Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Reviewer 1
Caricato da
Christer John UyCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Reviewer 1
Caricato da
Christer John UyCopyright:
Formati disponibili
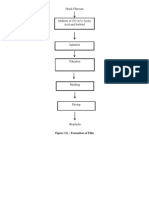
Crystallization
Is a solid-liquid separation
process, in which mass transfer
of a solute from the liquid
solution to a pure crystalline
phase occurs
Is the formation of solid
particles within a homogenous
phase
Is a unit operation used to
separate solutes from solution
Is the removal of a solute such
as a salt from a solution by
precipitating the solute from the
solution
Adsorption involves, in general, the
accumulation (or depletion) of solute
molecules at an interface (including
gas-liquid interfaces, as in foam
fractionation, and liquid-liquid
interfaces, as in detergency).
Adsorbate or solute - the material
being adsorbed
Adsorbent - the solid material being
used as the adsorbing phase
Physical Adsorption
Illustration
Industrial Application
Powdered salt for food industry
Production of sugar
Silicon crystal wafer production
Adsorption
A component of a gas or liquid
stream is removed and
adsorbed by a solid adsorbent
A separation process that
involves concentration of a
solute from a bulk vapor or
liquid phase on to the surface of
a porous solid.
The most common form of gassolid equilibrium
Occurs when the intermolecular
forces of attraction between the
fluid molecules and the solid
surface are greater than
attractive forces between
molecules of the fluid itself
A physical separation
process in which the adsorbed
material is not chemically
altered.
Reversible Process
The adsorption rate is generally
quite rapid
Also known as Van der Waals
adsorption
Chemical adsorption/ Chemisorption
Results from a chemical
interaction between the
adsorbate and adsorbent.
Only a monomolecular layer of
adsorbate appears on the
adsorbing medium
Frequently irreversible
Also known as Activated
adsorption
Factors Affecting Adsorption
Surface area of adsorbent
Adsorbate Solubility
Contact time or residence time.
Size of the molecule with
respect to size of the pores.
pH
Presence of other solutes
Commercial adsorbents
1. Activated carbon. This is a
microcrystalline material made
by thermal decomposition of
wood, vegetable shells, coal,
and so on, and has surface
areas of 300 to 1200 m2/g with
average pore diameters of 10 to
60 . Organics are mostly
adsorbed by activated carbon.
2. Silica gel. This adsorbent is
made by acid treatment of
sodium silicate solution and
then drying. It has a surface
area of 600-800 m2/g and
average pore diameters of 20 to
50 . It is primarily used to
dehydrate gases and liquids and
to fractionate hydrocarbons.
3. Activated alumina. To prepare
this material, hydrated
aluminum oxide is activated by
heating to drive off the water. It
is used mainly to dry gases and
liquids. Surface areas range
from 200-500 m2/g, with
average pore diameters of 20 to
140 .
4. Molecular sieve zeolites. These
zeolites are porous crystalline
aluminosilicates that form an
open crystal lattice containing
precisely uniform pores, which
makes it different from other
types of adsorbents, which have
a range of pore sizes. Different
zeolites have pore sizes from
about 3 to 10 . Zeolites are
used for drying, separation of
hydrocarbons, mixtures, and
many other applications.
5. Synthetic polymers or resins.
These are made by
polymerizing two major types of
monomers. Those made from
aromatics such as styrene and
divinylbenzene are used to
adsorb nonpolar organics from
aqueous solutions. Those made
from acrylic esters are usable
with more-polar solutes in
aqueous solutions.
Industrial Applications
Column Contact use a bed of
adsorbent to purify solutions
Slurry Contact use a powdered
adsorbent slurry to adsorb
desired materials
Gas Purifications
Gas bulk separation
Liquid purifications
Liquid bulk separation
Air-conditioning
Decolorization of cane refinery
syrups
Production of liquid sugar
Recovery of flavonoids, plant
extracts, colorants, aroma
Recovery of antibiotics and
proteins
Synthetic resin
Water purification
Waste water treatment
Centrifugation
Centrifugation is a process by which
solid particles are settled or filtered
from a liquid using centrifugal force as
a driving force.
It could also be that liquid particles are
settled from another liquid using the
same driving force.
Depending on the rotational speed
and distance from the axis of rotation,
the centrifugal force can be many
times greater than the force of gravity,
allowing even very small particles or
particles slightly denser than the fluid
to settle.
