Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
CH CW Ammoniaandurea
Caricato da
Ahmed AliTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
CH CW Ammoniaandurea
Caricato da
Ahmed AliCopyright:
Formati disponibili
The Physical Sciences Initiative
Manufacture of Ammonia & Urea in IFI, Cobh
Option 1a of the syllabus deals with Industrial Chemistry. Eight class periods are
recommended.
Option 1A .1: The General Principles of Industrial Chemistry (3 periods). This
looks at the characteristics of effective and successful industrial processes.
Option 1A .2: A Case Study (5 periods) of one of three Irish industrial processes:
1)
Manufacture of Ammonia (NH3) and Urea (CO(NH2)2) by IFI
in
Cobh.
2)
Manufacture of Nitric Acid (HNO3) and Calcium Ammonium
Nitrate (CAN) by IFI in Arklow.
3)
Manufacture of Periclase (MgO) by Premier Periclase in
Drogheda.
It is strongly recommended that students visit a local plant.
General Principles of Industrial Chemistry
Section 1A.1 of the syllabus states that when undertaking a case study of a particular
chemical industry, the following ten characteristics must be studied:
1)
Feedstock (raw materials, preparation).
2)
Rate (temperature and pressure variables, catalysts etc.).
3)
Production Yield (temperature and pressure variables,
catalysts).
4)
Co-Products (separation of, disposal or sale).
The Physical Sciences Initiative
1
The Physical Sciences Initiative
5)
Waste Disposal and Effluent Control (waste water treatment,
emission control).
6)
Quality Control.
7)
Safety (monitoring of hazards, safety features, safety training on
on site).
8)
Costs (fixed costs, variable costs, cost reduction by use of heat
exchangers, catalysts, recycling and selling of useful
co-products, cost of waste disposal).
9)
Site Location.
10)
Suitable Materials for the Construction of a Chemical Plant
(unreactive, resistant to corrosion).
The Physical Sciences Initiative
2
The Physical Sciences Initiative
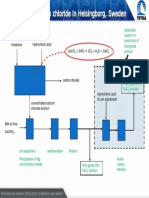
Manufacture of Ammonia by IFI in Cobh, Co. Cork
In 1979 NET established a combined ammonia and urea plant at Marino Point near
Cork using natural gas from the Kinsale gas field.
Type of process
The process is continuous, with the raw materials being fed in continuously and the
ammonia being continuously produced.
Feedstock : Raw materials are purified and may also have to undergo chemical
reactions in order to form the feedstock, from which the products are made.
1) Feedstock (raw materials, preparation)
The raw materials are compressed air, natural gas and water.
Compressed air is the source of nitrogen; natural gas and water are the sources of
hydrogen.
The air is filtered beforehand to remove dust. The water is passed through ion
exchange resins to deionise it. The sources of methane are the Kinsale gas field and
North Sea gas. A typical natural gas analysis from the Kinsale field is as follows:
CH4 (99%)
C2H6 and C3H8 (0.3%)
Inert gases (N2, He, Ar) 0.7%.
The gas is piped underwater to Inch beach in East Cork, from where Bord Gais
supplies it by underground pipeline to IFI.
Natural gas is odourless, so for safety reasons Bord Gais adds a tiny amount of
mercaptans which contains sulfur (9 ppm) to ensure the gas is detectable.
Removal of Sulfur From Natural Gas
Since the active sites on the catalysts are easily poisoned, IFIs first step must be to
remove the sulfur compounds from the natural gas. This is brought about by mixing
the incoming gas with hydrogen (recycled from later on in the process) and passing it
The Physical Sciences Initiative
3
The Physical Sciences Initiative
over a cobalt/nickel/alumina catalyst. Hydrogen sulfide is formed and removed by
reaction with zinc oxide.
ZnO(s) + H2S(s) ZnS + H2O(g)
Primary Steam Reforming
Methane gas is heated with steam in the presence of a catalyst to produce hydrogen.
In this reaction 90% of the methane gas is consumed. The reaction occurs by
introducing methane and steam into a reforming furnace. This furnaces contains 520
catalyst filled tubes and is fired by 198 forced draught burners.
CH4(g) + H2O(g)
CO(g) + 3H2(g)
H= +210 kJ/mol
Secondary Reforming
The remaining 10% of the methane is then reacted with air in a refractory- lined
vessel in the presence of heat and a catalyst.
