Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Control Narrative-Rev 1.1
Caricato da
Moeed IqbalDescrizione originale:
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Control Narrative-Rev 1.1
Caricato da
Moeed IqbalCopyright:
Formati disponibili
Star Engineering Services
Detailed Engineering
SMR/SOR/SIN nbr.
ENGINEERING TEAM
CONSTRUCTION TEAM
PROJECT TEAM
SURV EY T EA M
Control Narrative and Philosophy
1/11
page:
COMPANY
ref. number
Rev.
CONTRACTOR ref:
--
Status
1.0
--
Discipline
INS
22-Dec-15
Doc. Type
PRO
--
(Syst.) Unit
Rev. date
Control Narrative and Philosophy
Remarks:
1.0
22-12-15
Dtail Engineering
Moeed Iqbal
Rev.
Date
Description
Issued by
Reviewed by
Approved by
This document is the property of Star Engineering Services and must not be reproduced, stored or disclosed without permission from COMPANY.
Star Engineering Services
Detailed Engineering
SMR/SOR/SIN nbr.
ENGINEERING TEAM
CONSTRUCTION TEAM
PROJECT TEAM
SURV EY T EA M
Control Narrative and Philosophy
2/11
page:
COMPANY
ref. number
Rev.
CONTRACTOR ref:
--
Status
1.0
--
Discipline
INS
22-Dec-15
Doc. Type
PRO
--
(Syst.) Unit
Rev. date
TABLE OF CONTENTS
1.
Objective...........................................................................................................................................4
2.
Reference Documents ......................................................................................................................4
3.
Abbreviations....................................................................................................................................4
4.
Introduction .....................................................................................................................................5
5.
Description of Facility .......................................................................................................................5
6.
5.1.
Overview .......................................................................................................................... 5
5.2.
Description ........................................................................................................................ 5
5.3.
Process Flow Diagram ........................................................................................................ 6
Operation and Control Philosophy ....................................................................................................7
6.1.
Washing ........................................................................................................................... 7
6.2.
Sterilization ....................................................................................................................... 8
6.1.1.
6.1.2.
6.1.3.
6.2.1.
6.2.2.
6.2.3.
6.3.
Description ................................................................................................................................. 8
Procedure................................................................................................................................... 8
Equipments ............................................................................................................................... 8
Service ............................................................................................................................. 8
6.3.1.
6.3.2.
6.3.3.
6.4.
Description ................................................................................................................................. 7
Procedure................................................................................................................................... 7
Equipments ............................................................................................................................... 7
Filling ......................................................................................................................................... 8
Mixing Ingredients .................................................................................................................... 10
Sample Testing ......................................................................................................................... 10
Bottle Packing ................................................................................................................. 11
6.4.1.
6.4.2.
Description ............................................................................................................................... 11
Procedure................................................................................................................................. 11
This document is the property of Star Engineering Services and must not be reproduced, stored or disclosed without permission from COMPANY.
Star Engineering Services
Detailed Engineering
SMR/SOR/SIN nbr.
ENGINEERING TEAM
CONSTRUCTION TEAM
PROJECT TEAM
SURV EY T EA M
Control Narrative and Philosophy
3/11
page:
COMPANY
ref. number
Rev.
CONTRACTOR ref:
--
Status
1.0
--
Discipline
INS
22-Dec-15
Doc. Type
PRO
--
(Syst.) Unit
Rev. date
LIST OF TABLES
Table 1: Reference Documents ................................................................................................................4
Table 2: Cleaning and Washing Equipments ...........................................................................................7
Table 3: Sterilization Equipments.............................................................................................................8
Table 4: Equipments used in Adding- Initial .............................................................................................9
Table 5: Equipments used in Adding - Solid ............................................................................................9
Table 6: Equipments used in Mixing ...................................................................................................... 10
Table 7: Equipments used in Packing .................................................................................................... 11
This document is the property of Star Engineering Services and must not be reproduced, stored or disclosed without permission from COMPANY.
Star Engineering Services
Detailed Engineering
SMR/SOR/SIN nbr.
ENGINEERING TEAM
CONSTRUCTION TEAM
PROJECT TEAM
SURV EY T EA M
Control Narrative and Philosophy
COMPANY
ref. number
CONTRACTOR ref:
--
1.
