Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
10 1001@jama 2013 276214
Caricato da
Mutia FatinTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
10 1001@jama 2013 276214
Caricato da
Mutia FatinCopyright:
Formati disponibili
Clinical Review & Education
JAMA Clinical Challenge
Scalp Pustules in a Patient Receiving Chemotherapy
Hsien-Yi Chiu, MD; Hsien-Ching Chiu, MD
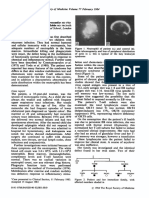
Figure 1.
An 85-year-old man with lung cancer (stage IV, adenocarcinoma) was
treated with the epidermal growth factor receptor [EGFR] inhibitor gefitinib (250 mg/d). One month later, he developed a few erythematous follicular papules with focal scaling on the scalp, which intermittently improved and worsened and were reasonably well controlled with
topical corticosteroids. Neither pustules nor hair loss was noted. The
cancer was well controlled for 31 months, when his tumor recurred and
he was switched to erlotinib (150 mg/d). Subsequently, the number of
scalp lesions increased, accompanied by pustules and hair loss. Numerous pustules with an erythematous
Quiz at jama.com
base were observed on the scalp vertex
(Figure 1). Paronychia, pruritus, and dry skin
were also present, but the pustular eruption was limited to the scalp.
Neither fever nor other constitutional symptoms were noted. Testing
of the scalp lesions using a potassium hydroxide (KOH) preparation produced negative results.
1068
JAMA September 11, 2013 Volume 310, Number 10
Downloaded From: http://jama.jamanetwork.com/ by a Boston University User on 09/10/2013
WHAT WOULD YOU DO NEXT?
A. Do nothing; the rash usually resolves
over time
B. Obtain a bacterial culture from the pustules
C. Obtain a Tzanck smear from the pustules
D. Obtain a skin biopsy from the pustules
jama.com
JAMA Clinical Challenge Clinical Review & Education
Diagnosis
EGFRinduced acneiform eruption
What to Do Next
D. Obtain a skin biopsy from the pustules
The key clinical feature is the consideration of EGFR inhibitor
induced acneiform eruption when evaluating papulopustular scalp
lesions in cancer patients treated with EGFR inhibitors.
Discussion
EGFR inhibitors are increasingly used to treat advanced malignancy.
However, the adverse effects of some EGFR inhibitors diminish quality of life or result in dose modification or discontinuation of these
drugs. EGFR inhibitors, which play an important role in skin and hair
follicle metabolic signaling, can cause distinctive cutaneous adverse
effectsincludingacneiformeruptions,paronychia,trichomegaly,brittle
and curly hairs, xerosis, and mucositis.1 Studies among EGFR inhibitor knockout mice showed thinning of skin and poorly defined stratification of the epidermis and altered terminal differentiation of the
epidermis and hair follicles.2 EGFR inhibitorinduced skin toxicities can
be paradoxically helpful because they reflect response to tumor
treatment,3 but studies have found a dissociation between the efficacy of gefitinib and early skin toxicity.4-6 A recent study demonstrated that the relationship between EGFR inhibitorinduced acneiform eruption and tumor response might decrease over time.1
Fifty percent to 70% of patients treated with EGFR inhibitors develop acneiform eruptions or papulopustular rashes.7 Because EGFR
mediates hair growth cycles and the inflammatory process, its inhibition seems to cause acneiform eruption resulting from abnormal keratinization, follicular retention and rupture, and subsequent failure
to ameliorate inflammation.2 The hair is usually not affected, but reports exist of hair texture changes, including fine, brittle, and curly
hair.8 Transgenic mice expressing inactive mutant EGFR in skin and
hairfollicleshaveshort,wavyhairandwhiskercurling.9 Extensivescalp
acneiform eruption, as was seen in this patient, can occur.8,9
EGFRinduced acneiform eruption can present with hair loss
with numerous scalp pustules resembling scalp infections, particularly tinea capitis. The differential diagnosis of scalp pustules in-
cludes dissecting cellulitis, folliculitis decalvans, fungal
infections, erosive pustular dermatosis, and acute localized exanthematous pustulosis. To determine which of these entities
is present, a skin biopsy is obtained after KOH testing and obtaining fungal cultures to evaluate for tinea capitis. Erlotinib was
not discontinued in this patient. EGFR inhibition likely
caused the skin lesions, because pustules first appeared afFigure 2. Skin biopsy from the scalp
ter the initiation of erlotinib; rerevealing diffuse inflammatory cell
sults of fungal and bacterial
infiltration around hair follicles and in
testing were negative, as was
the dermis, consisting of predominant
the response to antifungal
lymphoplasmacytic cells and focal
drugs; and the skin biopsy findneutrophils (hematoxylin-eosin,
original magnification 40).