Chemical Procedures and Processes
Types of Centrifuges
Fuel and Biofuel Industry
Centrifugal Settling or Sedimentation
Under centrifugal force, the
solid phase assumed to be
denser than the liquid phase
settles out to the bowl wall.
Concurrently, the lighter, more
buoyant liquid phase is
displayed towards the smaller
diameter.
Centrifugal Filtration
In a filtering centrifuge,
separating solids from liquid
does not require a density
difference between the two
phases.
In both cases, the solids and
liquids move toward the bowl
under centrifugal force.
But in this type, the solids are
retained by the filter medium,
while the liquid flows through
the cake solids and the filter.
Chemical processing which
produces raw products such as
acids, salts, oil refinery byproducts, polymers, oil-watersolids, and so on.
Fuel and Biofuel industry
including synthetic fuels,
biodiesel, ethanol, cellulosic
ethanol, algae biomass
dewatering; fuel and lube oil
purification
Food Technology and Processing
Food Processing which deals
with refining of vegetable oils,
dairy (milk, cheese, etc.);
poultry, swine and beef
rendering; yellow, white, and
brown grease separation; fruit
and vegetable juice; beer, wine
and liquor clarification, etc.
Pharmaceutical and Biotechnological
Industries
Pharmaceutical and
Biotechnology industries that
manufacture drugs, vaccines,
medicines, penicillin, mycelia, Ecoli bacteria, algae, enzymatic
waste
Drying
Industrial Applications
Refers to the removal of
relatively small amount of water
from materials (Evaporation
large amount)
Water is usually removed as
vapor by air
Water Processing
Wastewater processing deals
with separation of municipal,
farm, DAF (dissolved air
flotation), trap grease, drilling
mud, and environmental
wastewater sludges.
May also refer to the removal of
other organic liquids, such as
benzene, from solids
Used in preservation techniques
known as Dehydration
Classification of Drying Processes
Batch the material is inserted
into the drying equipment and
drying proceeds for a given
period of time
Continuous the material is
continuously added to the dryer
and dried material is
continuously removed
Category of Drying Processes
Heat is added by direct contact
with heated air at atmospheric
pressure, and the water vapor
formed is removed by the air
Vacuum drying, the removal of
water proceeds more rapidly at
low pressure and heat is added
indirectly by contact with a
metal wall or by radiation.
Freeze drying, water is sublimed
from frozen material
Applications:
Serve as a preservation techniques product must contain less than 10% of
moisture to suppress the development
of unwanted microorganisms.
e.g.
Dried Salt 0.5%H2O; Dried
Casein (powdered milk) - 8% H2O
For Non Food Products:
use to remove the excess water in
wood (as part of Timber
processing), paper and pulp
industry, to prevent the
development of the molds
reduction in volume and weight of
the material
Extraction
a process where two immiscible or
partly miscible liquids are brought
in contact with each other so that
the soluble substance(s) in one
liquid (raffinate phase) passes into
the other liquid (extract phase) by
diffusion.
What are the reasons to use
extraction?
Separation not feasible by
distillation
Break azeotropes
Energy requirements of
distillation are prohibitive
A complex distillation sequence
is required
The material is heat sensitive
The material is non-volatile
Applications:
Biotechnology - Recovery of
carboxylic acids from biomass
such as fermentation broths,
recovery of oil from algae
broths, extraction of valuable
products from fermentation
broth.
Pharmaceuticals- Recovery of
active materials from
fermentation broths, purification
of vitamin products
Chemical- Washing of
acids/bases, polar compounds
from organics; Recovery of
acrylic acid; Recovery of tightly
hydrogen-bonded organics from
water such as formaldehyde,
formic acid and acetic acid
Effluent Treatment- Recovery
of phenol, DMF, DMAC;
Recovery of acetic acid from
dilute solutions
Polymer processingRecovery of caprolactam for
nylon manufacture, separation
of catalyst from reaction
products
Petroleum- Lube oil quality
improvement, separation of
aromatics/ aliphatics (BTX),
separation of olefins/ paraffins,
separation of structural isomers
Food- Decaffeination of coffee
and tea, separation of essential
oils (flavors and fragrances )
Inorganic- Purification of
phosphoric acid
Metals- Recovery of cobalt and
nickel, recovery of rare earth
elements
Nuclear- Purification of
uranium
Filtration
It is the separation of a fluid-solids
mixture involving passage of most
of the fluid through a porous
barrier, which retains most of the
solid particulates contained in the
mixture.
