Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Types of Corrosion
Caricato da
GaneshDescrizione originale:
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Types of Corrosion
Caricato da
GaneshCopyright:
Formati disponibili
CHAPTER 2
TYPES OF CORROSION
2.1
INTRODUCTION
It is a great challenge to classify the types of corrosion in a uniform
way. One can divide the corrosion on the basis of appearance of corrosion damage, mechanism of attack, industry section, and preventive
methods. There are many types and causes of corrosion. In the oil and
gas production industries, corrosion is usually classified into four different types. This classification is mostly based on the types of corrosion
in the oil field (see Oxford and Foss, 1958):
1. Sweet corrosion
2. Sour corrosion
3. Oxygen corrosion
4. Electrochemical corrosion
One can also classify corrosion types as follows (see http://www.corrosiondoctors.org/Forms/fretting.
htm):
1. Uniform attack
2. Pitting corrosion
3. Crevice corrosion
4. Galvanic corrosion
5. Erosional corrosion
6. Fretting corrosion
7 . Cavitation
35
36
Chapter 2
Types of Corrosion
8. Intergranular corrosion
9. Stress corrosion
10. Dealloying (selective leaching)
11. Environmental cracking
12. Fatigue
13. Exfoliation
According to Bertness et al. (1989, p. 559), destruction of metal is
influenced by various physical and chemical factors which localize and
increase corrosion damage.
The conditions which promote corrosion include:
1. Energy differences in the form of stress gradients or chemical reactivities across the metal surface in contact with corrosive solution.
2. Differences in concentration of salts or other corrodants in electrolytic solution.
3. Differences in the amount of deposits, either solid or liquid, on the
metal surfaces, which are insoluble in the electrolytic solutions.
4. Temperature gradients over the surface of the metal in contact with
corrosive solution.
5. Compositional differences in the metal surface.
In this chapter, the authors tried to cover all of the above conditions
and types of corrosion, giving a brief introduction for each. One should
realize, however, that in practice usually more than one type of corrosion takes place.
2.2
SWEET CORROSION
Sweet corrosion is a common type of corrosion and can be defined as
the deterioration of metal due to contact with carbon dioxide, fatty
acids, or other similar corrosive agents, but excluding hydrogen sulfide
H2S. One can recognize the sweet corrosion by pitting on the steel.
Carbon dioxide systems are one of the most common environments in
the oilfield industry where corrosion occurs. Carbon dioxide reacts with
the moisture in the environment and forms a weak carbonic acid
(H2C03)in water, which then reacts with metal (however, this reaction
occurs very slowly):
CO, + H,O ++H,CO,
2.3 Sour Corrosion 37
Fe+ H 2 C 0 3+ FeCO,+ H2?
(2.3)
Corrosion rates in a COz system can reach very high levels (thousands of mils per year), but corrosion can be effectively inhibited. The
reaction of CO, with water will decrease pH*, which will cause corrosion. With increasing solubility of carbon dioxide in water, corrosion
will increase,The solubility is determined by the temperature, pressure,
and composition. Pressure increases the solubility, whereas temperature decreases the solubility. Sweet corrosion always occurs in gas-condensate wells. Condensate wells produce water with pH below 7 at the
wellhead and commonly as low as 4 at the bottom of some wells (see
Oxford and Foss, 1958). This is due to:
1. High COz contents of gas; usually up to 3%.
2. High total pressure, ranging from 1,000 to 8,OOOpsi at the
wellhead.
3. Presence of organic acids, such as acetic acid (CH3COOH).
Erosion by high-velocity gas stream aggravates the corrosion.
2.3
SOUR CORROSION
The deterioration of metal due to contact with hydrogen sulfide and
moisture is called sour corrosion. Although hydrogen sulfide is not corrosive by itself, it becomes a severely corrosive agent in the presence
of water. The general equation of sour corrosion can be expressed as
follows:
H2S+Fe+ H 2 0+FeSx + 2H+ H 2 0
(2.4)
One can recognize the sour corrosion by the black deposit, i.e., iron
sulfide deposit on the surface of steel. The scale tends to cause faster
corrosion because the iron sulfide plays the role of cathode, whereas
the steel acts as the anode where corrosion occurs. Sour corrosion
results in deep pitting on the equipment. Also, hydrogen released in the
38
Chapter 2
Types of Corrosion
above reaction can embrittle the steel (cause a sulfide stress cracking
[SSC]). The presence of C 0 2 and/or O2 aggravates sour corrosion. The
incidents of sour corrosion in refineries vary according to the sulfur
content of oil blends used. Amine units are the hot spots for sour
corrosion. The following refinery units are the places where sour corrosion is considerable (http://www.ionscience.com/Pages/HydroApp.