CH4 + 2O2 CO2 + 2H2O
H = -
The gases leaving this stage now are
hydrogen (produced in reaction 1),
nitrogen (obtained by removing oxygen from the air in the above reaction)
and,
some carbon monoxide and carbon dioxide. These carbon oxides must be
removed because they would poison the active sites on the catalyst used in the
production of ammonia.
To arrive at the pure feedstock (hydrogen and nitrogen), the following steps
remove the oxides of carbon.
Removal of Carbon Monoxide
CO(g) + H2O(g)
CO2(g) + H2(g)
H = -
This reaction is carried out using heat and a catalyst. The amount of CO is reduced to
0.2%.
The Physical Sciences Initiative
4
The Physical Sciences Initiative
An advantage of this reaction is the extra hydrogen production. This is another
exothermic reaction, so it allows for more heat recovery, again emphasising the
thermal efficiency of the ammonia process.
Removal of CO2
The gas is scrubbed with a hot potassium carbonate solution under high pressure and
high temperature.
CO2(g) + K2CO3(aq) + H2O(l) 2KHCO3(aq)
The potassium hydrogencarbonate solution is then removed.
Methanation
The gas mixture leaving the absorption column is then catalytically hydrogenated.
The aim is to convert any remaining CO and CO2 into methane. Methanation is
necessary as even trace amounts of CO and CO2 would poison the catalyst in the
ammonia synthesis.
CO9g) + 3H2(g) CH4(g) + H2O(g)
H = - 210 kJ
This is the reverse of the steam reforming process and is exothermic. The gas mixture
is now ready for ammonia synthesis. The mixture is composed of 24.7% N2, 74% H2,
1% CH4 and 0.3% inert gases (e.g. Ar).
2) Rates and 3) Product Yield
N2 + 3H2
2NH3 H = -
In theory to obtain a high yield of ammonia from this exothermic reaction, the
favourable conditions are low temperature, high pressure and the presence of a
catalyst. However, to achieve an acceptable rate of reaction, a compromise is reached
whereby a higher temperature, high pressure and a catalyst is used.
The Physical Sciences Initiative
5
The Physical Sciences Initiative
High pressure (100 to 200 atm) is achieved using a large steam driven compressor.
A catalyst of finely divided iron is used. Catalyst promoters are also used. High
temperature is used.
Only 17% of the nitrogen and hydrogen react to form ammonia. The unreacted
nitrogen and hydrogen is recycled back into the reaction vessel. The ammonia is
cooled, liquefied and stored at -33C at atmospheric pressure or is sent directly to the
urea plant.
1500 tonnes of ammonia are produced everyday at Marino Point. 40% of this is used
to make urea on site. The remainder goes by train to Arklow or by ship to Belfast.
4)
Co-Products
The co-product in the synthesis of ammonia is carbon dioxide. It is removed from the
synthesis gas as potassium hydrogencarbonate. To release the carbon dioxide the
potassium hydrogencarbonate solution is heated and the pressure is reduced.
2KHCO3(aq) CO2(g) + H2O(l) + K2CO3(aq)
Uses of carbon dioxide: It is used for the manufacture of urea on site and sold to soft
drinks manufacturers and breweries.
The Physical Sciences Initiative
6
The Physical Sciences Initiative
Manufacture of Urea by IFI in Cobh
Urea Production in IFI
1) Feedstock
Anhydrous ammonia and potassium hydrogencarbonate solution are the raw materials,
both of which are produced in IFI, Marino Point. The potassium hydrogencarbonate
solution is heated and the pressure is reduced. This releases the carbon dioxide
2KHCO3(aq) CO2(g) + H2O(l) + K2CO3(aq)
The feedstock is ammonia and carbon dioxide.
2) Rates and 3) Product Yield
Ammonia and carbon dioxide react to form ammonium carbamate. A high temperature
and pressure favour a higher reaction rate, but to obtain a high yield of ammonium
carbamate low temperature and high pressure are needed. Hence a compromise is
reached.
This reaction takes place in the carbamate condenser. It is carried out at high pressure to
push the reaction forward and the heat produced by the exothermic reaction is removed
as it is formed to ensure completion of the reaction.
2NH3 + CO2
2COONH4
The next reaction occurs in the urea reactor. Ammonium Carbamate loses water to form
urea.