ings were consistent with EGFR
inhibitorinduced acneiform
eruption. Topical and oral antibiotics and corticosteroids may be used
to treat EGFR inhibitorinduced acneiform eruption. Topical recombinant human epidermal growth factor may also be effective.10
Patient Outcome
Initially, despite the negative KOH fungal preparation, the scalp rash
was diagnosed as tinea capitis. The treatment response to oral and
topical antifungal agents was poor. A skin biopsy showed a dense
perifollicular, perivascular, and interstitial infiltrate consisting of predominant lymphoplasmacytic cells and focal neutrophils (Figure 2).
Periodic acidSchiff staining did not reveal spores or hyphae. Repeated fungal and bacterial cultures were negative, as were responses to KOH testing of the hairs and pustules. The skin lesion significantly improved with 1 month of doxycycline. However, the
patient continued to have episodic flares of scalp pustules approximately 2 to 3 times a year.
ARTICLE INFORMATION
REFERENCES
Author Affiliations: Department of Dermatology,
National Taiwan University Hospital Hsin-Chu
Branch, Hsin-Chu, Taiwan (H.-Y. Chiu); Department
of Dermatology, National Taiwan University
Hospital, Taipei, Taiwan (H.-C. Chiu).
1. Osio A, Mateus C, Soria JC, et al. Cutaneous
side-effects in patients on long-term treatment
with epidermal growth factor receptor inhibitors.
Br J Dermatol. 2009;161(3):515-521.
Corresponding Author: Hsien-Ching Chiu, MD,
Department of Dermatology, National Taiwan
University Hospital, 7 Chung-Shan South Rd, Taipei
100, Taiwan (hcchiu1003@ntu.edu.tw).
Section Editor: Huan J. Chang, MD, Associate Editor.
Conflict of Interest Disclosures: The authors have
completed and submitted the ICMJE Form for
Disclosure of Potential Conflicts of Interest and
none were reported.
Submissions: We encourage authors to submit
papers for consideration as a JAMA Clinical
Challenge. Please contact Dr Chang at tina.chang
@jamanetwork.org.
2. Lacouture ME. Mechanisms of cutaneous
toxicities to EGFR inhibitors. Nat Rev Cancer.
2006;6(10):803-812.
3. Peuvrel L, Bachmeyer C, Reguiai Z, et al.
Semiology of skin toxicity associated with
epidermal growth factor receptor (EGFR) inhibitors.
Support Care Cancer. 2012;20(5):909-921.
4. Kris MG, Natale RB, Herbst RS, et al. Efficacy of
gefitinib, an inhibitor of the epidermal growth
factor receptor tyrosine kinase, in symptomatic
patients with nonsmall cell lung cancer: a
randomized trial. JAMA. 2003;290(16):2149-2158.
5. Fukuoka M, Yano S, Giaccone G, et al. Multiinstitutional randomized phase II trial of gefitinib for
previously treated patients with advanced non
small-cell lung cancer (the IDEAL 1 Trial) [corrected].
J Clin Oncol. 2003;21(12):2237-2246.
jama.com
Downloaded From: http://jama.jamanetwork.com/ by a Boston University User on 09/10/2013
6. Giovannini M, Gregorc V, Belli C, et al. Clinical
significance of skin toxicity due to EGFR-targeted
therapies [published online June 22, 2009].
J Oncol. doi:10.1155/2009/849051.
7. Robert C, Soria JC, Spatz A, et al. Cutaneous
side-effects of kinase inhibitors and blocking
antibodies. Lancet Oncol. 2005;6(7):491-500.
8. Graves JE, Jones BF, Lind AC, Heffernan MP.
Nonscarring inflammatory alopecia associated with
the epidermal growth factor receptor inhibitor
gefitinib. J Am Acad Dermatol. 2006;55(2):
349-353.