4/11
page:
Rev.
Status
1.0
--
Discipline
INS
22-Dec-15
Doc. Type
PRO
--
(Syst.) Unit
Rev. date
Objective
The main purpose of this document is to provide the control narrative of pharmaceutical batching
process mentioned in the Drawing Apses Auto C-SP-786-01-02.
2.
Reference Documents
Sr. No.
1
3.
Document number
Apses Auto C-S-786-01-02
Abbreviations
PFD
BOM
PSI
RTD
HMI
Title
SP Piping Layout
Table 1: Reference Documents
Process Flow Diagram
Bill of Material
Pounds per Square Inch
Resistance Temperature Detectors
Human Machine Interface
This document is the property of Star Engineering Services and must not be reproduced, stored or disclosed without permission from COMPANY.
Star Engineering Services
Detailed Engineering
SMR/SOR/SIN nbr.
ENGINEERING TEAM
CONSTRUCTION TEAM
PROJECT TEAM
SURV EY T EA M
Control Narrative and Philosophy
COMPANY
ref. number
CONTRACTOR ref:
--
4.
5/11
page:
Rev.
Discipline
INS
22-Dec-15
Doc. Type
PRO
--
(Syst.) Unit
Status
1.0
Rev. date
--
Introduction
Star Engineering Services was established in 1994, with a spirit to promote technical skills and to assist
the process industries in all sectors such as:
Pharmaceutical
Food
Textile
Chemical
Star Engineering Services take up the jobs assigned with full assurance, confidence, keeping in view the
time schedule strictly. Work with technique is real sense of Professionalism, Company with its trained
technical staff feel pleasure to accept and under take the challenging critical jobs which can reward a
good reputation in industrial sectors, indeed company pride in mentioning that there has been absolutely
no complaints on the completed projects and Clients, Now they are our life time Clients. Company also
provides Operation and Maintenance services in Electrical , Mechanical and Civil disciplines.
5. Description of Facility
5.1. Overview
The pharmaceutical batch process occurs by mixing of selective inputs at specific temperature and
weight to achieve a desired product.
The inputs that are feeding to the chemical reactor (tank) are as follow:
Distilled water
Nitrogen /Sterilized filter
Raw material or chemical
The outputs that are coming out from the chemical reactor are as follow:
Final product
5.2. Description
The hot distilled water will enter in the vessel (tank) via a distilled water pump and then introduce a
chemical ingredient in the tank and mixed. The product is mixed using an agitator motor. After mixing
introduce the nitrogen in the mixture. After the completion of process the final product will come out
and pass through the chiller to bring the temperature at 11C and then goes for packing.
This document is the property of Star Engineering Services and must not be reproduced, stored or disclosed without permission from COMPANY.
Star Engineering Services
Detailed Engineering
SMR/SOR/SIN nbr.
ENGINEERING TEAM
CONSTRUCTION TEAM
PROJECT TEAM
SURV EY T EA M
Control Narrative and Philosophy
6/11
page:
COMPANY
ref. number
Rev.
CONTRACTOR ref:
--
Status
1.0
--
Discipline
INS
22-Dec-15
Doc. Type
PRO
--
(Syst.) Unit
Rev. date
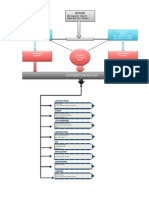
5.3. Process Flow Diagram
Cleaning
Washing
Sterilization
Service
Filling
Adding Initial Ingredients
Distilled water+ Nitrogen
Adding Solid Ingredients
Mixing
Sample Testing
Packing
Final Product
Figure 1: Process Flow Diagram
This document is the property of Star Engineering Services and must not be reproduced, stored or disclosed without permission from COMPANY.
Star Engineering Services
Detailed Engineering
SMR/SOR/SIN nbr.
ENGINEERING TEAM
CONSTRUCTION TEAM
PROJECT TEAM
SURV EY T EA M
Control Narrative and Philosophy
7/11
page:
COMPANY
ref. number
Rev.