Suspended solid particles in a fluid
of liquid or gas are physically or
mechanically removed by using a
porous medium that retains the
particles as a separate phase or
cake and passes the clear filtrate.
The suspended solid particles can
be very fine or much larger, very
rigid or plastic particles, spherical
or very irregular in shape
aggregates of particles or
individual particles.
The feed or slurry solution may
carry a heavy load of solid
particles or a very small amount.
Filtration and filters can be
classified several ways:
1. By driving force. The filtrate is
induced to flow through the
filter medium by hydrostatic head
(gravity), pressure applied upstream of
the filter medium, vacuum or reduced
pressure applied downstream of the
filter medium, or centrifugal force
across the medium. Centrifugal
filtration is closely related to
centrifugal sedimentation, and both
are discussed later under
Centrifuges.
2. By filtration mechanism.
Although the mechanism for
separation and accumulation of solids
is not clearly understood, two models
are generally considered and are the
basis for the application of theory to
the filtration process. When solids are
stopped at the surface of a filter
medium and pile upon one another to
form a cake of increasing thickness,
the separation is called cake
filtration. When solids are trapped
within the pores or body of the
medium, it is termed depth, filtermedium, or clarifying filtration.
3. By objective. The process goal of
filtration may be dry solids (the cake is
the product of value), clarified liquid
(the filtrate is the product of value), or
both. Good solids recovery is best
obtained by cake filtration, while
clarification of the liquid is
accomplished by either depth or cake
filtration.
4. By operating cycle. Filtration may
be intermittent (batch) or continuous.
Batch filters may be operated with
constant-pressure driving force, at
constant rate, or in cycles that are
variable with respect to both pressure
and rate. Batch cycle can vary greatly,
depending on filter area and solids
loading.
5. By nature of the solids. Cake
filtration may involve an accumulation
of solids that is compressible or
substantially incompressible,
corresponding roughly in filter-medium
filtration to particles that are
deformable and to those that are rigid.
The particle or particle aggregate size
may be of the same order of
magnitude as the minimum pore size
of most filter media (1 to 10 m and
greater), or may be smaller (1 m
down to the dimension of bacteria and
even large molecules). Most filtrations
involve solids of the former size range;
those of the latter range can be
filtered, if at all, only by filter-medium
type filtration or by ultrafiltration
unless they are converted to the
former range by aggregation prior to
filtration.
Applications:
Metal and mineral processing
Filtration of seawater
Fouling prevention in furnaces
Settling/Sedimentation
(Particle-Fluid Separation)
The particles are separated from the
fluid by gravitational forces acting on
the particles.
The particles can be solid particles or
liquid drops. The fluid can be a liquid
or gas and it may be at rest of motion.
The purpose is to remove the particles
from the fluid stream so that the fluid
is free of particle contaminants.
Sedimentation is the partial separation
or concentration of suspended solid
particles from a liquid by gravity
settling.
Differential Settling and Separation of
Solids in Classification
1. Sink-and-float methods. A liquid
is used whose density is
intermediate between the
heavy or high-density material
and that of the light-density
material. In this liquid, the
heavy particles will not float but
settle out from the medium,
while the light particles will
float.
2. Differential settling methods.
The separation of solid particles
into several size fractions based
upon their settling velocities in
a particular medium is called
differential settling or
classification. The density of the
medium is less than that either
of the two substances to be
separated.
Applications
Removal of solids from liquid
sewage wastes
Settling of crystals from the
mother liquor
Settling of solid particles from a
liquid food
Settling of a slurry from a
soybean leaching process
Mining
Biological science
Beverage production
Air pollution control
Leaching
Leaching is concerned with the
extraction of a soluble constituent
from a solid by means of a solvent.
In leaching, soluble material is
dissolved from its mixture with an
inert solid by means of a liquid
solvent.
The method used for the extraction
is determined by the proportion of
soluble constituent present, its
distribution throughout the solid,
the nature of the solid and the
particle size.
the amount of soluble material
removed is often greater than in
ordinary filtration washing, and the
properties of the solids may
change considerably during the
leaching operation.
Processes Concerned
Dissolving the soluble
constituent.
Separating the solution, so
formed, from the insoluble solid
residue.
Washing the solid residue in
order to free it of unwanted
soluble matter or to obtain as
much of the soluble material as
possible as the product.
