Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
2003 Csec Chem Paper 01
Caricato da
Perry SinCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
2003 Csec Chem Paper 01
Caricato da
Perry SinCopyright:
Formati disponibili
-21.
Which ofthe following apparatus/instruments
is most suitable for obtaining both cooking
oil and water from a- mixture of the two?
(A)
(B)
(C)
(D)
2.
II.
III.
IV.
(A)
(B)
(C)
(D)
Protons' and neutrons are found in
the-nucleus.
Electrons can be found anywhere
outside the nucleus.
%,
The number of protons always
equa l s th e number of neutrons.
The number of protons always
equals the number of electrons.
III.
(A)
(B)
(C)
(D)
6.
I and III
I and IV
II and III
III and IV
Which ofthe following compounds may be
conveniently prepared by precipitation?
Barium sulphate
Sodium sulphate
Copper (II) sulphate
Magnesium nitrate
In descending Group VI I ofthe periodic table,
which TWO ofthe fol lowing trends occur?
I.
II.
Filter funnel
Separating funnel
Fractionating column
Volumetric flask
Which TWO of the following statements are
true about the arrangement of electrons,
protons and neutrons in an atom?'
1.
3.
5,
I V.
The reactivity of the elements
increases
(A)
(B)
(C)
(D)
I and Ill
I and IV
II and III
II and IV
Dilute sulphuric acid is classified as a strong
acid because it
(A)
produces 2 moles of hydrogen ions
per mole of acid
(B)
requires 2 moles ofsodium hydroxide
for neutralisation
(C)
is almost completely ionised and is
a good conductor of electricity
ionises to give both hydrogen ions
and sulphate ions.
(D)
7.
When a saturated solution of copper (II)
sulphate is cooled , crystals of copper (II)
sulphate-5 -waterbegin to form because solubilityofcopper(II) sulphate -5-water
(A)
(B)
4.
Which of the atoms represented below by
theirelectronicconfiguration willmost readily
form a positive ion?
(C)
(D)
(A)
(B)
(C).
(D)
2,8,1
2,8,2
2,8,7
2,8,8
The density of the elements increases
The relative atomic mass of the It
elements decreases
The melting point of the elements
increases
increases with decreasing temperature
decreases with increasing temperature
decreases with decreasing temperature
increases with increasing temperature
GO ONTO THE NEXT ['AGE
00260[/F2003
-33
8.
Thearrangementofelements in the periodic
table is based on
,(A)
(B)
(C)
(D)
10.
Which of the following statements about sodium metal are CORRECT?
1.
relative atomic mass
atomicnumber
massnumber
relative molecular mass
II.
III.
9.
IV.
When iron (III) sulphate reacts with aqueous
potassium iodide,abrown colourationofiodine
isproduced. Whichofthefollowingdeductions
is correct?
/-(A)
Iron (III) sulphate is a reducing
agent.
.. (B)
The iodide ion, I,' has been oxidised to iodine.
l(C)
The.i,on,(III) sulphate has lost
electrons.
(D)
The iodide ion , I, has gained electrons.
(A)
(B)
(C)
(D)
11.
I and III only
land IV only
II and Ill only
II and IV only
When aqueous silver nitrate is added to an
aqueous solution ofmagnesium chloride, a
white precipitate forms. The ionic equation
forthe formationofthis precipitate is
(A)
Mg+(aq) + NO3 (aq ) = MgNO3(s)
(B)
Age+(aq) + 2Cl (aq) = AgCI2(s)
(C)
Mgt+(aq)+2i O3(aq) = Mg
(D)
12.
It contains Na ions and mobile
electrons.
ItcontainsNa ionsandmobile
electrons.
It contains cations which repel each
other.
It contains anions which repel each
other.
(NO3 k)
Ag+(aq) + Cl (aq) = AgCI(s)
In which TWO of the following equations are the UNDERLINED reagents acting as
oxidising agents?
1.
2H2 (aq) + SO2(aq) = 2H2O(I) + 3S(s)
It.
1-12OZ(l) + H2SO4(aq) + 2KI(aq) = K2SO4(aq) + 2I-I,O(1) + I2(s)
III.
2FeC l ,(aq) + C 12(g) = 2FeC 1,( aq)
IV.
C60 (s) + H2 (g) = Cu(s) + H20(1)
(A)
_(B)
(C)
(D)
I and Ill
I and IV
-II and Ili
II a rid-[_V
GO ON TO THE NEXT PAGE
002601 /F 2063
tl
-413.