CONTRACTOR ref:
--
Status
1.0
Discipline
INS
22-Dec-15
Doc. Type
PRO
--
(Syst.) Unit
Rev. date
--
6. Operation and Control Philosophy
This section should be read in conjunction with the PFDs, BOM. Title description and Document number
for reference documents i.e. PFDs and BOM is mentioned in section-1.2 of this document.
The operational steps of this pharmaceutical bathing process are divided in three major steps. These
steps are as follow:
Cleaning
Sterilization
Service
Packing
6.1. Washing
6.1.1.
Description
The main purpose of cleaning is to wash the vessel after batch complete or usage.
6.1.2.
Procedure
The distilled water is continuously heated at 90oC by heat exchanger. When the operator will give
command of Washing from HMI, the inlet valve will open and start filling the vessel. After the desired
level set point the dead end valve and drain valve will open. The hot distilled water will discharge from
vessel via drain valve. The distilled water temperature in vessel should be 40o C.
The amount of distilled water entered in the vessel is based on time. The time is selected by the
operator. The total amount of distilled water used is monitored and totalized by flow transmitter. After
completion of washing process the dead end valve and drain valve will close.
6.1.3.
Sr No
1
2
3
4
Equipments
Equipment List
Dead End Valve
Inlet Valve
Drain valve
Flow Transmitter
Table 2: Cleaning and Washing Equipments
This document is the property of Star Engineering Services and must not be reproduced, stored or disclosed without permission from COMPANY.
Star Engineering Services
Detailed Engineering
SMR/SOR/SIN nbr.
ENGINEERING TEAM
CONSTRUCTION TEAM
PROJECT TEAM
SURV EY T EA M
Control Narrative and Philosophy
8/11
page:
COMPANY
ref. number
Rev.
CONTRACTOR ref:
--
Status
1.0
--
Discipline
INS
22-Dec-15
Doc. Type
PRO
--
(Syst.) Unit
Rev. date
6.2. Sterilization
6.2.1.
Description
The main purpose of this step is to sterilize the vessel in order to kill the bacteria before the process
starts. The sterilization is done by introducing a pressurized steam and holding a steam at a steam for
specific time.
6.2.2.
Procedure
The operator will press Sterilization button from HMI. The pressure switch will allow the steam to enter
in the vessel if the pressure lies between the required pressure. The steam will be hold in the vessel for
a specific time according to company requirement. The steam is released out from dead end valve and
drain valve. If the vessel is pressurized more than the set pressure then the safety valve will open and
maintain pressure within desired set points.
When the sterilization process will complete then the dead end valve and drain valve will close.
6.2.3.
Equipments
Sr No
1
2
3
4
Equipment List
Dead End Valve
Drain Valve
Safety valve
Vessel
6.3. Service
Table 3: Sterilization Equipments
The service includes the following steps:
Adding Ingredients
Mixing Ingredients
Sample Testing
6.3.1.
Filling
6.3.1.1. Adding Initial Ingredients
Description
The main purpose of this step is to add the initial ingredients according to the batch requirements and
unit specifications.
This document is the property of Star Engineering Services and must not be reproduced, stored or disclosed without permission from COMPANY.
Star Engineering Services
Detailed Engineering
SMR/SOR/SIN nbr.
ENGINEERING TEAM
CONSTRUCTION TEAM
PROJECT TEAM
SURV EY T EA M
Control Narrative and Philosophy
9/11
page:
COMPANY
ref. number
Rev.
CONTRACTOR ref:
--
Status
1.0
Discipline
INS
22-Dec-15
Doc. Type
PRO
--
(Syst.) Unit
Rev. date
--
Procedure
The operator will press Add initial ingredients button form HMI. The inlet valve on the distilled water
pipe will open to feed hot distilled water (40O C) the vessel. The inlet valve will open and pump will start
to add distilled water in the vessel for time specific.
After the addition of water the nitrogen will enter in the vessel. The pressure switch will allow nitrogen
to enter if the pressure lies between 19psi to 150psi. The pressure gauge is also used to monitor the
nitrogen pressure. The nitrogen entered for a specific time. The pressure regulator will maintain nitrogen
line pressure according to the process requirements.