NH2COONH4
CO(NH2)2 + H2O
= +
This is a slow reaction and must be carried out at high temperature to make it
economical. Since it is an endothermic reaction anyway, high temperature favours it.
Steam is used to provide the heat needed for this reaction.
The solution formed in the urea reactor contains urea, ammonium carbamate and water.
The Physical Sciences Initiative
7
The Physical Sciences Initiative
The residual ammonium carbamate is decomposed into ammonia and carbon dioxide by
heat and these are released as gases, which are recycled to the carbamate condenser,
where they react.
Concentration of the Aqueous Urea
The solution of urea is transferred to an evaporator, where the water is evaporated under
vacuum to give molten urea.
Prilling of the Urea Melt
The molten product is sprayed downwards from the top of a tall prill tower (50m) against
the upward flow of air. Air is drawn up through the tower by fans in the roof. The cool
air solidifies the urea into prills (very small spherical particles).
4) Co-Products
There are no co-products.
STAGES 5 TO 10 REFER TO THE PRODUCTION OF BOTH
AMMONIA AND UREA.
5) Waste Disposal and Effluent Control
Waste water treatment at IFI is carried out within the plant to facilitate recycling of the
ammonia and carbon dioxide back into the production stream. Ammonia plant effluent is
treated with ion exchange resins where ammonia is replaced by hydrogen ions and carbon
dioxide is replaced by hydroxide ions. A total of 255 m3 of water per hour is recovered,
treated and reused in this way.
Urea plant effluent is treated with steam to hydrolyse urea to ammonia and carbon
dioxide, which is then recycled.
The Physical Sciences Initiative
8
The Physical Sciences Initiative
Air emissions are monitored for the presence of ammonia, oxides of nitrogen and urea
dust.
Emissions include:
a) Carbon dioxide, which is emitted to the atmosphere;
b) Steam, emitted through steam vents;
c) Nitrous oxides (flue gases);
d) Ammonia, which is flamed and emitted as nitrogen;
e) Water, removed from the urea in the evaporator and from washing the ion
exchangers in the demineralising plant
6) Quality Control
Detailed analyses of the gas mixtures at all stages of the ammonia and urea production
are carried out using gas chromatography and infra red spectroscopy. This is done to
monitor the performance of the catalysts in particular.
7) Safety
There are two alarm systems, a fire alarm and an alarm to indicate gas release. Part of the
workforce is specifically trained in first aid and fire fighting. The site is designed so as to
allow access by vehicles (e.g. fire engines) to central parts of the plant.
8 ) Costs
Water
4.5 million litres of fresh water is needed everyday. This is bought from the local council.
Cost Reduction
The Physical Sciences Initiative
9
The Physical Sciences Initiative
Cost reductions are achieved by recycling in both processes and by the sale of the useful
co-product carbon dioxide in the case of the ammonia process. The use of heat
exchangers and catalysts also help to reduce costs.
Heat Exchangers
Some of the reactions are exothermic, e.g. ammonia synthesis. The heat is recovered
using heat exchangers. A heat exchanger is a collection of tubes in a circle with water in
them. The tubes are then surrounded by the hot gas (or liquid).
The heat is recovered by producing approximately 300 tonnes per hour of steam at 100
atm of pressure in a number of high pressure boilers. This provides the steam required for
the reaction in the primary reformer, and also is a source of power for operating some
process equipment in the plant. Approximately 45 MW of power is provided in this way.
This is equivalent to the electrical consumption of a town like Tralee.
Catalysts
Recent developments in technology have resulted in manufacturers producing more
effective catalysts, which allow the reactions to occur at lower temperatures and so
reduce costs.
Fixed costs are those that do not depend on the level of production. These include labour
costs and the cost of plant depreciation and repayment of loans. Variable costs depend on
the level of production. They include the costs of raw materials, heat and electricity, and
the costs of waste disposal.
Natural Gas
Up to the year 2000 all the natural gas was obtained from Kinsale, but this has begun to
run out, so now some of it is obtained from the North Sea. The cost of the gas has
therefore increased due to increased transport costs.
9) Site Location
Why Marino Point?
1) Convenient Supply of natural gas from the Kinsale field.
The Physical Sciences Initiative
10
The Physical Sciences Initiative
2) Good supply of skilled personnel in the Cork area.
3) There is a sea water cooling system available.