When crystals of potassium nitrate are
dissolved in water, the temperature of the
17.
Which ofthe following can exactly neutral ise
20 cm' of 2.0 mol dm-'sodium hydroxide?
solution decreases because
(A)
(B)
(C)
(D)
14.
The high melting point of ionic compounds
may be due to the
(A)
(B)
(C)
(D)
15.
little energy is required to break
down the crystal, structure of the
potassium nitrate
heat is always absorbed when a
substance dissolves ,
the energy content of dissolved
potassium nitrate is higher than
that of solid potassium nitrate
potassium nitrate is colder than
water
arrangement of ions
large numbers of ions
attraction among ions
movement of ions
I.
10 cm' of 4.0 mol dm-' hydrochloric
acid
it.
l0'cm' of2.0 mol dm-3 sulphuric acid
III.
(A)
I bnly
(B) ' II only
(C)
I and 11 only
(D), , II and III only
18.
Some -calcium carbonate was reacted with
excess dilute hydrochloric acid. The volume
of carbon dioxide evolved was recorded and
plotted against time. Which of the following
graphs represents this reaction?
(A)
Total volume
of CO2/Cm 3
Which of the following statements about
chemical reactions is NOT correct?
(A)
(B)
(C)
(D)
Energy is given out when bonds break
and taken in when bonds form.
Chemical reactions involve the
making and breaking of bonds.
Endothermic reactions take energy
from.the surroundings.
Exothermic reactions give energy
to the surroundings.
20 cm' of 4.0 mol dm' nitric acid
Time/ mins
(B)
Total volume'
of CO2/cm3
Titnel mins
(C)
16.
A substance that conducts an electric current
but remains chemically unchanged is
(A)
(B)
(C)
(D)
aqueous copper (II) sulphate
copper
sulphur
solid sodium chloride
Time/ mins
(D)
Total volume
of CO2 / cm3
Time/ mins
GO ON TO THE NEXT PAGE
002601/F2003
- 519.
In which of the following processes is
fractional distillation NOT used?
(A)
(B)
(C)
(D)
20.
22. . The mass of an element produced in
electrolysis is proportional to the
'Refining of crude petroleum
Separation of methanol from a
methanol-water mixture
Separation of chlorophyll ina leaf
extract
Separation of liquid air into
nitrogen and oxygen
Graphite can be used as a lubricant because of
(A)
(B)
(C)
(D)
strong attraction between the hexagonal layers of carbon atoms
weak attraction between the hex.,
gonal layers of carbon atoms
the loose electrons which can move
throughout the, lattice
strong attraction within the hexagonal layers of carbon atoms
II.
III.
IV.
quantity of electricity which has
been passed
volume of the electrolyte
mass of the electrolyte
mass of the electrode
(A)
(B)
(C)
(D)
I only
II and IV only
I and III only
III and IV only
I.
23.
Which of the following statements BEST
characterises a catalyst?
(A) .
(B)
(C)
(D)
21.
It increases the activation energy
of the reaction.
It alters the quantity of the products
formed.
It is always unchanged physically
at the end of a reaction.
It is always unchanged chemically
Which of the following sulphates are insoluble?
1.
II.
III .
IV.
Barium suiphate
Magnesium sulphate
Lead sul p hate
Aluminium sulphate
(A)
(B)
I and III only
I and H only
(C)
(D)
II and IV only
III and IV only
at the end of a reaction.
24.
Which of the following operations results in
sublimation?
(A)
(B)
(C)
(D)
25.'
Heating of solid sodium chloride
Cooling of oxygen gas
Heating of ethanol .
Heating of iodine crystals
How many neutrons and electrons does the
2+
24
particle
12
have?
Neutrons
(A)
(B)
(C)
12
12
24
(D)
24
Electrons
10
12
10
12
0
GO ON TO TFIE NEXT PAGE
002601 /F 200
-626.
Which of the following sources of energy is
the MOSTcommonly used in the world today?
(A)
(B)
(C)
(D)
Nuclear
Fossil fuels
Biogas
Gasohol
30.
A sample of substance N was placed in solution
M. Which ofthe'followingdiagrams represents
pairs of experiments that would be most
suitable to determine how the concentration of
M affects the rate of the reaction?
II
27.
Study the following thermochemical equation.
(A)
X+Y = Z, AH=--B_kJ
x cm3of
1 mol dm-3
solution
of M
x cm3 of
2moldm-3
solution
of M
1gofN
IgofN
Which of the following methods can be used
to compute the value of - B kJ?