Equipments
Sr No
1
2
3
4
5
Equipment List
Pressure Switch
Pressure Regulator
Heat exchanger
Pressure gauge
Vessel
Table 4: Equipments used in Adding- Initial
6.3.1.2. Adding Solid Ingredients
Description
The main purpose of this step is to add the solid ingredients according to the batch requirements and
unit specifications.
Procedure
The operator will press Add solid ingredients button form HMI.
The chemical is added in the vessel according to process requirement and the load cell is used to weight
and monitor the amount of chemical added.
The lid will then be closed after introducing chemical in the vessel.
Equipments
Sr No
1
2
Equipment List
Vessel
Load Cell
Table 5: Equipments used in Adding - Solid
This document is the property of Star Engineering Services and must not be reproduced, stored or disclosed without permission from COMPANY.
Star Engineering Services
Detailed Engineering
SMR/SOR/SIN nbr.
ENGINEERING TEAM
CONSTRUCTION TEAM
PROJECT TEAM
SURV EY T EA M
Control Narrative and Philosophy
page:
COMPANY
ref. number
Rev.
CONTRACTOR ref:
--
6.3.2.
Status
1.0
--
10/11
Discipline
INS
22-Dec-15
Doc. Type
PRO
--
(Syst.) Unit
Rev. date
Mixing Ingredients
Description
The main purpose of this step is to mix the ingredients according to the batch requirements and unit
specifications.
Procedure
The operator will press Mixing button form HMI. The agitator motor will start to mixing the chemical, hot
distilled water and the Nitrogen. The nitrogen will only enter if the pressure lies between 19 PSI to 150
PSI. The pressure switch and canal filter housing is used in the same line.
The mixing process is time based. The RTD and Level Sensor is used to monitor the mixing process and
for safety of high pressure the safety vent valve.
The safety valve will ensure the pressure of vessel.
Equipments
Sr No
1
2
3
4
5
6
Equipment List
Agitator Motor
Level Sensor
RTD
Safety Valve
Pressure Gauge
Canal Filter Housing
Table 6: Equipments used in Mixing
6.3.3.
Sample Testing
The sample batch testing is done when the process of mixing is complete. If the sample is perfect then
it will proceed to packing otherwise it will mixed again or process personal will take the decision.
The sample is taken at the drain.
This document is the property of Star Engineering Services and must not be reproduced, stored or disclosed without permission from COMPANY.
Star Engineering Services
Detailed Engineering
SMR/SOR/SIN nbr.
ENGINEERING TEAM
CONSTRUCTION TEAM
PROJECT TEAM
SURV EY T EA M
Control Narrative and Philosophy
page:
COMPANY
ref. number
Rev.
CONTRACTOR ref:
--
Status
1.0
--
11/11
Discipline
INS
22-Dec-15
Doc. Type
PRO
--
(Syst.) Unit
Rev. date
6.4. Bottle Packing
6.4.1.
Description
The packing is done after the batch test is successful and the fluid is forced towards the packing
machine.
6.4.2.
Procedure
The operator will select packing from the HMI. The dead end valve will open and centrifugal pump 2000
Lit\Hr will start. The running feedback of pump will open the pack line valve and chillier line valves. The
final product will flow towards packing line passed through the chillier so that the temperature of
product will drop from 40O C to 11O C.
If the operator will stop the bottle packing line then the pack line valve and chillier line valve will close
and circulation line valve will open. The pump will allow the final product to continuously circulate
between vessel and circulation line.
Equipments
Sr No
1
2
3
4
5
6
Equipment List
Valve
Pressure Gauge
Packing line valve
Chiller line valve
Circulation line valve
Gauge
Table 7: Equipments used in Packing
This document is the property of Star Engineering Services and must not be reproduced, stored or disclosed without permission from COMPANY.