htm#B ac):
1. Amine columns
2. CDU lower reflux circuits
3. Catalytic crackers
4. Coker gas recovery systems
5. Diesel drier overhead accumulators
6. Fuel gas mixing drums and knockout drums
7. Flame knockout drums
8. Compressor suction drums
9. Gas compressors
10. Hot wells
11. Gadoil pre-saturators, strippers, and absorbers
12. Hydrocracker reactor effluent air coolers
13. High-pressure sour gas separators
14. Low-pressure sour gas separators
15. Off-gas knockout pots
16. On-site flare knockout drums
17. Recycle gas knockout drums
18. VBU flare knockout drums
19. Sour fuel gas knockout drums
20. Sour gas gathering lines, absorber towers, knockout drums, flash
drums
21. Sour water strippers
22. Sour flare lines
23. Vacuum off-gas knockout drums
2.4
CLASSES OF CORROSION
Numerous types of steel destruction can result from the corrosion
process, which are listed under the following classes of corrosion:
Uniform attack. The entire area of the metal corrodes uniformly resulting in thinning of the metal.his often occurs to drill pipe, but usually
2.4
Classes of Corrosion 39
is the least damaging of different types of corrosive attacks. Uniform
rusting of iron and tarnishing of silver are examples of this form of
corrosion attack.
Crevice corrosion. Crevice corrosion is an example of localized attack
in the shielded areas of metal assemblies such as pipes and collars,
rod pins and boxes, tubing, and drill pipe joints. Crevice corrosion is
caused by concentration differences of corrodants over a metal
surface. Electrochemical potential differences result in selective
crevice or pitting corrosion attack. Oxygen dissolved in drilling fluid
promotes crevice and pitting attack of metal in the shielded areas of
drill string and is the common cause of washouts and destruction
under rubber pipe protectors.
Pitting corrosion. Pitting is often localized in a crevice but can also
occur on clean metal surfaces in a corrosive environment. An example
of this type of corrosion attack is the corrosion of steel in highvelocity seawater, low-pH aerated brines, or drilling fluids. Upon
formation of a pit, corrosion continues as in a crevice but, usually, at
an accelerated rate.
Intergranular corrosion. Metal is preferentially attacked along the
grain boundaries. Improper heat treatment of alloys or hightemperature exposure may cause precipitation of materials or nonhomogeneity of the metal structure at the grain boundaries, which
results in preferential attack. Weld decay is a form of intergranular
attack. The attack occurs in a narrow band on each side of the weld
owing to sensitizing or changes in the grain structure due to welding.
Appropriate heat-treating or metal selection can prevent the weld
decay. Ringworm corrosion is a selective attack which forms groove
around the pipe near the box or external upset end. This type of
selective attack is avoided by annealing the entire pipe after the
upset is formed.
Galvanic or two-metal corrosion. Galvanic corrosion may occur when
two different metals are in contact in a corrosive environment. The
attack is usually localized near the point of contact.
Selective leaching. Selective leaching occurs when one component of
an alloy is removed by the corrosion process. An example of this type
of corrosion is the selective corrosion of zinc in brass.
Cavitation corrosion. Cavitation damage results in a sponge-like
appearance with deep pits in the metal surface. The destruction may
be caused by purely mechanical effects in which pulsating pressures
cause vaporization with formation and collapse of bubbles at the
metal surface. The mechanical working of the metal surface causes
40
Chapter 2
Types of Corrosion
destruction, which is amplified in a corrosive environment. This type
of corrosion attack, example of which are found in pumps, may be
prevented by increasing the suction head on the pumping equipment.
A net positive suction head should always be maintained not only
to prevent cavitation damage, but also to prevent possible suction of
air into the flow stream. The latter can aggravate corrosion in many
environments.
Erosion-corrosion. The combination of erosion and corrosion results
in severe localized attack of metal. Damage appears as smooth
groove or hole in the metal, such as in a washout of the drill pipe,
casing, or tubing. The washout is initiated by pitting in a crevice
which penetrates the steel. The erosion-corrosion process completes
the metal destruction. The erosion process removes protective films
from the metal and exposes clean metal surface to the corrosive
environment. This accelerates the corrosion process. Impingement
attack is a form of erosion-corrosion process, which occurs after the
breakdown of protective films. High velocities and presence of abrasive suspended material and the corrodants in drilling and produced
fluids contribute to this destructive process. The combination of wear
and corrosion may also remove protective surface films and accelerate localized attack by corrosion. This form of corrosion is often
overlooked or recognized as being due to wear. The use of inhibitors
can often control this form of metal destruction. For example, inhibitors are used extensively for protection of downhole pumping equipment in oil wells.
Corrosion due to variation in fluid flow. Velocity differences and turbulence of fluid flow over the metal surface cause localized corrosion. In addition to the combined effects of erosion and corrosion,
variation in fluid flow can cause differences in concentrations of corrodants and depolarizers, which may result in a selective attack of
metals. For example, selective attack of metal occurs under the areas
which are shielded by deposits from corrosion, i.e., scale, wax, bacteria, and sediments, in pipelines and vessels.