I.
H.
III.
(A)
(B)
(C)
(D)
Energy of Z minus the energy of X.
and Y
Energy of X and Y minus the energy
of Z
The sum of the energies of X, Y
and Z
I only
I and II only
III only
II and III only
()
x cm13of
cold
(C)
II
x cm3 of
hot solution
of I mol dm-3
or m
solution
1 mol dm3
of M.
-1gofN
28.
I g of N
Which of the following processes provides
evidence in support of the particulate nature
of matter?
II
(A)
(B)
(C)
(D)
Diffusion
Precipitation
Condensation
Filtration
(D);
1gof N
29.
xcm3of
1 mol dm
solution
of M
2gN
Sulphuric acid forms the sodium salts NaHSO4
and Na2SO4. Its basicity is therefore
(A)
(B)
(C)
(D)
I
2
3
4
GO ON TO THE NEXT PAGE
002601 /F 2003
-731.
Atoms of isotopes , X and Y, have
1.
II.
III.
the same number of electrons..
the same number of protons.
different numbers of neutrons.
(A)
(B)
(C)
(D)
III only
II and III only
I and II only
I, Hand III
35.
All of the following gases are colourless
EXCEPT
(A)
02
(B)
32.
33.
Which ofthe following could produce carbon
dioxide and carbon monoxide as pollutants?
I.
II.
III.
'IV.
Geothermal energy
Solar energy
Fossil fuels
Biogas
(A)
I only
(B)
(C)
H only
II and III only
(D)
III and IV only
At 1 atmosphericpressure,,the particles move
fastest in a
(A)
(B)
(C)
(D)
34.
36.
37.
(C)
NO2
(D)
NH3
Which of the following elements reacts most
vigorously with I-t (aq) ions to give hydrogen
gas?
(A)
(B)
(C)
Zinc
Lead
Iron
(D)
Copper
Which of the following is a weak electrolyte?
(A)
(B)
(C)
(D)
Molten lead
Aqueous ethanoic acid
Dilute hydrochloric acid
Molten lead bromide
liquid at 25 C
gas at 25 C
liquid at 100 C
gas at 100 C
Which of the following pairs of acids is
monobasic?
(A)
HCl and CH*3CO2H
(B)
HCl and H2SO4
(C)
HNO3 and H2S04
(D)
CH3 *CO,H and HO2000,H
GO ON TO THE NEXT PAGE
002601 /F 2003
-840.
Item 38 refers to the following diagrams.
will(produce blue precipitate with aqueous
sodium hydroxide?
Dilute sulphuric acid \
. C\
' Which of the following aqueous solutions
(A)
(B)
(C)
(D)
Calcium
carbonate
41.
11
Calcium nitrate
Iron (11) nitrate
Copper (11) nitrate
Aluminium nitrate
Which of the following statements is true?
(A)
(B)
(C)
(D)
Ammonia is an acidic gas.
Ammonia is soluble in water and
functions as a base.
Nitric acid is used as a fertilizer and
a reducing agent.
Nitric acid contains two. replaceable
hydrogen atoms.
III
42.
Aluminium is more reactive than iron yet,
after a while, a piece of aluminium left
exposed to the atmosphere becomes corrosion resistant whilst a similar piece of
iron continues to corrode. This is because
the
Dilute hydrochloric
acid
IV
(S
Calci um
aluminium has reacted completely
(A)
(B) . iron oxide formed is more reactive
_31
r-11
than the aluminium oxide formed
(C)
carbonate Z
38.
In which ofthe experiments would the amount
of carbon dioxide collected be GREATEST?
(A)
(B)
(C)
(D)
39.
I
II
III
[V
Which of the following gives an alkaline
reaction with moist litmus paper?
(A)
(B)
(C)
(D)
Ammonia
Nitrogen (IV) oxide
Hydrogen chloride
. Water
(D)
3.
oxide film on aluminium protects the
metal from further corrosion but the
oxide film on iron does not
iron oxide reacts with the iron below
but aluminium oxide does not react
with the aluminium below
Which of the following methods is used for
the extraction of metals at the top of the
electrochemical 'series?
(A)
(13)
(C)
(D)
Electrolysis of molten chloride
Electrol y sis of aqueous chloride
Electrolysis of molten oxide
Reduction of oxide with carbon
GO ON TO THE, NEXT PAGE.
00260 l /F 2003
C 4
9
44.
Which of the following metals will NOT
react with water under any conditions?
(A)
(B)
(C)
(D)
48.
A compound has the following structural
formula.
