Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Fluidization - Wikipedia, The Free Encyclopedia
Caricato da
Dila AdilaCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Fluidization - Wikipedia, The Free Encyclopedia
Caricato da
Dila AdilaCopyright:
Formati disponibili
3/9/2015
FluidizationWikipedia,thefreeencyclopedia
Fluidization
From Wikipedia, the free encyclopedia
This article includes a list of references[1], but its sources remain unclear
because it has insufficient inline citations. Please help to improve[2] this
article by introducing[3] more precise citations. (March 2014)
[4]
Schematic drawing of a fluidized bed reactor
Fluidization (or fluidisation) is a process similar to liquefaction[5] whereby a granular
material[6] is converted from a static solid[7]-like state to a dynamic fluid[8]-like state.
This process occurs when a fluid (liquid[9] or gas[10]) is passed up through the granular
material.
chromeextension://iooicodkiihhpojmmeghjclgihfjdjhj/front/in_isolation/reformat.html
1/7
3/9/2015
FluidizationWikipedia,thefreeencyclopedia
When a gas flow is introduced through the bottom of a bed of solid particles, it will move
upwards through the bed via the empty spaces between the particles. At low gas
velocities, aerodynamic drag[11] on each particle is also low, and thus the bed remains in
a fixed state. Increasing the velocity, the aerodynamic drag forces will begin to
counteract the gravitational forces, causing the bed to expand in volume as the particles
move away from each other. Further increasing the velocity, it will reach a critical value
at which the upward drag forces will exactly equal the downward gravitational forces,
causing the particles to become suspended within the fluid. At this critical value, the bed
is said to be fluidized and will exhibit fluidic behavior. By further increasing gas velocity,
the bulk density of the bed will continue to decrease, and its fluidization becomes more
violent, until the particles no longer form a bed and are conveyed upwards by the gas
flow.
When fluidized, a bed of solid particles will behave as a fluid, like a liquid or gas. Like
water[12] in a bucket[13]: the bed will conform to the volume of the chamber, its surface
remaining perpendicular to gravity[14]; objects with a lower density than the bed density
will float on its surface, bobbing up and down if pushed downwards, while objects with a
higher density sink to the bottom of the bed. The fluidic behavior allows the particles to
be transported like a fluid, channeled through pipes[15], not requiring mechanical
transport (e.g. conveyor belt[16]).
A simplified every-day-life example of a gas-solid fluidized bed[17] would be a hot-air
popcorn popper[18]. The popcorn kernels[19], all being fairly uniform in size and shape,
are suspended in the hot air rising from the bottom chamber. Because of the intense
mixing of the particles, akin to that of a boiling liquid, this allows for a uniform
temperature of the kernels throughout the chamber, minimizing the amount of burnt
popcorn. After popping, the now larger popcorn particles encounter increased
aerodynamic drag which pushes them out of the chamber and into a bowl.
The process is also key in the formation of a sand volcano[20] and fluid escape structures
in sediments[21] and sedimentary rocks[22].
Applications
Most of the fluidization applications use one or more of three important characteristics
of fluidized beds: 1) Fluidized solids can be easily transferred between reactors. 2) The
chromeextension://iooicodkiihhpojmmeghjclgihfjdjhj/front/in_isolation/reformat.html
2/7
3/9/2015
FluidizationWikipedia,thefreeencyclopedia
intense mixing within a fluidized bed means that its temperature is uniform. 3) There is
excellent heat transfer between a fluidized bed and heat exchangers immersed in the
bed.
In 1920s, the Winkler process was developed to gasify coal in a fluidized bed, using
oxygen. It was not commercially successful.
The first large scale commercial implementation, in the early 1940s, was the fluid
catalytic cracking (FCC)[23] process, which converted heavier petroleum[24] cuts into
gasoline[25]. Carbon-rich "coke[26]" deposits on the catalyst[27] particles and deactivates
the catalyst in less than 1 second[28]. The fluidized catalyst particles are shuttled
between the fluidized bed reactor and a fluidized bed burner where the coke deposits are
burned off, generating heat for the endothermic[29] cracking reaction.
By the 1950s fluidized bed technology was being applied to mineral and metallurgical
processes such as drying, calcining[30], and sulfide roasting[31].
In the 1960s, several fluidized bed processes dramatically reduced the cost of some
important monomers[32]. Examples are the Sohio process for acrylonitrile[33] and the
oxychlorination process for vinyl chloride[34]. These chemical reactions are highly
exothermic and fluidization ensures a uniform temperature, minimizing unwanted side
reactions, and efficient heat transfer to cooling tubes, ensuring a high productivity.