4) Transport of products easy.
The adjacent railway line is used to transfer ammonia to Arklow. There is a jetty to ship
products to Belfast and to the export markets. Marino Point is situated in a deep water
harbour, so ships up to 25,000 tonnes can be used.
10) Construction Materials
Mild steel on a deep solid concrete foundation is used in some parts of the plant.
Stainless steel with a very high specification is used in parts of the plant like compressors
and reaction chambers, so that corrosion can only occur at a very slow rate.
Uses of Ammonia
1) Manufacture of urea.
2) Manufacture of nitric acid.
3) Manufacture of fertilisers.
Uses of Urea
1) Used on runways as a non-corrosive de-icer.
2) Manufacture of MDF (medium density fibreboard).
3) Used as a nitrogenous fertilisers.
Industrial Synthesis of Ammonia
Natural Gas
Desulfurisation
The Physical Sciences Initiative
11
The Physical Sciences Initiative
Steam
Carbon Monoxide and
Hydrogen Formed
Air
Oxygen Removed From Air
By Methane
Steam
Carbon Monoxide is
Converted to Carbon Dioxide
Carbon Dioxide Removal
N2 & H2
Recycling
Pump
Ammonia
To Storage
Tanks
N2 & H2
Ammonia
Condenser
Reaction
Chamber
Pump
Compressor
The Physical Sciences Initiative
12
Potrebbero piacerti anche
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Evaluation of Dissolved Air Flotation Unit For OilDocumento8 pagineEvaluation of Dissolved Air Flotation Unit For OilAhmed AliNessuna valutazione finora
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- Water Treatment Processes and Chemical CalculationsDocumento6 pagineWater Treatment Processes and Chemical CalculationsAhmed AliNessuna valutazione finora
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Ijrerd A085 PDFDocumento4 pagineIjrerd A085 PDFAhmed AliNessuna valutazione finora
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (894)
- 04-Control of Volatile Organic Compounds (VOCs)Documento187 pagine04-Control of Volatile Organic Compounds (VOCs)Ahmed AliNessuna valutazione finora
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Problems: CHEM1020Documento45 pagineProblems: CHEM1020Ahmed AliNessuna valutazione finora
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Thermochemistry Problems CalculatorDocumento17 pagineThermochemistry Problems CalculatorAhmed AliNessuna valutazione finora
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Airlift Bioreactors and Mechanically Stirred Tanks Are Widely Used in BioprocessingDocumento2 pagineAirlift Bioreactors and Mechanically Stirred Tanks Are Widely Used in BioprocessingAhmed AliNessuna valutazione finora
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Applications of Egg Shell and Egg Shell Membrane As AdsorbentsDocumento13 pagineApplications of Egg Shell and Egg Shell Membrane As AdsorbentsAhmed AliNessuna valutazione finora
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (587)
- Project 33Documento8 pagineProject 33Ahmed AliNessuna valutazione finora
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (265)
- URUAE Full Proceeding Sept (1) - 12-14-110-114 PDFDocumento5 pagineURUAE Full Proceeding Sept (1) - 12-14-110-114 PDFAhmed AliNessuna valutazione finora
- Investigation of Surfactant Effect On The Operational Characteristics PDFDocumento11 pagineInvestigation of Surfactant Effect On The Operational Characteristics PDFAhmed AliNessuna valutazione finora
- Study On The Hydrodynamics of Stirred Vessels PDFDocumento6 pagineStudy On The Hydrodynamics of Stirred Vessels PDFAhmed AliNessuna valutazione finora
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- J. Basic. Appl. Sci. Res., 1 (11) 2314-2318, 2011Documento5 pagineJ. Basic. Appl. Sci. Res., 1 (11) 2314-2318, 2011Ahmed AliNessuna valutazione finora
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- Ms-09-Development of A Paper Recycling ProcessDocumento7 pagineMs-09-Development of A Paper Recycling ProcesshidaiNessuna valutazione finora