HHHH
I
I
I
Magnesium
A l u mi n i um
Iron
Copper
H-C-C-C-C-O-H
I
I
I
HH
H-C-H
45.
H-C-H
Which of the following observations would
you expect to make when excess sodium
hydroxide solution is added to a solution
containing zinc ions?
(A)
(B)
(C)
(D)
H
Which TWO of the following statements are
correct? The compound
An alkaline'gas produced
White precipitate soluble in excess
White precipitate insoluble in excess
A green precipitate insoluble inexcess
Items 46-47 refer to the following organic
compounds.
(A)
(B)
(C)
(D)
Ethanol
Ethene
Ethanoic acid
Ethyl ethanoate
49.
I..
II.
III.
IV.
is an alcohol.
is a branched alkane.
can react with sodium.
Js an unreactive substance.
(A)
I and III
(B)
(C)
(D)
I and IV
H and III
II and IV
Which of the following would you expect
to form an addition polymer?
In answering items. 46-47 a particular choice
from the above maybe made more than once,
once, or not at all.
(A)
Which of the organic compounds
(B)
46.
undergoes addition reactions?
47.
is immiscible with waterand is sweet-smelling?
CH,=CH
CN
O
CH3 - C-NH2
0
I
(C)
C17H35
(D)
-C - OH
H
I
CH3' C- CH3
I
CH 3
J
GO ON TO THE NEXT PAGE
002601 /F 2003
-1050.
Ethanol is NOT used asa
(A)
(B)
(C)
(D)
51.
.55.
beverage
fuel
lubricant
solvent
In whichofthe following istheamide linkage
present?
(A)
(B)
(C)
(D)
Polythene
Nylon
Starch
Terylene
56.
52.
The equation shown above represents a
reaction which is classified as
53.
Terylene
Nylon
Starch
Polythene
(A)
(B)
(C)
(D)
I only
I and IV only
I, II and III only
II, III and IV only
Which of the following substances gives a
blue colouration with iodine?
Fats..
Starches
Proteins
Polyamides
Item 57 refers to the following diagram of
an experiment.
addition
neutralisation
polymerisation
substitution
Yeast may be used in the fermentation of
starch to ethanol because
(A)
it is acidic
(B)
it contains enzymes which act on
starch
yeast itself produces the ethanol
it fixes the oxygen needed in the
reaction from the atmosphere
(C)
(D)
57.
54.
1.
II.
III.
I V.
(A)
(B)
(C)
(D)
-H H
HH
I
I
I
I
H-C-C-H +C12-->H-C-C-C1 +HCI
I
i
I
I
H H
H H
(A)
(B)
(C)
(D)
Which ofthe following is/are condensation
polymers?
The process by which large molecules of
hydrocarbon are broken up into smaller
molecules is called
(A)
(B)
(C)
saponification
cracking
polymerization
(D)
condensation
002601/F2003
The gas produced can be BEST identified
ilLiquid Xis
(A)
(B)
(C)
li me water
litmusselution
bromine water
(D)
sodium hydroxide solution
GOON TO TI-IF NEXT PAGE
- HItems 58 - 59 refer to the following diagram which represents three conversions
in organic chemistry
Ethyl ethanoate
II
Ethanoic acid
III
Ethanol
58.
59.
Which ofthe following equations BEST represents Conversion I?
(A)
CH3000H + C2H50H -a CH3000C2H5 + H2O
(B)
CH3COOH + C2HSOH
(C)
CH3000H+C2H5OH - CH3000C2H5 +H2O
(D)
CZHSCOOH +C2H5OH - C2H5000C-H5 H2O
C2H5000CH3 + H2O
For Conversion II, ethyl ethanoate is heated under reflux with
another compound. The name of the compound is
sodium chloride
ethanol
fatty acids
sodium hydroxide
60.
The compound propene, CH 3 CH=CH 2'
reacts with bromine to produce
(A)
CH3CBrCHBr + HBr
(B)
CH3CHBrCH2Br + HBr
(C)
CH3C1-12CHBr2
(D)
CH3CHBrCH,Br
IF YOU FINISH BEFORE TIME IS CALLED , CHECK YOUR WORK ON THIS TEST.