In the late 1970s, a fluidized bed process for the synthesis of polyethylene[35]
dramatically reduced the cost of this important polymer[36], making its use economical in
many new applications. The polymerization reaction generates heat and the intense
mixing associated with fluidization prevents hot spots where the polyethylene particles
would melt. A similar process is used for the synthesis of polypropylene[37].
Currently, most of the processes that are being developed for the industrial production of
carbon nanotubes[38] use a fluidized bed.1
A new potential application of fluidization technology is chemical looping
combustion[39], which has not yet been commercialized. One solution to reducing the
potential effect of carbon dioxide[40] generated by fuel combustion[41] (e.g. in power
chromeextension://iooicodkiihhpojmmeghjclgihfjdjhj/front/in_isolation/reformat.html
3/7
3/9/2015
FluidizationWikipedia,thefreeencyclopedia
stations[42]) on global warming[43] is carbon dioxide sequestration[44]. Regular
combustion[45] with air[46] produces a gas that is mostly nitrogen[47] (as it is air's main
component at about 80% by volume), which prevents economical sequestration.
Chemical looping uses a metal[48] oxide[49] as a solid oxygen[50] carrier. These metal
oxide particles replace air (specifically oxygen[51] in the air) in a combustion reaction
with a solid, liquid or gaseous fuel in a fluidized bed, producing solid metal particles from
the reduction[52] of the metal oxides and a mixture of carbon dioxide and water vapor[53],
the major products of any combustion reaction. The water[54] vapor is condensed,
leaving pure carbon dioxide which can be sequestered. The solid metal particles are
circulated to another fluidized bed where they react with air (and again, specifically
oxygen in the air), producing heat and oxidizing[55] the metal particles to metal oxide
particles that are recirculated to the fluidized bed combustor.
Fluidization has many applications with the use of ion exchange[56] particles for the
purification and processing of many industrial liquid streams. Industries such as food &
beverage, hydrometallurgical, water softening, catalysis, bio-based chemical etc. use ion
exchange as a critical step in processing. Conventionally ion exchange has been used in a
packed bed where a pre-clarified liquid passes downward through a column. Much work
has been done at the University of Western Ontario in London Ontario, Canada on the
use of a continuous fluidized ion exchange system, named "Liquid-solid circulating
fluidized bed" (LSCFB), recently being called "Circulating fluidized ion exchange" (CFIX).
This system has widespread applications extending the use of traditional ion exchange
systems because it can handle feed streams with large amounts of suspended solids due
to the use of fluidization.23
External links
Notes
1. Jump up ^ International Journal of Chemical Reactor Engineering[57]
2. Jump up ^ Prince; A. Bassi; C. Haas (2012). Biotechnology Progress 28 (1). Missing or
empty |title= (help)
3. Jump up ^ Mazumder; Zhu, Ray (April 2010). "Optimal design of liquid-solid
circulating fluidized bed for continuous protein recovery". Powder Technology 199 (1):
chromeextension://iooicodkiihhpojmmeghjclgihfjdjhj/front/in_isolation/reformat.html
4/7
3/9/2015
FluidizationWikipedia,thefreeencyclopedia
3247. doi[58]:10.1016/j.powtec.2009.07.009[59].