Stress corrosion. The stress corrosion is produced by the combined
effects of stress and corrosion on the behavior of metals. An example
of stress corrosion is that local action cells are developed due to the
residual stresses induced in the metal and adjacent unstressed metal
in the pipe. Stressed metal is anodic, whereas the unstressed metal
is cathodic. The degree to which these stresses are induced in pipes
varies with (1)the metallurgical properties, (2) cold work, (3) weight
of the pipe, (4)effects of slips, notch effects at tool joints, and ( 5 )
2.4
Classes of Corrosion 41
presence of H2S gas. In the oil fields, H,S-induced stress corrosion
has been instrumental in bringing about sudden failure of drill
pipes.
2.4.1
Stress-Induced Corrosion
Because of extra energy supplied by the stress, the portion of the same
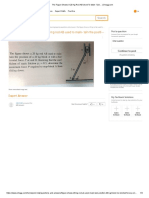
metal exposed to a higher stress acts as anode. This can be demonstrated by placing a bent nail into seawater (Figure 2.1). The initial
points of corrosion attack (formation of rust) will be the more stressed
areas, i.e., the head, the point, and middle portion of the nail as shown
in Figure 2.1, with discoloration of water with rust in the vicinity.
In general, the more stressed part of a metal structure acts as anode
where corrosion occurs. Thus, stress begets rust!
Hydrogen collects on the pipe as a film of atomic hydrogen which
quickly combines with itself to form molecular hydrogen gas (H,). The
hydrogen gas molecules are too large to enter the steel and, therefore,
usually bubble off harmlessly.
In the presence of sulfide, however, hydrogen gradient into the steel
is greatly increased. The sulfide and higher concentration of hydrogen
atoms work together to maximize the number of hydrogen atoms that
enter the steel. Once in the steel, atomic hydrogen is converted to
SALT WATER
Figure 2.1 Bent nail in seawater showing the flow of electrons (and
electrical current)-stress cell.
42
Chapter 2
Types of Corrosion
molecular hydrogen, which gives rise to a high stress in the metal
(hydrogen-induced stress). Presence of atomic hydrogen in steel reduces
the ductility of the steel and causes it to break in a brittle manner.
The amount of atomic hydrogen required to initiate sulfide stress
cracking appears to be small, possibility as low as 1ppm. Sufficient
hydrogen must be available, however, to establish a differential gradient required to initiate and propagate a crack. The H2S concentration
as low as 1-3 ppm can produce cracking of highly stressed and highstrength steels (Wilhelm and Kane, 1987).
According to Cron and Marsh (1983, p. 1035), very low concentrations of H,S (0.1 ppm) and low partial pressures (0.001 atm) can cause
SSC. The time to failure is decreased with increasing concentration of
H2S and increasing stress. Maintaining a high temperature prevents
cracking, because SSC does not occur at temperatures above =180"F.
Stress-corrosion cracking can occur in most alloys, and the corrodants which promote stress cracking may differ (few in number for each
alloy). Cracking can occur in both acidic and alkaline environments,
usually in the presence of chloride ion and/or oxygen.
Engineers should monitor the condition of tubular goods (casing,
pipelines, etc.) in areas that are subsidence-prone due to fluid (oil, water)
withdrawal (see Chilingarian et al., 1995).Faults and fractures form as a
result of subsidence, which can cause appreciable stress in the casing for
example. In turn, stressed metals are corrosion-prone.
2.5
TYPES OF CRACKING IN DRILLING AND
PRODUCING ENVIRONMENTS
Hydrogen embrittlement (sulfide cracking) and corrosion fatigue are
two forms of cracking that are associated with drilling and producing
environments.
2.5.1 Hydrogen Embrittlement (Sulfide Cracking)
Hydrogen embrittlement occurs as a sudden cracking of metal, caused
by the entrapment of hydrogen within the lattice structure. The cracking of the metal may proceed in a stepwise rupturing manner. The corrosion process in an acid environment produces atomic hydrogen:
Fe+2H++ Fe2++2Ho (atoms)
(2.5)
2.5
Types of Cracking in Drilling and Producing Environments 43
Fe - 2e- + Fez+ (anode)
(2.6)
2H++2e-+ 2 H o (cathode)
(2.7)
Some of the hydrogen atoms that are formed in the cathodic reaction
penetrate the metal with subsequent formation of molecular hydrogen.
The remaining hydrogen atoms combine to form molecules of hydrogen gas at the metal surface. The adsorption of hydrogen atoms by the
metal causes a loss in ductility and cracking of high-strength steels.