9. Costa DB, Kobayashi S, Schumer ST.
Erlotinib-associated alopecia in a lung cancer
patient. J Thorac Oncol. 2007;2(12):1136-1138.
10. Shin JU, Park JH, Cho BC, Lee JH. Treatment of
epidermal growth factor receptor inhibitorinduced
acneiform eruption with topical recombinant
human epidermal growth factor. Dermatology.
2012;225(2):135-140.
JAMA September 11, 2013 Volume 310, Number 10
1069
Potrebbero piacerti anche
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- Persons With Disabilities Nonetheless Often Remain Excluded From DRM Policies andDocumento3 paginePersons With Disabilities Nonetheless Often Remain Excluded From DRM Policies andMutia FatinNessuna valutazione finora
- Ene 12799Documento8 pagineEne 12799Mutia FatinNessuna valutazione finora
- Laterality: Asymmetries of Body, Brain and CognitionDocumento11 pagineLaterality: Asymmetries of Body, Brain and CognitionMutia FatinNessuna valutazione finora
- Laterality: Asymmetries of Body, Brain and CognitionDocumento11 pagineLaterality: Asymmetries of Body, Brain and CognitionMutia FatinNessuna valutazione finora
- Cluster Headache2Documento6 pagineCluster Headache2epiipo91Nessuna valutazione finora
- Schizophr Bull 2008 Shepard 799 800Documento2 pagineSchizophr Bull 2008 Shepard 799 800Mutia FatinNessuna valutazione finora
- Relationship Between Cesarean Delivery Rate and Maternal and Neonatal MortalityDocumento8 pagineRelationship Between Cesarean Delivery Rate and Maternal and Neonatal MortalityMutia FatinNessuna valutazione finora
- BMJ h3728 FullDocumento14 pagineBMJ h3728 FullMutia FatinNessuna valutazione finora
- 10 1016@j Neuropsychologia 2014 07 027Documento13 pagine10 1016@j Neuropsychologia 2014 07 027Mutia FatinNessuna valutazione finora
- The Association Between BMI and COPD: The Results of Two Population-Based Studies in Guangzhou, ChinaDocumento6 pagineThe Association Between BMI and COPD: The Results of Two Population-Based Studies in Guangzhou, ChinaMutia FatinNessuna valutazione finora
- 1 s2.0 S2215001315300081 MainDocumento6 pagine1 s2.0 S2215001315300081 MainMutia FatinNessuna valutazione finora
- 10 1016@j Neuropsychologia 2014 07 027Documento13 pagine10 1016@j Neuropsychologia 2014 07 027Mutia FatinNessuna valutazione finora
- 178 Full PDFDocumento2 pagine178 Full PDFMutia FatinNessuna valutazione finora
- 90 210 1 SMDocumento4 pagine90 210 1 SMMutia FatinNessuna valutazione finora
- 93 216 1 SMDocumento3 pagine93 216 1 SMMutia FatinNessuna valutazione finora
- WHO South-East Asia Journal of Public HealthDocumento111 pagineWHO South-East Asia Journal of Public HealthMutia Fatin100% (1)
- JPG 150007Documento1 paginaJPG 150007Mutia FatinNessuna valutazione finora
- Preventing Stroke in People With Atrial FibrillationDocumento1 paginaPreventing Stroke in People With Atrial FibrillationMutia FatinNessuna valutazione finora
- Virulence Factors of Pathogenic BacteriaDocumento9 pagineVirulence Factors of Pathogenic BacteriaMutia FatinNessuna valutazione finora
- Trauma Tulang Belakang, Dr. AzhDocumento31 pagineTrauma Tulang Belakang, Dr. AzhIlham RistanandaNessuna valutazione finora
- Fungal Virulence FactorsDocumento21 pagineFungal Virulence FactorsMutia FatinNessuna valutazione finora
- Guidelines Acne VulgarisDocumento14 pagineGuidelines Acne Vulgarisriena456Nessuna valutazione finora
- Host Pathogen InteractionDocumento11 pagineHost Pathogen InteractionMutia FatinNessuna valutazione finora
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (119)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- PESCI Recalls PDFDocumento9 paginePESCI Recalls PDFDanishNessuna valutazione finora