Links
1. http://en.wikipedia.org/wiki/Wikipedia:Citing_sources
2. http://en.wikipedia.org/wiki/Wikipedia:WikiProject_Fact_and_Reference_Check
3. http://en.wikipedia.org/wiki/Wikipedia:When_to_cite
4. http://en.wikipedia.org/wiki/File:Fluidized_Bed_Reactor_Graphic.svg
5. http://en.wikipedia.org/wiki/Liquefaction
6. http://en.wikipedia.org/wiki/Granular_material
7. http://en.wikipedia.org/wiki/Solid
8. http://en.wikipedia.org/wiki/Fluid
9. http://en.wikipedia.org/wiki/Liquid
10. http://en.wikipedia.org/wiki/Gas
11. http://en.wikipedia.org/wiki/Drag_(physics)
12. http://en.wikipedia.org/wiki/Water
13. http://en.wikipedia.org/wiki/Bucket
14. http://en.wikipedia.org/wiki/Gravity
15. http://en.wikipedia.org/wiki/Water_pipe
16. http://en.wikipedia.org/wiki/Conveyor_belt
17. http://en.wikipedia.org/wiki/Fluidized_bed
18. http://en.wikipedia.org/wiki/Popcorn_maker
19. http://en.wikipedia.org/wiki/Popcorn
20. http://en.wikipedia.org/wiki/Sand_volcano
21. http://en.wikipedia.org/wiki/Sediment
22. http://en.wikipedia.org/wiki/Sedimentary_rock
23. http://en.wikipedia.org/wiki/Fluid_catalytic_cracking
24. http://en.wikipedia.org/wiki/Petroleum
25. http://en.wikipedia.org/wiki/Gasoline
chromeextension://iooicodkiihhpojmmeghjclgihfjdjhj/front/in_isolation/reformat.html
5/7
3/9/2015
FluidizationWikipedia,thefreeencyclopedia
26. http://en.wikipedia.org/wiki/Coke_(fuel)
27. http://en.wikipedia.org/wiki/Catalyst
28. http://en.wikipedia.org/wiki/Second
29. http://en.wikipedia.org/wiki/Endothermic
30. http://en.wikipedia.org/wiki/Calcination
31. http://en.wikipedia.org/wiki/Roasting_(metallurgy)
32. http://en.wikipedia.org/wiki/Monomers
33. http://en.wikipedia.org/wiki/Acrylonitrile
34. http://en.wikipedia.org/wiki/Vinyl_chloride
35. http://en.wikipedia.org/wiki/Polyethylene
36. http://en.wikipedia.org/wiki/Polymer
37. http://en.wikipedia.org/wiki/Polypropylene
38. http://en.wikipedia.org/wiki/Carbon_nanotubes
39. http://en.wikipedia.org/wiki/Chemical_looping_combustion
40. http://en.wikipedia.org/wiki/Carbon_dioxide
41. http://en.wikipedia.org/wiki/Combustion
42. http://en.wikipedia.org/wiki/Power_station
43. http://en.wikipedia.org/wiki/Global_warming
44. http://en.wikipedia.org/wiki/Carbon_sequestration
45. http://en.wikipedia.org/wiki/Combustion
46. http://en.wikipedia.org/wiki/Air
47. http://en.wikipedia.org/wiki/Nitrogen
48. http://en.wikipedia.org/wiki/Metal
49. http://en.wikipedia.org/wiki/Oxide
50. http://en.wikipedia.org/wiki/Oxygen
51. http://en.wikipedia.org/wiki/Oxygen
52. http://en.wikipedia.org/wiki/Redox
53. http://en.wikipedia.org/wiki/Water_vapor
54. http://en.wikipedia.org/wiki/Water
chromeextension://iooicodkiihhpojmmeghjclgihfjdjhj/front/in_isolation/reformat.html
6/7
3/9/2015
FluidizationWikipedia,thefreeencyclopedia
55. http://en.wikipedia.org/wiki/Redox
56. http://en.wikipedia.org/wiki/Ion_exchange
57. http://www.bepress.com/ijcre/vol3/R3/
58. http://en.wikipedia.org/wiki/Digital_object_identifier
59. http://dx.doi.org/10.1016%2Fj.powtec.2009.07.009
Get a free Evernote account to save this article and
view it later on any device.
Create account
chromeextension://iooicodkiihhpojmmeghjclgihfjdjhj/front/in_isolation/reformat.html
7/7
Potrebbero piacerti anche
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Plate Heat Exchanger - Wikipedia, The Free EncyclopediaDocumento6 paginePlate Heat Exchanger - Wikipedia, The Free EncyclopediaDila AdilaNessuna valutazione finora
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Penukar Ion 07Documento34 paginePenukar Ion 07Dila AdilaNessuna valutazione finora
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
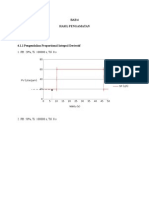
- Bab 4 Hasil Pengamatan: 1. PB: 20%, Ti: 100000 S, TD: 0 SDocumento9 pagineBab 4 Hasil Pengamatan: 1. PB: 20%, Ti: 100000 S, TD: 0 SDila AdilaNessuna valutazione finora
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Mark S. Bentley, Norbert I. Kömle, Günter Kargl, Erika Hütter Space Research Institute, 8042 Graz, AustriaDocumento1 paginaMark S. Bentley, Norbert I. Kömle, Günter Kargl, Erika Hütter Space Research Institute, 8042 Graz, AustriaDila AdilaNessuna valutazione finora
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Industrial Application of MicrobesDocumento3 pagineIndustrial Application of MicrobesDila AdilaNessuna valutazione finora
- Detailed Lesson Plan in Science IiiDocumento3 pagineDetailed Lesson Plan in Science Iiicharito riveraNessuna valutazione finora