Materials, which interfere with the pairing of atoms of hydrogen to
form hydrogen gas at cathodic areas of metal, enhance the penetration
of atomic hydrogen into the steel. Hydrogen sulfide in drilling fluids
supplies sulfide ions, which prevent the pairing of hydrogen atoms to
form hydrogen gas. Thus, the penetration of atomic hydrogen into steels
is promoted by the presence of hydrogen sulfide. The time to failure by
hydrogen embrittlement is shortened by increasing:
1. Concentration of hydrogen sulfide
2. Stress
3. Strength and hardness of steel
This behavior is illustrated by Hudgins et al. (1966) in Figures 2.2
and 2.3. The embrittlement (sulfide stress cracking [SSC]) occurs generally in steels with yield strengths above 90,000 psi or above Rockwell
C-20-22 hardness. Lower-strength steels are not subject to SSC;
however, they are subject to hydrogen blistering.
2.5.2
Hydrogen Blistering
Hydrogen penetration of low-strength steel may cause blistering, which
appears as bumps on the metal surface. The hydrogen atoms form
hydrogen molecules at points of defect in the metal. The hydrogen gas
cannot penetrate into nor escape from the crystalline structure. The
pressure of hydrogen gas increases sufficiently to part the metal, create
a void. and raise a blister on the steel surface.
2.5.3
Corrosion Fatigue
Cyclic stresses at high levels cause fatigue failure of metal. In a
corrosive environment, the progress of fatigue is accelerated by
44 Chapter 2 Types of Corrosion
40
35
u 30
[L
In-
5 25
I"
20
15
Applied stresses
Expressed as % of yield deformation
k Y
I
Week
I
Month
I
Figure 2.2 Relationship (approximate) between hardness and time to
failure a t different applied stresses, expressed as percentage of yield
deformation, for carbon steels in 5% NaCl solutions containing 3,000 ppm
H2S (After Hudgins, 1969, p. 42, figure 1; courtesy of Materials Protection).
electrochemical corrosion. Corrosion fatigue is the combined dction of
corrosion and fatigue (cyclic stressing), which results in early fracture
of metal. Corrosion fatigue is the principal cause of drill pipe failures.
By establishing limits to the stresses repeatedly applied to metal, failure
by metal fatigue in a noncorrosive environment can be avoided. The
endurance limit is the maximum cyclic stress level which can be applied
to a metal without a fatigue failure. In an environment with continued
corrosion, instead of exhibiting an endurance limit, metal will fail due
to the growth of corrosion fatigue cracks.The time to failure or number
of stress cycles necessary to cause failure is decreased with increasing
severity of corrosive environment and level of stress as illustrated in
Figure 2.4.
Corrosion fatigue is enhanced by corrodants in a corrosive environment which cause pitting of steel, such as oxygen and acid gases (H2S
and C02), which are often present in drilling and produced fluids.
Increasing the strength of steels will not improve the resistance to corrosion fatigue. Instead, it may shorten the number of cycles to failure.
2.5
Types of Cracking in Drilling and Producing Environments
45
40
35
30
U
v;
25
VI
0
C
I"
20
l5
Stress level
130% Yield detorm
10
0.5
50
10
500 1,000
100
Time to tailure, hours
Figure 2.3 Relationship (approximate) between hardness and time to
failure for carbon steels in 5% NaCl solution containing various
concentrations of H2S. Stress level = 130% of yield deformation (After
Hudgins, 1969, p. 43, figure 2; courtesy of Materials Protection).
lo4
lo5
106
10'
Cy cl es for failure ( N )
Figure 2.4 Relationship between endurance strength of steel stress and
number of cycles needed for the occurrence of failure in corrosive and
noncorrosive environments (After Bertness, 1957, p.131, figure 1; courtesy
of Division of Production, American Petroleum Institute).
46
Chapter 2
Types of Corrosion
High-strength steels are more susceptible to pitting than the lowstrength steels.
Notches, such as the ones caused by tongs or slips, and corrosion
under protectors will accelerate corrosion fatigue failure. The relationship of fatigue to environment, tensile strength, and surface condition
of steel is illustrated in Figure 2.5.
Figure 2.5 Relationship between endurance limit and tensile strength for
polished, notched, and corroding specimens (applied to ordinary
corrodible steels) (After Battelle Memorial Institute, 1949, p. 78, figure 93;
courtesy of John Wiley and Sons, Inc.).
References and Bibliography 47
Inhibitors, which reduce the corrosion and the entry of corrosiongenerated hydrogen into the rods, can reduce the frequency of corrosion fatigue failures provided that stress is within a reasonable range.
Examples of field test of inhibitors for control of corrosion fatigue are
discussed by Martin (1980,1983) (also see Chapter 4).
REFERENCES A N D BIBLIOGRAPHY
Annand, R. R., 1981. Corrosion Characteristics and Control in Deep, Hot Gas
Wells. Southwestern Petroleum Short Course.
Battelle Memorial Institute, 1949. Prevention of the Failure of Metals Under
Repeated Stress. New York, NY. Wiley, 295 pp.