00260 1 /F 2003
Potrebbero piacerti anche
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (120)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Chm557 Laboratory Report: Experiment 4 The Aldol Condensation Reaction: Preparation of DibenzalacetoneDocumento17 pagineChm557 Laboratory Report: Experiment 4 The Aldol Condensation Reaction: Preparation of DibenzalacetonesyafNessuna valutazione finora
- The Precious Metals We Prefer To IgnoreDocumento10 pagineThe Precious Metals We Prefer To Ignoreadamdwaldrop100% (3)
- Circle Theorem Booklet PDFDocumento16 pagineCircle Theorem Booklet PDFPerry Sin100% (1)
- Course Outline: Course Title: Linear Algebra Course Code: EDME3201 (ME32A) Level: 3 Semester: 3 Credits: 3Documento3 pagineCourse Outline: Course Title: Linear Algebra Course Code: EDME3201 (ME32A) Level: 3 Semester: 3 Credits: 3Perry SinNessuna valutazione finora
- Full Maths SbaDocumento9 pagineFull Maths SbaPerry Sin86% (22)
- 3850 - Mathematics - Stage - 1 - Question - Paper - Set 1 PDFDocumento19 pagine3850 - Mathematics - Stage - 1 - Question - Paper - Set 1 PDFPerry SinNessuna valutazione finora
- Lesson Plan September 24 To 28Documento19 pagineLesson Plan September 24 To 28Perry SinNessuna valutazione finora
- PROGRAMME ADVISING SOE MATHS Model A 2019-20 - Teacher TrainedDocumento8 paginePROGRAMME ADVISING SOE MATHS Model A 2019-20 - Teacher TrainedPerry SinNessuna valutazione finora
- Unicellular PDFDocumento1 paginaUnicellular PDFPerry SinNessuna valutazione finora
- 2004 Csec Chem Paper 01Documento10 pagine2004 Csec Chem Paper 01Perry SinNessuna valutazione finora
- 3850 Mathematics Stage 1 Marking Guide Set 1Documento1 pagina3850 Mathematics Stage 1 Marking Guide Set 1Perry SinNessuna valutazione finora
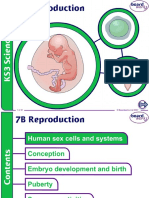
- 7B ReproductionDocumento31 pagine7B ReproductionPerry Sin50% (2)
- Pick An ElementDocumento1 paginaPick An ElementPerry SinNessuna valutazione finora
- The Gastrointestinal System PDFDocumento2 pagineThe Gastrointestinal System PDFPerry SinNessuna valutazione finora
- 2003 Csec Chem Paper 01 PDFDocumento10 pagine2003 Csec Chem Paper 01 PDFMaya SinghNessuna valutazione finora
- The Respiratory System PDFDocumento2 pagineThe Respiratory System PDFPerry SinNessuna valutazione finora
- The Menstrual Cycle PDFDocumento6 pagineThe Menstrual Cycle PDFPerry Sin100% (1)
- The Nervous System PDFDocumento3 pagineThe Nervous System PDFPerry SinNessuna valutazione finora
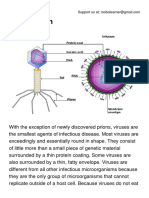
- Virus PDFDocumento2 pagineVirus PDFPerry SinNessuna valutazione finora
- Plant Cell PDFDocumento10 paginePlant Cell PDFPerry SinNessuna valutazione finora
- Types of Neurons PDFDocumento2 pagineTypes of Neurons PDFPerry Sin100% (2)
- The Urinary System PDFDocumento2 pagineThe Urinary System PDFPerry Sin100% (1)
- The Immune System PDFDocumento2 pagineThe Immune System PDFPerry SinNessuna valutazione finora
- The Endocrine System PDFDocumento3 pagineThe Endocrine System PDFPerry SinNessuna valutazione finora
- Mitosis Cell Division PDFDocumento8 pagineMitosis Cell Division PDFPerry SinNessuna valutazione finora
- White Blood Cells (WBC) PDFDocumento3 pagineWhite Blood Cells (WBC) PDFPerry SinNessuna valutazione finora
- The Heart PDFDocumento5 pagineThe Heart PDFPerry SinNessuna valutazione finora
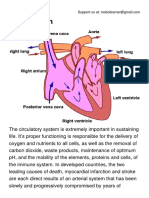
- The Circulatory System PDFDocumento4 pagineThe Circulatory System PDFPerry SinNessuna valutazione finora
- The Breathing System PDFDocumento2 pagineThe Breathing System PDFPerry SinNessuna valutazione finora
- The Cardiovascular System PDFDocumento2 pagineThe Cardiovascular System PDFPerry SinNessuna valutazione finora