- The Seventh House in AstrologyDocumento6 pagineThe Seventh House in Astrologytratak100% (1)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- OKM 54MP FlyerDocumento1 paginaOKM 54MP FlyerJohnsonNessuna valutazione finora
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Reservoir Rock PropertiesDocumento148 pagineReservoir Rock Propertiesiscribdusername100% (7)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- NCP DehydrationDocumento4 pagineNCP DehydrationYnah Sayoc100% (2)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- RocketsDocumento2 pagineRocketsAlin VoicuNessuna valutazione finora
- Ec210b Pub20021241-I PDFDocumento1.046 pagineEc210b Pub20021241-I PDFCholif 'oliph' Fadhilah100% (16)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Parker Valve Safety CrownDocumento11 pagineParker Valve Safety Crownayman akrabNessuna valutazione finora
- 159 SnackDocumento97 pagine159 SnackGuy PlaterNessuna valutazione finora
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- Department of Mechanical EnginneringDocumento11 pagineDepartment of Mechanical EnginneringViraj SukaleNessuna valutazione finora
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- CH Six Global Transportation Planning and ExecutionDocumento41 pagineCH Six Global Transportation Planning and ExecutionDsh ShNessuna valutazione finora
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The Russian Review - 2020 - ROTH EY - Listening Out Listening For Listening in Cold War Radio Broadcasting and The LateDocumento22 pagineThe Russian Review - 2020 - ROTH EY - Listening Out Listening For Listening in Cold War Radio Broadcasting and The LateOkawa TakeshiNessuna valutazione finora
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- EI6704: UNIT 5 NotesDocumento19 pagineEI6704: UNIT 5 NotesMadhu MithaNessuna valutazione finora
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Wps Gtaw Monel b127 b164Documento2 pagineWps Gtaw Monel b127 b164Srinivasan Muruganantham67% (3)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Regulatory Framework For Water Dams in QuebecDocumento2 pagineRegulatory Framework For Water Dams in QuebecRaveeNessuna valutazione finora
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- ANG Coupe Coco Mangue PassionDocumento1 paginaANG Coupe Coco Mangue PassionRicardo Rovira ChalerNessuna valutazione finora
- The Sparkle EffectDocumento22 pagineThe Sparkle EffectVida Betances-ReyesNessuna valutazione finora
- EASL 2021 Version 4 NewDocumento691 pagineEASL 2021 Version 4 NewGupse Köroğlu AdalıNessuna valutazione finora
- Hot Process Liquid SoapmakingDocumento11 pagineHot Process Liquid SoapmakingPanacea PharmaNessuna valutazione finora
- Modeling Plastics in ANSYS (Compatibility Mode) PDFDocumento14 pagineModeling Plastics in ANSYS (Compatibility Mode) PDFashutosh.srvNessuna valutazione finora
- Fill in The Table by Determining The Functions of The Following ItemsDocumento6 pagineFill in The Table by Determining The Functions of The Following ItemsJessabel CandidatoNessuna valutazione finora
- Gastritis: Department of Gastroenterology General Hospital of Ningxia Medical University Si Cen MDDocumento82 pagineGastritis: Department of Gastroenterology General Hospital of Ningxia Medical University Si Cen MDAvi Themessy100% (1)
- Part 66 B1 - MODULE 4 3 SERVOMECHANISMSDocumento26 paginePart 66 B1 - MODULE 4 3 SERVOMECHANISMSyaman91100% (1)
- Blank BPSU TemplateDocumento6 pagineBlank BPSU TemplateClarina Alviz BerganteNessuna valutazione finora
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Questionpaper Unit1WCH01 January2018 IAL Edexcel ChemistryDocumento24 pagineQuestionpaper Unit1WCH01 January2018 IAL Edexcel Chemistryshawon.sbd06Nessuna valutazione finora
- EV Connect What Is EVSE White PaperDocumento13 pagineEV Connect What Is EVSE White PaperEV ConnectNessuna valutazione finora
- Earthing ResistanceDocumento4 pagineEarthing ResistanceNeeraj Purohit100% (1)
- Pahlavi PoemDocumento9 paginePahlavi PoemBatsuren BarangasNessuna valutazione finora
- Study Antimicrobial Activity of Lemon (Citrus Lemon L.) Peel ExtractDocumento5 pagineStudy Antimicrobial Activity of Lemon (Citrus Lemon L.) Peel ExtractLoredana Veronica ZalischiNessuna valutazione finora
- Ground PlaneDocumento1 paginaGround Planeaeronautical rajasNessuna valutazione finora
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)