Potrebbero piacerti anche
- Control NarrativeDocumento19 pagineControl NarrativeVilla VallejosNessuna valutazione finora
- Distributed Computer Control System: Proceedings of the IFAC Workshop, Tampa, Florida, U.S.A., 2-4 October 1979Da EverandDistributed Computer Control System: Proceedings of the IFAC Workshop, Tampa, Florida, U.S.A., 2-4 October 1979T. J. HarrisonNessuna valutazione finora
- Process Control NarrativeDocumento28 pagineProcess Control NarrativeGeetha_jagadish30100% (3)
- Process Control NarrativeDocumento57 pagineProcess Control Narrativea_attarchi100% (1)
- Advanced Process Control A Complete Guide - 2020 EditionDa EverandAdvanced Process Control A Complete Guide - 2020 EditionNessuna valutazione finora
- Process Control Narrative SampleDocumento12 pagineProcess Control Narrative Samplefri_13th100% (2)
- Control Narratives GDC-121-8Documento89 pagineControl Narratives GDC-121-8Romner CórdovaNessuna valutazione finora
- Operating & Control PhilosophyDocumento16 pagineOperating & Control PhilosophyPrasadNessuna valutazione finora
- CH2MHILL - Process Control Philosohpy - Pinon Ridge ProjectDocumento23 pagineCH2MHILL - Process Control Philosohpy - Pinon Ridge ProjectyyuliusNessuna valutazione finora
- LIMA-J-SPE-0001 Instrument Specification For Process Control System - Rev 1ADocumento25 pagineLIMA-J-SPE-0001 Instrument Specification For Process Control System - Rev 1AIkhtiander Ikhtiander100% (2)
- Process Control NarrativesDocumento29 pagineProcess Control NarrativesSergei LisiyNessuna valutazione finora
- DCS Control System StandardDocumento33 pagineDCS Control System StandardMauro MLRNessuna valutazione finora
- Instr 12107 Instrument Drawings and DocumentsDocumento37 pagineInstr 12107 Instrument Drawings and DocumentsMeliana Butar-Butar100% (2)
- I Et 6000.67 0000 800 Pdy 001 eDocumento178 pagineI Et 6000.67 0000 800 Pdy 001 epitucha_hs100% (1)
- List of Instrumentation Project Engineering DocumentsDocumento19 pagineList of Instrumentation Project Engineering DocumentsVraja KisoriNessuna valutazione finora
- 2.1.1.5 Instrument IndexDocumento18 pagine2.1.1.5 Instrument IndexpeckonetNessuna valutazione finora
- SPC-0804.02-60.05 Rev D2 DCS SpecificationDocumento31 pagineSPC-0804.02-60.05 Rev D2 DCS SpecificationharmlesdragonNessuna valutazione finora
- SPC-0804.02-99.02 Rev D2 Preparation of P&I DDocumento37 pagineSPC-0804.02-99.02 Rev D2 Preparation of P&I DPadakandla Suman100% (1)
- Instrument Engineering DesignDocumento25 pagineInstrument Engineering DesignPi Mos100% (1)
- Instrument Air Supply SystemsDocumento12 pagineInstrument Air Supply SystemsDragan Gasic67% (3)
- Nioec-Sp-00-50 Criteria For Process and MechanicsDocumento37 pagineNioec-Sp-00-50 Criteria For Process and MechanicsCalNessuna valutazione finora
- PCEDO001Documento12 paginePCEDO001Nestor GalianoNessuna valutazione finora
- Control System Specification Energy Equity KeeraDocumento42 pagineControl System Specification Energy Equity KeeraIkhtiander IkhtianderNessuna valutazione finora
- Process Control and Shutdown Philosophy PDFDocumento12 pagineProcess Control and Shutdown Philosophy PDFIdil FitriNessuna valutazione finora
- Icm-En-1600 - Field Instrument InstallationDocumento22 pagineIcm-En-1600 - Field Instrument Installationnghiemta18Nessuna valutazione finora
- 02 0186 06 PB 005 A Control Philosophy Rev 0 FaridhDocumento40 pagine02 0186 06 PB 005 A Control Philosophy Rev 0 FaridhVijay Rajaindran100% (1)