Bertness, T. A., 1957. Reduction of failures caused by corrosion in pumping
wells. API Dril. Prod. Pract., 37: 129-135.
Bertness, T. A., Chilingar, G. V., and Al-Bassam, M., 1989. Corrosion in drilling
and producing operations. In: G. V. Chilingar, J. Robertson, and S . Kumar
(Editors), Surface Operations in Petroleum Production, IZ, Amsterdam:
Elsevier, pp. 283-317.
Chilingar, G. V. and Beeson, C. M., 1969. Surface Operations in Petroleum
Production. New York, NY American Elsevier, 397 pp.
Cron,C.J. and Marsh, G.A., 1983.Overview of economics and engineering aspects
of corrosion in oil and gas production. J. Pet. Technol.,June: 1033-1041.
Davis, J. B., 1967. Petroleum Microbiology. Amsterdam: Elsevier, 604 pp.
Dean, H. J., 1977.Avoiding drilling and completion corrosion. Pet. Eng., lO(9):
23-28.
Deming, H. G., 1940. Fundamental Chemistry. New York and London: John
Wiley and Sons, Inc., 756 pp.
Doig, K. and Wachter, A. P., 1951. Bacterial casing corrosion in the Ventura
Field. Corrosion, 7 :221-224.
Fontana, M. G. and Greene, N. D., 1967. Corrosion Engineering. New York,
NY McGraw-Hill, 391 pp.
Gatzke, L. K. and Hausler, R. H., 1983. Gas well corrosion inhibition with KP
223/KP 250. NACE Annual Con$, April 18-22, Anaheim, CA.
Hackerman, N. and Snavely, E. S., 1971. Fundamentals of inhibitors. In: NACE
Basic Corrosion Course. NACE, Houston, TX, (9): 1-25.
Hilliard, H. M., 1980. Corrosion control in Cotton Valley production. SOC.Pet.
Eng. Cotton Valley Symp., SPE 9062,Tyler, TX, May 21: 4 pp.
Hudgins, C. M., 1969.A review of corrosion problems in the petroleum industry. Muter. Prot., 8(1): 41-47.
Hudgins, C. M., McGlasson, R. L., Mehdizadeh,,.'F and Rosborough, W. M.,
1966.Hydrogen sulfide cracking of carbon and alloy steels. Corrosion, 22(8):
238-251
Ironite Products Co., 1979. Hydrogen Suljide Control. 41 pp.
May, P. D., 1978. Hydrogen sulfide control. Drilling-DCW,April.
48
Chapter 2
Types of Corrosion
Martin, R. L., 1979. Potentiodynamic polarization studies in the field. Muter.
Perform., 18(3): 41-50.
Martin, R. L., 1980.Inhibition of corrosion fatigue of oil well sucker rod strings.
Muter. Perform., 19(6): 20-23.
Meyer, F. H., Riggs, 0.L., McGlasson, R. L., and Sudbury, J. D. 1958. Corrosion
products of mild steel in hydrogen sulfide environments. Corrosion, 14(2):
109t-115 t.
National Association of Corrosion Engineers, 1979. Corrosion Control in
Petroleum Production, NACE TPC Publ. No. 5: 101 pp.
NGAA (Natural Gasoline Association of America), 1953. Condensate Well
Corrosion. Tulsa, OK: N.G.A.A., 203 pp.
Oxford, W. F. Jr. and Foss, R. E., 1958. Corrosion of Oil- and Gas-Well Equipment. Dallas, TX: Division of Production, American Petroleum Institute,
87 PP.
Rahman, S. S. and Chilingarian, G.V., 1995. Casing Design: Theory and Practice.
Elsevier, Amsterdam, The Netherlands, 373 pp.
Ray, J. D., Randall, B. V., and Parker, J. C., 1978. Use of Reactive Iron Oxide to
Remove H2S from Drilling Fluid. 53rd Annu. Fall Tech. Conf. SOC.Pet. Eng.
AIME, Oct. 1-3, Houston, TX: 4 pp.
Rhodes, F. H. and Clark, J. M., 1936. Corrosion of metals by water and carbon
dioxide under pressure. Znd. Eng. Chem., 28(9): 1078-1079.
Simpson, J. P., 1979. A new approach to oil-base muds for lower-cost drilling.
J. Pet. Technol.,31(5): 643-650.
Snavely, E. S., 1971. Chemical removal of oxygen from natural waters. J. Pet.
Technol.,23(4): 443-446.
Snavely, E. S. and Blount, F. E., 1969. Rates of reaction of dissolved oxygen
with scavengers in sweet and sour brines. Corrosion, 25(10): 397-404.
Staehle, R. W., 1978.$70 billion plus or minus $21 billion (editorial). Corrosion,
34(6): 1-3.
Uhlig, H. H. (Editor), 1948. The Corrosion Handbook. New York, N Y Wiley,
1188 pp.