- Najib Khalife - Advances in TPET and Its Immunomodulatory Effect in NMDDocumento6 pagineNajib Khalife - Advances in TPET and Its Immunomodulatory Effect in NMDMarina ShinkoNessuna valutazione finora
- National Mental Health ProgrammeDocumento9 pagineNational Mental Health ProgrammeSharika sasi0% (1)
- CHN Exercise 1 - Paola Mariz P. CeroDocumento3 pagineCHN Exercise 1 - Paola Mariz P. CeroPaola Mariz P. CeroNessuna valutazione finora
- Faktor PICODocumento1 paginaFaktor PICOAlldo SaodalaNessuna valutazione finora
- Nursing Standards for Labour RoomDocumento3 pagineNursing Standards for Labour RoomRenita ChrisNessuna valutazione finora
- MCQ1FULLDocumento176 pagineMCQ1FULLtheintrov100% (11)
- Restraints Assignment on Definitions, Types, Indications and Nurses RoleDocumento8 pagineRestraints Assignment on Definitions, Types, Indications and Nurses RolemacmohitNessuna valutazione finora
- Standards of Medical Care in Diabetes-2022Documento10 pagineStandards of Medical Care in Diabetes-2022Adina SimionNessuna valutazione finora
- Psik Soal UtsDocumento1 paginaPsik Soal UtsNaurahSalsabil / 20Nessuna valutazione finora
- How to Keep Your Heart HealthyDocumento11 pagineHow to Keep Your Heart HealthyLarissa RevillaNessuna valutazione finora
- Lazy Leucocyte SyndromeDocumento2 pagineLazy Leucocyte SyndromeDragos BourosNessuna valutazione finora
- SBI Insurance Violating PMJAY NormsDocumento4 pagineSBI Insurance Violating PMJAY NormsAntar SinghNessuna valutazione finora
- Psychiatric and Mental Health Nursing - Wikipedia PDFDocumento83 paginePsychiatric and Mental Health Nursing - Wikipedia PDFJanani AmmuNessuna valutazione finora
- Assessment 7Documento5 pagineAssessment 7api-525782290Nessuna valutazione finora
- MKSAP 16 - NeurologyDocumento333 pagineMKSAP 16 - NeurologyBacanator100% (4)
- Associate Professor:Ivan Bonet. Obstetric and Gynecology Associate Professor:Ivan Bonet. Obstetric and GynecologyDocumento31 pagineAssociate Professor:Ivan Bonet. Obstetric and Gynecology Associate Professor:Ivan Bonet. Obstetric and Gynecologyivan0% (1)
- CCIM MD Siddha Syllabus for Nanju Maruthuvam SpecialtyDocumento16 pagineCCIM MD Siddha Syllabus for Nanju Maruthuvam SpecialtyJithinNessuna valutazione finora
- Силлабус PChD 1 engDocumento22 pagineСиллабус PChD 1 engaaccvNessuna valutazione finora
- Drugs Acting On The Gastrointestinal System PDFDocumento18 pagineDrugs Acting On The Gastrointestinal System PDFMarc De JesusNessuna valutazione finora
- Drug StudyDocumento17 pagineDrug StudyTherese ArellanoNessuna valutazione finora
- Sush Unity Haemotology-1700Documento51 pagineSush Unity Haemotology-1700Dr-Jahanzaib GondalNessuna valutazione finora
- Nerves Injury Post Neuroaxial AnesthesiaDocumento11 pagineNerves Injury Post Neuroaxial AnesthesiaElisabeth SabajoNessuna valutazione finora
- Comparative Efficacy of Non-Sedating Antihistamine Updosing in Patients With Chronic UrticariaDocumento6 pagineComparative Efficacy of Non-Sedating Antihistamine Updosing in Patients With Chronic UrticariadregleavNessuna valutazione finora
- ImgDocumento1 paginaImgLIDIYA MOL P V100% (1)
- 4-5TH JANUARY 2023: Organized byDocumento3 pagine4-5TH JANUARY 2023: Organized byvivien kate perixNessuna valutazione finora
- ThoracentesisDocumento4 pagineThoracentesisCyntia Theresia Lumintang100% (1)
- Health Apps - A ToolkitDocumento3 pagineHealth Apps - A ToolkitAlexandra WykeNessuna valutazione finora
- Amanita PhalloidesDocumento15 pagineAmanita PhalloidesJair Carrillo100% (1)