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- DF Manual of PulverizerDocumento3 pagineDF Manual of PulverizerAhmed AliNessuna valutazione finora
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Cool Lab ManualDocumento12 pagineCool Lab Manualgiri_shwetaNessuna valutazione finora
- Produce Food Grade Calcium Chloride from Limestone Using HClDocumento1 paginaProduce Food Grade Calcium Chloride from Limestone Using HClAhmed AliNessuna valutazione finora
- Design and Construction of Waste Paper' Recycling PlantDocumento12 pagineDesign and Construction of Waste Paper' Recycling PlantAhmed Ali100% (1)
- Evap DesignDocumento16 pagineEvap DesignAhmed Ali100% (3)
- An Example of Sizing of A Heat Exchanger in Which A Stream Undergoes Both A Phase Change and Temperature Change in The Vapor And/or LiquidDocumento4 pagineAn Example of Sizing of A Heat Exchanger in Which A Stream Undergoes Both A Phase Change and Temperature Change in The Vapor And/or LiquidJoshua JohnsonNessuna valutazione finora
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Hariom ReportDocumento60 pagineHariom Reportहरिओम हरी100% (2)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2219)
- Progress of Styrene ProductionDocumento2 pagineProgress of Styrene ProductionAhmed AliNessuna valutazione finora
- Xylenes Material 2520balanceDocumento8 pagineXylenes Material 2520balanceAhmed AliNessuna valutazione finora
- 19890204Documento11 pagine19890204Ahmed AliNessuna valutazione finora
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Urea Synthesis ProcessDocumento5 pagineUrea Synthesis ProcessrajmaneamitNessuna valutazione finora
- Benzene Toluene EquilibriumDocumento2 pagineBenzene Toluene EquilibriumAhmed AliNessuna valutazione finora
- Optimize The ProductionDocumento103 pagineOptimize The Productionferik2Nessuna valutazione finora
- Al Zoubi2015Documento9 pagineAl Zoubi2015Ahmed AliNessuna valutazione finora
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (119)
- 1992 Lazaridis Daf Metal IonsDocumento16 pagine1992 Lazaridis Daf Metal IonsAhmed AliNessuna valutazione finora
- Equity Research Report On Bajaj Finserv LTD Authored By: Mohammad Reza PoyaDocumento6 pagineEquity Research Report On Bajaj Finserv LTD Authored By: Mohammad Reza Poyashraddha anandNessuna valutazione finora
- List of Standards and Guidelines For Drilling, Well Construction & Well OperationsDocumento2 pagineList of Standards and Guidelines For Drilling, Well Construction & Well OperationsInternational Association of Oil and Gas Producers100% (1)
- Heat Exchanger (Oil To Water) Installation Information: Description: Power RequirementsDocumento4 pagineHeat Exchanger (Oil To Water) Installation Information: Description: Power Requirements413666436qq.comNessuna valutazione finora
- Inphorm Online: Click Here To AccessDocumento10 pagineInphorm Online: Click Here To AccessconimecNessuna valutazione finora
- Ncpfirst - X-Form - PDF Example: (Epc - Dedicated Front Cover Sheet Here)Documento12 pagineNcpfirst - X-Form - PDF Example: (Epc - Dedicated Front Cover Sheet Here)Anil100% (1)
- FertiOne Manual (30.11.15)Documento36 pagineFertiOne Manual (30.11.15)YacineNessuna valutazione finora
- L7 Voltage Regulator, Half Wave Rectifier S2 1617Documento20 pagineL7 Voltage Regulator, Half Wave Rectifier S2 1617Bhagyalaxmi patilNessuna valutazione finora
- Home Automation and Surveillance: A ReviewDocumento4 pagineHome Automation and Surveillance: A ReviewATSNessuna valutazione finora
- W SeatDocumento2 pagineW SeatwalleyranNessuna valutazione finora
- Lista Precios Ahu Mas Accesorios Sinclair 2020Documento80 pagineLista Precios Ahu Mas Accesorios Sinclair 2020Jonathan ArboledaNessuna valutazione finora
- Thermal Protector For Motor: Ballast For Fluorescent and Temperature Sensing ControlsDocumento1 paginaThermal Protector For Motor: Ballast For Fluorescent and Temperature Sensing ControlsPasilius OktavianusNessuna valutazione finora
