital Heart DiseasesMox SwanNessuna valutazione finora
- ANEURYSMDocumento31 pagineANEURYSMPooja Sahu100% (1)
- SCLEROTHERAPYDocumento439 pagineSCLEROTHERAPYVishal Gaikwad100% (2)
- Lesson 12 HeartDocumento9 pagineLesson 12 HeartEly FructuosoNessuna valutazione finora
- Zero To Finals MedicineDocumento352 pagineZero To Finals MedicinePlay100% (23)
- Total Anomalous Pulmonary Venous ConnectionDocumento30 pagineTotal Anomalous Pulmonary Venous Connectionwiyay34652ceoshubcomNessuna valutazione finora
- Hypertention Urgency/emergencyDocumento35 pagineHypertention Urgency/emergencyMohamad al-fazmi100% (1)
- CVD BleedDocumento25 pagineCVD BleedArla Donissa-Donique Castillon Alvior100% (1)
- SCIENCE 9 Q1 WK1 2 The Respiratory and Circulatory SystemsDocumento42 pagineSCIENCE 9 Q1 WK1 2 The Respiratory and Circulatory SystemsDanielle SibayNessuna valutazione finora
- AHA Examination Auscultation Part IVDocumento42 pagineAHA Examination Auscultation Part IVHananya ManroeNessuna valutazione finora
- Clinical Approach To A Neonate With Cyanosis - Review - IJPDocumento11 pagineClinical Approach To A Neonate With Cyanosis - Review - IJPSridhar KaushikNessuna valutazione finora
- Form 3 Science Chapter 2Documento9 pagineForm 3 Science Chapter 2Mohamad Harith100% (5)
- Referat Hipertensi - GeriatriDocumento21 pagineReferat Hipertensi - GeriatriAndrew SoerijadiNessuna valutazione finora
- PeripheralVascularDisease NotesDocumento6 paginePeripheralVascularDisease NotesNoah PontinoNessuna valutazione finora
- AngioplastyDocumento38 pagineAngioplastyBuyung HamzahNessuna valutazione finora
- Physiology Practical Measurement of Blood PressureDocumento6 paginePhysiology Practical Measurement of Blood PressureAli MohamedNessuna valutazione finora
- Resurgence of Beta Blocker-Focus On HTDocumento47 pagineResurgence of Beta Blocker-Focus On HTAkhil SharmaNessuna valutazione finora
- Contoh Draft Rubdown Acara SimposiumDocumento3 pagineContoh Draft Rubdown Acara SimposiumdryusiaspanNessuna valutazione finora
- Anaesthetic Considerations in Cardiac Patients Undergoing Non Cardiac SurgeryDocumento7 pagineAnaesthetic Considerations in Cardiac Patients Undergoing Non Cardiac Surgerysatria wibawaNessuna valutazione finora
- Rasio Kadar Trigliserida-Kolesterol HDL Serum Tinggi Meningkatkan Keparahan Klinis Penderita Stroke Iskemik AkutDocumento5 pagineRasio Kadar Trigliserida-Kolesterol HDL Serum Tinggi Meningkatkan Keparahan Klinis Penderita Stroke Iskemik AkutYasmeen BiandaNessuna valutazione finora
- Transport System Question and AnswersDocumento12 pagineTransport System Question and Answerskumara guruparanNessuna valutazione finora
- Sacubitril Valsartan The Role of NeprilyDocumento11 pagineSacubitril Valsartan The Role of NeprilySalvanabilaNessuna valutazione finora
- Term Paper On Heart DiseaseDocumento4 pagineTerm Paper On Heart Diseaseea9k5d7j100% (1)
- Dr. Naitik Trivedi & Dr. Upama Trivedi: Cardiovascular SystemDocumento20 pagineDr. Naitik Trivedi & Dr. Upama Trivedi: Cardiovascular SystemAhmed ImranNessuna valutazione finora
- Chapter 24 - Alterations of Cardiovascular Function in Children - Nursing Test BanksDocumento19 pagineChapter 24 - Alterations of Cardiovascular Function in Children - Nursing Test BanksNeLNessuna valutazione finora
- Assessing The Heart and Abdomen ScriptDocumento5 pagineAssessing The Heart and Abdomen Scriptfvfwmy6mqmNessuna valutazione finora
- Centralretinalarteryocclusion 150821150708 Lva1 App6891 PDFDocumento50 pagineCentralretinalarteryocclusion 150821150708 Lva1 App6891 PDFDhivya SekarNessuna valutazione finora
- Structure of ThymusDocumento6 pagineStructure of ThymusShubham Pareshkumar KadiwalaNessuna valutazione finora