V2 , x2 V1 , x1
extractor
L0 , N 0 , y0 , B L1 , N1 , y1 , B
There are four important factors
to be considered:
Particle size- It is generally
desirable that the range of particle
size should be small so that each
particle requires approximately the
same time for extraction and, in
particular, the production of a large
amount of fine material should be
avoided as this may wedge in the
interstices of the larger particles
and impede the flow of the solvent.
Solvent- The liquid chosen should
be a good selective solvent and its
viscosity should be sufficiently low
for it to circulate freely.
Temperature- In most cases, the
solubility of the material which is
being extracted will increase with
temperature to give a higher rate
of extraction.
Agitation of the fluid- Agitation
of the solvent is important because
this increases the eddy diffusion
and therefore the transfer of
material from the surface of the
particles to the bulk of the solution,
as discussed in the following
section. Further, agitation of
suspensions of fine particles
prevents sedimentation and more
effective use is made of the
interfacial surface.
Potrebbero piacerti anche
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Untold Truth About COCONUT OilDocumento37 pagineUntold Truth About COCONUT Oillihu74100% (4)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Thermo ProblemsDocumento8 pagineThermo ProblemsChrister John UyNessuna valutazione finora
- Pharmaceutics 1 Mcqs PDF Download 1Documento5 paginePharmaceutics 1 Mcqs PDF Download 143 Mokal Prasad.100% (2)
- 1ci3pqr1q 814635Documento5 pagine1ci3pqr1q 814635Santosh50% (2)
- Wetlands: Prepared By: Benedict J. Salazar Christer John M. UyDocumento30 pagineWetlands: Prepared By: Benedict J. Salazar Christer John M. UyChrister John UyNessuna valutazione finora
- Biodiesel Settling AccelerationDocumento6 pagineBiodiesel Settling AccelerationCharles CivinelliNessuna valutazione finora
- Gaseous FuelDocumento12 pagineGaseous FuelChrister John UyNessuna valutazione finora
- Calibration of Acid BuretteDocumento7 pagineCalibration of Acid BuretteChrister John Uy100% (2)
- Gaseous FuelDocumento12 pagineGaseous FuelChrister John UyNessuna valutazione finora
- Abstract UyDocumento1 paginaAbstract UyChrister John UyNessuna valutazione finora
- Seawater Quality Assessment and Identification of Pollution Sources Along the Central Coastal Area of Gabes Gulf (SE Tunisia)_ Evidence of Industrial Impact and Implications for Marine Environment ProtectionDocumento8 pagineSeawater Quality Assessment and Identification of Pollution Sources Along the Central Coastal Area of Gabes Gulf (SE Tunisia)_ Evidence of Industrial Impact and Implications for Marine Environment ProtectionChrister John UyNessuna valutazione finora
- Recrystallization & Melting Point Determination Purification of Crystalline Organic CompoundDocumento10 pagineRecrystallization & Melting Point Determination Purification of Crystalline Organic CompoundChrister John UyNessuna valutazione finora
- Second SOC of Guimaras Province (20181205) Smaller Opt PDFDocumento226 pagineSecond SOC of Guimaras Province (20181205) Smaller Opt PDFChrister John UyNessuna valutazione finora
- Op-Ed: Dolphin For Dinner? Poor Countries Increasing Dolphin Consumption Elizabeth Batt, January 8, 2012 SummaryDocumento3 pagineOp-Ed: Dolphin For Dinner? Poor Countries Increasing Dolphin Consumption Elizabeth Batt, January 8, 2012 SummaryChrister John UyNessuna valutazione finora
- Ideal Solns, Colig PropsDocumento19 pagineIdeal Solns, Colig PropsChrister John Uy50% (2)
- D3300 SG (En) 02Documento144 pagineD3300 SG (En) 02Christer John UyNessuna valutazione finora
- Change Does Not Roll in On The Wheels of InevitabilityDocumento2 pagineChange Does Not Roll in On The Wheels of InevitabilityChrister John UyNessuna valutazione finora
- Registration Form (Interbarkada)Documento2 pagineRegistration Form (Interbarkada)Christer John UyNessuna valutazione finora