Uhlig, H. H., 1965. Corrosion and Corrosion Control, 3rd ed. New York, N Y
Wiley, 371 pp.
Watkins, J. W. and Wright, J., 1953. Corrosive action on steel by gases dissolved
in water. Pet. Eng., 25(12, Nov.): B50-B57.
Wendt, R. P.,1979.The kinetics of Ironite Sponge H2S reactions. Pet. Div. Am.
Soc. Mech. Eng., Energy Technol. Con$, Houston, TX, Nov. 5-9,1978:7 pp.
Wendt, R. P., 1979. Control of hydrogen sulfide by alkalinity may be dangerous
to your health. Pet. Eng. Int., 51(6, May): 66-74.
Wendt, R. P., 1979. Alkalinity control of H2S in muds is not always safe. World
Oil, 188 (2, Aug.): 60-61.
Whitman, W., Russell, R., and Altieri, V., 1924. Effect of Hydrogen-Ion Concentration on the Submerged Corrosion of Steel. Znd. Eng. Chem., 16: 665.
Wilhelm, S. M. and Kane, R. D., 1987. Status Report: Corrosion Resistant
Alloys, Petroleum Engineering International, March: 3641.
Potrebbero piacerti anche
- Corrosion Failures: Theory, Case Studies, and SolutionsDa EverandCorrosion Failures: Theory, Case Studies, and SolutionsNessuna valutazione finora
- Types of CorrosionDocumento8 pagineTypes of CorrosionNiranjana SivaNessuna valutazione finora
- CorrosionDocumento8 pagineCorrosionGM VillaneaNessuna valutazione finora
- Corrosion Lab ReportDocumento21 pagineCorrosion Lab ReportJeremiah MolaletsiNessuna valutazione finora
- CorrosionDocumento14 pagineCorrosionChalakAhmedNessuna valutazione finora
- Paint FundamentalsDocumento19 paginePaint FundamentalsSaurabh SinghNessuna valutazione finora
- Corrosion - Erossion: Ulhas ThakurDocumento14 pagineCorrosion - Erossion: Ulhas ThakurmkkamarajNessuna valutazione finora
- Tech Coating SelectionDocumento8 pagineTech Coating SelectionKarthikeyan Shanmugavel100% (1)
- Failure Mechanisms of C-Steels (API 571)Documento18 pagineFailure Mechanisms of C-Steels (API 571)Mohmed AllamNessuna valutazione finora
- Corrosion (Compatibility Mode) 2Documento54 pagineCorrosion (Compatibility Mode) 2Omkar Kumar JhaNessuna valutazione finora
- 7 Forms of Corrosion IDocumento39 pagine7 Forms of Corrosion IJesus De la RosaNessuna valutazione finora
- Corrosion PDFDocumento51 pagineCorrosion PDFSuryansh SinghNessuna valutazione finora
- Under Standing MICDocumento36 pagineUnder Standing MICalkhiatNessuna valutazione finora
- Biological Induced CorrosionDocumento22 pagineBiological Induced CorrosionHemanth100% (1)
- Corrosion Coupon TestingDocumento5 pagineCorrosion Coupon TestingJefanny JaouhariNessuna valutazione finora
- Failure Modes of RBIDocumento5 pagineFailure Modes of RBIMuhammad OmarNessuna valutazione finora
- Smarter Materials Selection For Corrosion Control PDFDocumento12 pagineSmarter Materials Selection For Corrosion Control PDFAsyraf Nordin100% (1)
- Types of CorrosionDocumento1 paginaTypes of CorrosionprathapNessuna valutazione finora
- What Is CorrosionDocumento4 pagineWhat Is CorrosionOsransyah Os100% (1)
- CorrosionDocumento81 pagineCorrosionsureshs83Nessuna valutazione finora
- Lunit-V CorrosionDocumento14 pagineLunit-V CorrosionMidhunRameshThuvasseryNessuna valutazione finora
- Atmospheric Corrosion MechanismDocumento27 pagineAtmospheric Corrosion MechanismmghgolNessuna valutazione finora
- CorrosionDocumento20 pagineCorrosionndesigngmailNessuna valutazione finora
- Corrosion QuestionsDocumento12 pagineCorrosion Questionsblakk archimedes100% (1)
- In This Issue:: Focus On Cathodic Protection & Monitoring/Coating Applicators Winn & Coales (Denso) LTDDocumento40 pagineIn This Issue:: Focus On Cathodic Protection & Monitoring/Coating Applicators Winn & Coales (Denso) LTDVõ Quang Kiệt100% (1)
- Final Exam Pipeline CorrosionDocumento6 pagineFinal Exam Pipeline CorrosionAl-somuda' Ali Al-smmani100% (2)
- Corrosion Inhibitors - Principles, Mechanisms and Applications PDFDocumento16 pagineCorrosion Inhibitors - Principles, Mechanisms and Applications PDFleonardoNessuna valutazione finora