- I-005u3 System Control DiagramDocumento244 pagineI-005u3 System Control Diagramvishnumnair1Nessuna valutazione finora
- Process Control Valve EngineeringDocumento294 pagineProcess Control Valve EngineeringRAMSAHARE MALLAH100% (2)
- Instrument Data Sheets FormatsDocumento165 pagineInstrument Data Sheets Formatsrathnam.pm100% (1)
- Process Control PhilosophyDocumento30 pagineProcess Control Philosophyahmad santoso100% (2)
- Instrument HOOK - UP Drawing Basics - Industrial Automation - Industrial Automation, PLC Programming, Scada & Pid Control SystemDocumento2 pagineInstrument HOOK - UP Drawing Basics - Industrial Automation - Industrial Automation, PLC Programming, Scada & Pid Control SystemABVSAINessuna valutazione finora
- Process Control Philosophy Rev A PDFDocumento48 pagineProcess Control Philosophy Rev A PDFsswahyudi100% (2)
- P&IDs and Process Control NarrativesDocumento7 pagineP&IDs and Process Control Narrativesaugur8866124Nessuna valutazione finora
- P&ID Package (Acero)Documento38 pagineP&ID Package (Acero)Luis Gabriel Gomez Amortegui100% (2)
- SAMSUNG SEM-9461E - Process Design Manual - Preparation of P&ID and UFD Rev1 2005Documento292 pagineSAMSUNG SEM-9461E - Process Design Manual - Preparation of P&ID and UFD Rev1 2005d_maziero100% (2)
- Tankage InstrumentationDocumento30 pagineTankage InstrumentationChinmayNessuna valutazione finora
- 몽중1 P&ID 131227-제본파일 (링크 마크업Documento272 pagine몽중1 P&ID 131227-제본파일 (링크 마크업Lê Thành Chung100% (3)
- Specifications For DCS, ESD and InstrumentationDocumento219 pagineSpecifications For DCS, ESD and Instrumentationchandakbera100% (7)
- Alarm Management PDFDocumento51 pagineAlarm Management PDFBouazzaNessuna valutazione finora
- Example Instrument IndexDocumento1 paginaExample Instrument Indexsvnaik14Nessuna valutazione finora
- Instrumentation - Control Design BasisDocumento40 pagineInstrumentation - Control Design BasisRajesh Barkur100% (2)
- Safeguarding PhilosophyDocumento47 pagineSafeguarding PhilosophyAnonymous QSfDsVxjZ100% (8)
- Io ListDocumento4 pagineIo ListsswahyudiNessuna valutazione finora
- DB-000-13780-0002 - 01 Instrument Engineering Standards BookDocumento47 pagineDB-000-13780-0002 - 01 Instrument Engineering Standards BookNima SharifiNessuna valutazione finora
- Ip IO List ExampleDocumento1 paginaIp IO List ExampleRusla SipayungNessuna valutazione finora
- SIS ESD Sistems For Process Industries Using IEC 61508 Unit7 SIL SelectionDocumento100 pagineSIS ESD Sistems For Process Industries Using IEC 61508 Unit7 SIL Selection최재호Nessuna valutazione finora
- Alarm and Set PointDocumento18 pagineAlarm and Set Pointchemsac2Nessuna valutazione finora
- 06.standard Specification PDFDocumento308 pagine06.standard Specification PDFshareyhouNessuna valutazione finora
- Process Control and Shutdown PhilosophyDocumento14 pagineProcess Control and Shutdown PhilosophyJoanrenis SaranyaNessuna valutazione finora
- RFQ Dcs ImplementationDocumento11 pagineRFQ Dcs ImplementationUmer ZiauddinNessuna valutazione finora
- Alarm MGT PlantpaxDocumento51 pagineAlarm MGT PlantpaxDina MaulidaNessuna valutazione finora
- DHG-PVE-DD-1-IN-BOD-101 Design Basis For Instrumentation - A - 290714 PDFDocumento40 pagineDHG-PVE-DD-1-IN-BOD-101 Design Basis For Instrumentation - A - 290714 PDFhai_solincvnNessuna valutazione finora
- Instrument Design CriteriaDocumento41 pagineInstrument Design CriteriasanthaNessuna valutazione finora
- 00 Me SPC 0005 ADocumento12 pagine00 Me SPC 0005 Aandmar2011Nessuna valutazione finora