- The Digestive System PDFDocumento5 pagineThe Digestive System PDFPerry SinNessuna valutazione finora
- Meosis Cell Division PDFDocumento7 pagineMeosis Cell Division PDFPerry SinNessuna valutazione finora
- ChmistryDocumento27 pagineChmistryChooi YingNessuna valutazione finora
- DPP1 SBlock Advan6264893396548698825Documento4 pagineDPP1 SBlock Advan6264893396548698825Drushya SalunkeNessuna valutazione finora
- Qualitative Analysis of CationsDocumento23 pagineQualitative Analysis of CationsRaymond Godfrey DagwasiNessuna valutazione finora
- Chemistry Module Form 4Documento197 pagineChemistry Module Form 4Thanabalan MunuswamyNessuna valutazione finora
- Metal Oxide Chemistry and Synthesis 3ed 1994 - JolivetDocumento168 pagineMetal Oxide Chemistry and Synthesis 3ed 1994 - Jolivetnbenger100% (2)
- Aluminum and Magnesium Hydroxide Suspenion - Research and FormulationDocumento3 pagineAluminum and Magnesium Hydroxide Suspenion - Research and FormulationEllie Marie RoyalesNessuna valutazione finora
- Friday 23 May 2014 - Morning: As Gce Chemistry ADocumento12 pagineFriday 23 May 2014 - Morning: As Gce Chemistry AAneesa KumarNessuna valutazione finora
- Ias 4 4 275 284 PDFDocumento10 pagineIas 4 4 275 284 PDFHarjinder SinghNessuna valutazione finora
- Chemistry Form 4Documento37 pagineChemistry Form 4Sanusi Mohd100% (1)
- Mole Reactions and Stoichiometry MultipleDocumento25 pagineMole Reactions and Stoichiometry MultiplelinaNessuna valutazione finora
- University of Cambridge International Examinations General Certificate of Education Ordinary LevelDocumento20 pagineUniversity of Cambridge International Examinations General Certificate of Education Ordinary Levelmstudy123456Nessuna valutazione finora
- NCERT Solutions For Class 10 Science Chapter 2 Acids Bases and SaltsDocumento10 pagineNCERT Solutions For Class 10 Science Chapter 2 Acids Bases and SaltsshafiNessuna valutazione finora
- Chapter 5 Energy and Chemical Changes Form 4Documento29 pagineChapter 5 Energy and Chemical Changes Form 4mydqueNessuna valutazione finora
- Acetone PDFDocumento1 paginaAcetone PDFMulayam Singh YadavNessuna valutazione finora
- Vol 14, No 4 RHADocumento20 pagineVol 14, No 4 RHAKawchhar AhammedNessuna valutazione finora
- MCQ Based Questions Grade 10 - Acids, Bases and SaltsDocumento6 pagineMCQ Based Questions Grade 10 - Acids, Bases and SaltsMahi RajneNessuna valutazione finora
- PHDocumento24 paginePHDattatraya GutteNessuna valutazione finora
- RTS Chemistry SPM Question Bank Chapter 14Documento6 pagineRTS Chemistry SPM Question Bank Chapter 14dobbybibiNessuna valutazione finora
- UNIT III GastrointestinalagentsAntacidDocumento9 pagineUNIT III GastrointestinalagentsAntacidgalihNessuna valutazione finora
- Water Softening (IR)Documento15 pagineWater Softening (IR)Iser100% (2)
- ScienceDocumento19 pagineSciencedmarandiNessuna valutazione finora
- Alkali Activated MetakaolinDocumento15 pagineAlkali Activated MetakaolinMadihah Wan RazaliNessuna valutazione finora
- Analytical Chemistry QuestionsDocumento39 pagineAnalytical Chemistry QuestionsTahir HussainNessuna valutazione finora
- NEET UG Chemistry P Block ElementsDocumento47 pagineNEET UG Chemistry P Block ElementskamalNessuna valutazione finora
- 300731846Documento76 pagine300731846Aiv DeeNessuna valutazione finora
- The Elastic Property of Polyvinyl Alcohol Gel With Boric AcidDocumento7 pagineThe Elastic Property of Polyvinyl Alcohol Gel With Boric AcidHaroon RashidNessuna valutazione finora
- IGCSE Chem Summer 2016 Question Paper 63Documento12 pagineIGCSE Chem Summer 2016 Question Paper 63rNessuna valutazione finora
- Module Test (MT 02) Class - X: "Project Abhyudaya"Documento13 pagineModule Test (MT 02) Class - X: "Project Abhyudaya"Tarun SoniNessuna valutazione finora