- Article CO2CorrosionCHEM409 - Background of CO2 CorrosionDocumento4 pagineArticle CO2CorrosionCHEM409 - Background of CO2 Corrosionmohamed samyNessuna valutazione finora
- Coatings For Corrosion Protection - Offshore Oil and Gas OperationDocumento335 pagineCoatings For Corrosion Protection - Offshore Oil and Gas Operationsabari ramasamyNessuna valutazione finora
- 2011 03 15 Clariant - Corrosion Inhibitors and Corrosion Inhibitor SelectionDocumento31 pagine2011 03 15 Clariant - Corrosion Inhibitors and Corrosion Inhibitor SelectionVivek PatilNessuna valutazione finora
- PDFDocumento23 paginePDFDharmaraaj RajalinggamNessuna valutazione finora
- Rusting, Season Cracking, Waterline Attack, Crazing, Checking, ChalkingDocumento126 pagineRusting, Season Cracking, Waterline Attack, Crazing, Checking, ChalkingNuke FerdiliaNessuna valutazione finora
- Corrosion Prevention by Use of InhibitorsDocumento19 pagineCorrosion Prevention by Use of InhibitorsSai PradeepNessuna valutazione finora
- Self-Study On API RP 571 - Damage MechanismDocumento84 pagineSelf-Study On API RP 571 - Damage Mechanismrosli2503100% (2)
- B-GAS-GRADE - 2-Theory GoodDocumento34 pagineB-GAS-GRADE - 2-Theory GoodCERTS100% (1)
- Corrosion Inhibition Approach of Oil Production Systems in Offshore OilfieldsDocumento5 pagineCorrosion Inhibition Approach of Oil Production Systems in Offshore OilfieldsKArenNessuna valutazione finora
- Corrosion Type MicDocumento5 pagineCorrosion Type MicCharlie Chong100% (3)
- Trial Paper HandoutDocumento13 pagineTrial Paper HandoutPradeep Kumar BowmarajuNessuna valutazione finora
- CLSCC LiteratureDocumento62 pagineCLSCC LiteratureNakarin PotidokmaiNessuna valutazione finora
- A Study of Corrosion Rate of Stainless Steels AISI 316 and 306 Against HCL H2SO4 and Dead Sea WaterDocumento45 pagineA Study of Corrosion Rate of Stainless Steels AISI 316 and 306 Against HCL H2SO4 and Dead Sea WatermohdghNessuna valutazione finora
- Corrosion Issue89 LowResDocumento28 pagineCorrosion Issue89 LowResLvision100% (1)
- CuiDocumento6 pagineCuiأحمد صبحىNessuna valutazione finora
- Filming Corrosion Inhibitor For Oil and Gas FieldDocumento20 pagineFilming Corrosion Inhibitor For Oil and Gas FieldelsyakiebNessuna valutazione finora
- HHHGH HHH H K KM FK MDocumento17 pagineHHHGH HHH H K KM FK MIslam M. RedaNessuna valutazione finora
- Progress in Corrosion-The First 50 Years of The EFC - McintyreDocumento213 pagineProgress in Corrosion-The First 50 Years of The EFC - McintyreGustavo Adolfo Piñero Borges100% (1)
- Chemical Oxygen Demand in Waste WaterDocumento3 pagineChemical Oxygen Demand in Waste WaterGopal MallickNessuna valutazione finora
- Different Types of CorrosionDocumento19 pagineDifferent Types of CorrosionbabmirNessuna valutazione finora
- Corrosion Engineering: Dr. Khalid H. RashidDocumento87 pagineCorrosion Engineering: Dr. Khalid H. RashidHmid AljbreNessuna valutazione finora
- InhibitorDocumento33 pagineInhibitorMohd SyazwanNessuna valutazione finora
- Corrosion Quiz - IngDocumento5 pagineCorrosion Quiz - IngErik Alfiandy100% (1)
- Corrosion InhibitorsDocumento70 pagineCorrosion InhibitorsSundar Sk100% (2)
- BGAS - Question & AnswerDocumento27 pagineBGAS - Question & Answerthanhdk007Nessuna valutazione finora
- Corrosion of Stainless Steels of Cryogenic Hydrocarbon Flare Tips BurnersDocumento13 pagineCorrosion of Stainless Steels of Cryogenic Hydrocarbon Flare Tips Burnersravikanth_rNessuna valutazione finora
- Corrosion Management Issue151Documento32 pagineCorrosion Management Issue151Issam Mokrani100% (1)
- Corrosion and Materials in Hydrocarbon Production: A Compendium of Operational and Engineering AspectsDa EverandCorrosion and Materials in Hydrocarbon Production: A Compendium of Operational and Engineering AspectsNessuna valutazione finora
- Effect of Corrosion in StructuresDocumento32 pagineEffect of Corrosion in StructuresasvihariNessuna valutazione finora