- SSG NG01017365 GEN CS 2180 00001 - D01 - Inspection Datasheet For SteelDocumento9 pagineSSG NG01017365 GEN CS 2180 00001 - D01 - Inspection Datasheet For SteelDaniel DamboNessuna valutazione finora
- DRP001-OUF-SPE-W-000-017-B1 Material Certification RequirementsDocumento11 pagineDRP001-OUF-SPE-W-000-017-B1 Material Certification RequirementsDaniel MartinezNessuna valutazione finora
- MC20005-WHP-WI-P-0114 Liquid Seal Pot Datasheet Work Instruction - RevD1Documento8 pagineMC20005-WHP-WI-P-0114 Liquid Seal Pot Datasheet Work Instruction - RevD1nguyenmainamNessuna valutazione finora
- Tinrhert Field Development Project - Epc 1 Inlet Separation and Boosting Facility in OhanetDocumento19 pagineTinrhert Field Development Project - Epc 1 Inlet Separation and Boosting Facility in OhanetbelhaskaNessuna valutazione finora
- NPK-000-B1-MR-400138-K RFQ For Cyclone Hot Insulation R0Documento15 pagineNPK-000-B1-MR-400138-K RFQ For Cyclone Hot Insulation R0DangolNessuna valutazione finora
- SDA-S17893-BNYC1-PX-1206-00001 C01 Bonny Equipment Sizing Calculation ReportDocumento37 pagineSDA-S17893-BNYC1-PX-1206-00001 C01 Bonny Equipment Sizing Calculation ReportsegunNessuna valutazione finora
- Certificate: Vesta Energy Ltd. - 2017-2019 Safety Orientation (Third Party Contractors)Documento1 paginaCertificate: Vesta Energy Ltd. - 2017-2019 Safety Orientation (Third Party Contractors)Moeed IqbalNessuna valutazione finora
- Director General Medical Services Naval Headquarters IslamabadDocumento1 paginaDirector General Medical Services Naval Headquarters IslamabadMoeed IqbalNessuna valutazione finora
- TDG Class 7 For Portable Gauge Users Certificate-SignedDocumento1 paginaTDG Class 7 For Portable Gauge Users Certificate-SignedMoeed IqbalNessuna valutazione finora
- Occupational Health and Safety Act: Revised Statutes of Alberta 2000 Chapter O-2Documento44 pagineOccupational Health and Safety Act: Revised Statutes of Alberta 2000 Chapter O-2Moeed IqbalNessuna valutazione finora
- GD L ExemptionDocumento36 pagineGD L ExemptionMoeed IqbalNessuna valutazione finora
- Chapter - Continuous Sensors: V V V VDocumento19 pagineChapter - Continuous Sensors: V V V VMoeed IqbalNessuna valutazione finora
- 220 MW (CCPP), Combine Cycle Power Plant Korangi KarachiDocumento5 pagine220 MW (CCPP), Combine Cycle Power Plant Korangi KarachiMoeed IqbalNessuna valutazione finora
- National Police Check (NPC) Application Form: SECTION 1: Type of Check RequiredDocumento5 pagineNational Police Check (NPC) Application Form: SECTION 1: Type of Check RequiredAdil KhanNessuna valutazione finora
- Koerr Kymera System Electronic Control SystemsDocumento1 paginaKoerr Kymera System Electronic Control SystemsMoeed IqbalNessuna valutazione finora
- Acknowledge ApplicationDocumento1 paginaAcknowledge ApplicationMoeed IqbalNessuna valutazione finora
- Boiler: Distribution Bus BarDocumento2 pagineBoiler: Distribution Bus BarMoeed IqbalNessuna valutazione finora
- Control System Engineer ResumeDocumento6 pagineControl System Engineer ResumeMoeed IqbalNessuna valutazione finora
- Control System Engineer ResumeDocumento6 pagineControl System Engineer ResumeMoeed IqbalNessuna valutazione finora
- Automatic Street Light Control System (Electronics Project) - ProjectAbstracts - Com - Projects Ideas and DownloadsDocumento5 pagineAutomatic Street Light Control System (Electronics Project) - ProjectAbstracts - Com - Projects Ideas and DownloadsMoeed IqbalNessuna valutazione finora