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- Ncom N102Documento16 pagineNcom N102RixroxNessuna valutazione finora
- PRAI-M0-YT01-LA-7500 - As-Built - Data Sheet For Feed Water PumpDocumento3 paginePRAI-M0-YT01-LA-7500 - As-Built - Data Sheet For Feed Water PumpSuman GhoshNessuna valutazione finora
- High Voltage Engineering Ref ManualDocumento147 pagineHigh Voltage Engineering Ref Manualzeus009100% (1)
- Screw Compressors: Models: VEDocumento42 pagineScrew Compressors: Models: VEVoştinar IoanNessuna valutazione finora
- Removable Francis Turbine SealsDocumento4 pagineRemovable Francis Turbine SealsmachevelNessuna valutazione finora
- Green Building Rating Systems ExplainedDocumento42 pagineGreen Building Rating Systems ExplainedJake CerezoNessuna valutazione finora
- PHYSICSDocumento3 paginePHYSICSAndrew NibungcoNessuna valutazione finora
- Automotive Application Guide 2014 - BR PDFDocumento60 pagineAutomotive Application Guide 2014 - BR PDFbnc1100% (1)
- A380-LEVEL III - ATA 26 Fire - Smoke DetectionDocumento42 pagineA380-LEVEL III - ATA 26 Fire - Smoke DetectionAbolfazl Mazloomi100% (2)
- TSSR 2g Grahafamily3 PKMDocumento43 pagineTSSR 2g Grahafamily3 PKMHaryo WNessuna valutazione finora
- Need For Earthing and Double Insulation PDFDocumento2 pagineNeed For Earthing and Double Insulation PDFMeNessuna valutazione finora
- 08 Kobelco MARK 8 Mechatron Control SistemDocumento45 pagine08 Kobelco MARK 8 Mechatron Control SistemNadiel Aceto 46100% (1)
- Fuel ImbalanceDocumento7 pagineFuel Imbalancedarryl_baguioNessuna valutazione finora
- Accident Radio Logic GOIANIADocumento157 pagineAccident Radio Logic GOIANIACatalin CuraliucNessuna valutazione finora
- AQA QMS Pvt. LTD.: S No Company Name Membership NoDocumento11 pagineAQA QMS Pvt. LTD.: S No Company Name Membership NoDevasyrucNessuna valutazione finora
- ALSOQOOF IntroductionDocumento5 pagineALSOQOOF Introductionsudeesh kumarNessuna valutazione finora
- 415V MCCs and PMCCs Technical SpecificationDocumento114 pagine415V MCCs and PMCCs Technical SpecificationanmohieyNessuna valutazione finora
- DHA Anita MHA2 Paper2 Unit 3.1Documento34 pagineDHA Anita MHA2 Paper2 Unit 3.1Priyan TripathiNessuna valutazione finora
- Brazil Chapter 12: Agriculture, Minerals, Industries & TradeDocumento8 pagineBrazil Chapter 12: Agriculture, Minerals, Industries & TradeHaseebullahNessuna valutazione finora
- Asset Integrity Management for Offshore and Onshore StructuresDa EverandAsset Integrity Management for Offshore and Onshore StructuresNessuna valutazione finora
- Flow Analysis for Hydrocarbon Pipeline EngineeringDa EverandFlow Analysis for Hydrocarbon Pipeline EngineeringNessuna valutazione finora
- Practical Wellbore Hydraulics and Hole Cleaning: Unlock Faster, more Efficient, and Trouble-Free Drilling OperationsDa EverandPractical Wellbore Hydraulics and Hole Cleaning: Unlock Faster, more Efficient, and Trouble-Free Drilling OperationsValutazione: 5 su 5 stelle5/5 (1)
- Pocket Guide to Flanges, Fittings, and Piping DataDa EverandPocket Guide to Flanges, Fittings, and Piping DataValutazione: 3.5 su 5 stelle3.5/5 (22)
- Black Gold Stranglehold: The Myth of Scarcity and the Politics of OilDa EverandBlack Gold Stranglehold: The Myth of Scarcity and the Politics of OilNessuna valutazione finora
- Oil and Gas Pipelines and Piping Systems: Design, Construction, Management, and InspectionDa EverandOil and Gas Pipelines and Piping Systems: Design, Construction, Management, and InspectionValutazione: 4.5 su 5 stelle4.5/5 (16)
- Industrial Piping and Equipment Estimating ManualDa EverandIndustrial Piping and Equipment Estimating ManualValutazione: 5 su 5 stelle5/5 (7)
- Machinery Lubrication Technician (MLT) I and II Certification Exam GuideDa EverandMachinery Lubrication Technician (MLT) I and II Certification Exam GuideValutazione: 2 su 5 stelle2/5 (1)