- The University Wit To Win ItDocumento2 pagineThe University Wit To Win ItChrister John UyNessuna valutazione finora
- Potentiometer: Schematic SymbolDocumento1 paginaPotentiometer: Schematic SymbolChrister John UyNessuna valutazione finora
- Figure 3.6Documento1 paginaFigure 3.6Christer John UyNessuna valutazione finora
- Chapter 16 - SaltsDocumento3 pagineChapter 16 - SaltsschlemielzNessuna valutazione finora
- TautomerismDocumento3 pagineTautomerismShubham JainNessuna valutazione finora
- Rossari Biotech Limited-Company ProfileDocumento17 pagineRossari Biotech Limited-Company ProfileGajendra SharmaNessuna valutazione finora
- Product and Process Information: As Of: June 2003Documento9 pagineProduct and Process Information: As Of: June 2003avinashchauhan2695Nessuna valutazione finora
- Periodic TableDocumento1 paginaPeriodic TableNerisa Nurul BulanNessuna valutazione finora
- Ecology Class Notes - Biogeochemical Cycle (Nutrient Cycle) : December 2019Documento15 pagineEcology Class Notes - Biogeochemical Cycle (Nutrient Cycle) : December 2019farNessuna valutazione finora
- Physical Chemistry Practical: Laboratory ManualDocumento22 paginePhysical Chemistry Practical: Laboratory ManualSoham MukherjeeNessuna valutazione finora
- MSE 227 HW9 F10 SolutionsDocumento5 pagineMSE 227 HW9 F10 Solutionsputri nur shahidaNessuna valutazione finora
- Redox Reactn BalancingDocumento4 pagineRedox Reactn BalancingshahanasnizarNessuna valutazione finora
- Organic ReactionDocumento9 pagineOrganic Reactionliska ramdanawatiNessuna valutazione finora
- Cambridge IGCSE: Combined Science 0653/42Documento21 pagineCambridge IGCSE: Combined Science 0653/42ردينه فايزNessuna valutazione finora
- CAC Hyperfluid Plus (H2) : Superplasticiser For Low Grades Concrete Based On Poly-Carboxylic EtherDocumento2 pagineCAC Hyperfluid Plus (H2) : Superplasticiser For Low Grades Concrete Based On Poly-Carboxylic Ethersmkrishnan89Nessuna valutazione finora
- Assignment 6: Building Construction and Materials IvDocumento10 pagineAssignment 6: Building Construction and Materials IvIsha KarodiNessuna valutazione finora
- " U.V. Plast": Screen InksDocumento2 pagine" U.V. Plast": Screen InksJavier RealNessuna valutazione finora
- Chapter 19Documento26 pagineChapter 19tyobertsNessuna valutazione finora
- Lecture 5-6-Grossman and Jominy HardenabilityDocumento58 pagineLecture 5-6-Grossman and Jominy Hardenabilitychristofer kevinNessuna valutazione finora
- ω-Transaminases for the Production of Optically Pure Amines and Unnatural Amino AcidsDocumento9 pagineω-Transaminases for the Production of Optically Pure Amines and Unnatural Amino AcidsChamula K MasNessuna valutazione finora
- Idoc - Pub - Astm A570 Steel Grade 50 PDFDocumento1 paginaIdoc - Pub - Astm A570 Steel Grade 50 PDFFrancisco Javier Torres AlvaradoNessuna valutazione finora
- Borchers: Additive TIDocumento1 paginaBorchers: Additive TIEldhoseNessuna valutazione finora
- Chapter 7 Workplace HygieneDocumento29 pagineChapter 7 Workplace HygieneMariane Christine Joy BayonNessuna valutazione finora
- The Effect of Curing On The Properties of Metakaolin and Fly Ash-Based Geopolymer PasteDocumento7 pagineThe Effect of Curing On The Properties of Metakaolin and Fly Ash-Based Geopolymer PasteMuhammad Riaz AhmadNessuna valutazione finora
- Luanshya Akatiti DamDocumento6 pagineLuanshya Akatiti DamRamoutar (Ken) SeecharranNessuna valutazione finora
- Cartmay Service For You: FeaturesDocumento6 pagineCartmay Service For You: FeaturesSerhat YıldırımNessuna valutazione finora
- F4 1st Term ExamDocumento14 pagineF4 1st Term ExamSingapore TripNessuna valutazione finora
- Process of Deodourizing of Iso Prpopyl AlcoholDocumento4 pagineProcess of Deodourizing of Iso Prpopyl AlcoholMani ChemistNessuna valutazione finora
- TDS SHELL CATENEX SNR ARIZONAmotorsDocumento2 pagineTDS SHELL CATENEX SNR ARIZONAmotorsİbrahim MutafogluNessuna valutazione finora