- Resume - Niraj KachhadiaDocumento3 pagineResume - Niraj KachhadiaMoeed IqbalNessuna valutazione finora
- Bi RadsDocumento10 pagineBi RadsFeiky Herfandi100% (1)
- Jinko 570 Mono Facial Jkm570m-7rl4-VDocumento2 pagineJinko 570 Mono Facial Jkm570m-7rl4-VShahneela AnsariNessuna valutazione finora
- Osh e MeerDocumento3 pagineOsh e MeerfatduckNessuna valutazione finora
- Low Voltage Fixed and Automatic Power Factor Correction SystemsDocumento6 pagineLow Voltage Fixed and Automatic Power Factor Correction Systemszabiruddin786Nessuna valutazione finora
- Meditran SX Sae 15w 40 API CH 4Documento1 paginaMeditran SX Sae 15w 40 API CH 4Aam HudsonNessuna valutazione finora
- PYMS Is A Reliable Malnutrition Screening ToolsDocumento8 paginePYMS Is A Reliable Malnutrition Screening ToolsRika LedyNessuna valutazione finora
- Ca 2013 39Documento40 pagineCa 2013 39singh1699Nessuna valutazione finora
- Industrial Visit ReportDocumento8 pagineIndustrial Visit ReportAnuragBoraNessuna valutazione finora
- 351 UN 1824 Sodium Hydroxide SolutionDocumento8 pagine351 UN 1824 Sodium Hydroxide SolutionCharls DeimoyNessuna valutazione finora
- NASA ISS Expedition 2 Press KitDocumento27 pagineNASA ISS Expedition 2 Press KitOrion2015Nessuna valutazione finora
- HDFC Life Insurance (HDFCLIFE) : 2. P/E 58 3. Book Value (RS) 23.57Documento5 pagineHDFC Life Insurance (HDFCLIFE) : 2. P/E 58 3. Book Value (RS) 23.57Srini VasanNessuna valutazione finora
- The Preparation of Culture MediaDocumento7 pagineThe Preparation of Culture MediaNakyanzi AngellaNessuna valutazione finora
- Factors That Contribute To Successful BakingDocumento8 pagineFactors That Contribute To Successful BakingErrol San Juan100% (1)
- Cross Rate and Merchant RateDocumento26 pagineCross Rate and Merchant RateDivya NadarajanNessuna valutazione finora
- CAT Test Series - 2014Documento2 pagineCAT Test Series - 2014dimevsnNessuna valutazione finora
- Plyometric Training: Sports Med 2Documento9 paginePlyometric Training: Sports Med 2Viren ManiyarNessuna valutazione finora
- HSN-Lube 2007 PDFDocumento45 pagineHSN-Lube 2007 PDFCecilio Valderrama100% (3)
- Cuts of BeefDocumento4 pagineCuts of BeefChristopher EnriquezNessuna valutazione finora
- ISCO HDPE Full Line CatalogDocumento252 pagineISCO HDPE Full Line Catalogpvsreddy2002100% (1)
- House of Candy PresentationDocumento42 pagineHouse of Candy PresentationRohit JaroudiyaNessuna valutazione finora
- Learning Guide No 5Documento19 pagineLearning Guide No 5Menal JemalNessuna valutazione finora
- Logiq e r7 SMDocumento435 pagineLogiq e r7 SMAroNessuna valutazione finora
- What Does She/He Look Like?: Height Build AGEDocumento18 pagineWhat Does She/He Look Like?: Height Build AGEHenrich Garcia LimaNessuna valutazione finora
- 2016 Uptown Parksuites D3 PDFDocumento54 pagine2016 Uptown Parksuites D3 PDFArafat Domado100% (1)
- Electronic Price List June 2022Documento55 pagineElectronic Price List June 2022MOGES ABERANessuna valutazione finora
- Self Reflection 1Documento5 pagineSelf Reflection 1api-270873994Nessuna valutazione finora
- KL 4 Unit 6 TestDocumento3 pagineKL 4 Unit 6 TestMaciej Koififg0% (1)
- Water TreatmentDocumento13 pagineWater TreatmentBayuNessuna valutazione finora
- Lesson 4: Health and Fitness AdvertisingDocumento4 pagineLesson 4: Health and Fitness AdvertisingCatherineNessuna valutazione finora
- Mon AnhDocumento7 pagineMon AnhDavid NguyenNessuna valutazione finora