- Chemistry Unit - 2 NotesDocumento13 pagineChemistry Unit - 2 Notesjoshinihar19Nessuna valutazione finora
- Basics of Corrosion ControlDocumento9 pagineBasics of Corrosion ControlSenad Senna MuratovicNessuna valutazione finora
- PH INDICATOR PAPER BY IMMOBILIZING TURMERIC RHIZOME ETHANOLDocumento9 paginePH INDICATOR PAPER BY IMMOBILIZING TURMERIC RHIZOME ETHANOLKhaznah Khalishah HidayatNessuna valutazione finora
- The Figure Shows A 20 KG Rod AB Used To Main - Tain...Documento4 pagineThe Figure Shows A 20 KG Rod AB Used To Main - Tain...Amir PskNessuna valutazione finora
- Isolation and Purification of Metals Easy NotesDocumento4 pagineIsolation and Purification of Metals Easy NotesYogesh PatilNessuna valutazione finora
- Fire Resistance Evaluation of Lightweight Geopolymer ConcreteDocumento1 paginaFire Resistance Evaluation of Lightweight Geopolymer ConcreteUpadesh ShresthaNessuna valutazione finora
- Mind Map Convection Heat Transfer PDFDocumento1 paginaMind Map Convection Heat Transfer PDFMuhammad FawwazNessuna valutazione finora
- The Chemistry of LifeDocumento113 pagineThe Chemistry of LifeDeron C. De CastroNessuna valutazione finora
- MTT AssayDocumento2 pagineMTT AssayHameedhaNessuna valutazione finora
- Calorimetry and ThermochemistryDocumento12 pagineCalorimetry and ThermochemistryMae Joy PalmaNessuna valutazione finora
- Calculations Based On Fuel AnalysisDocumento3 pagineCalculations Based On Fuel AnalysisBrille Adrian FernandoNessuna valutazione finora
- Kinetic Isotope Effect: Rachel Nicholls 30/04/14Documento18 pagineKinetic Isotope Effect: Rachel Nicholls 30/04/14Joana SugiartoNessuna valutazione finora
- Characteristics, Applications, and Processing of Stress - Strain Behavior (I)Documento7 pagineCharacteristics, Applications, and Processing of Stress - Strain Behavior (I)nelson bessoneNessuna valutazione finora
- JM at Pro MergedDocumento2 pagineJM at Pro MergedFilos ProductionNessuna valutazione finora
- Electrophysical Phenomena in The Tribology of Polymers Sviridewok Kilmovich Kestelman (OPA 1999)Documento195 pagineElectrophysical Phenomena in The Tribology of Polymers Sviridewok Kilmovich Kestelman (OPA 1999)Shivangi NaikNessuna valutazione finora
- GATE Physics SyllabusDocumento3 pagineGATE Physics Syllabuskumar HarshNessuna valutazione finora
- Presentation On Utilities & Offsite Iffco, ParadeepDocumento22 paginePresentation On Utilities & Offsite Iffco, ParadeepSOCRATESNessuna valutazione finora
- Parametric and Kinetic Studies On The Decolorization of Aqueous Methyl Red Solution Using Electrolyzed WaterDocumento88 pagineParametric and Kinetic Studies On The Decolorization of Aqueous Methyl Red Solution Using Electrolyzed WaterJoyce EdrozoNessuna valutazione finora
- Aldehydes TheoryDocumento22 pagineAldehydes TheorynewspapermaekNessuna valutazione finora
- 3C25 Solid State Physics Old Notes 1 of 11 (UCL)Documento4 pagine3C25 Solid State Physics Old Notes 1 of 11 (UCL)ucaptd3Nessuna valutazione finora
- SprayBall Design - Pressure Drop - Ken Morrison ArticleDocumento6 pagineSprayBall Design - Pressure Drop - Ken Morrison ArticleHejoolju GrubsNessuna valutazione finora
- Tugas (Pokok Bahasan 4)Documento2 pagineTugas (Pokok Bahasan 4)Candra ErawanNessuna valutazione finora
- Organic Rankine Cycle System For Waste Heat Recovery From Twin Cylinder Diesel Engine ExhaustDocumento7 pagineOrganic Rankine Cycle System For Waste Heat Recovery From Twin Cylinder Diesel Engine ExhaustSabi SanthoshNessuna valutazione finora
- MCQ Thermal PDFDocumento6 pagineMCQ Thermal PDFpriya dharshiniNessuna valutazione finora
- Psychrometrics History. ASHRAE Journal Oct-2004Documento5 paginePsychrometrics History. ASHRAE Journal Oct-2004alvarellos92Nessuna valutazione finora
- Volumetric DilatometryDocumento14 pagineVolumetric DilatometryNasim MalekiNessuna valutazione finora
- Claisen Rearrangement Over The Past Nine Decades: Ana M. Martı N CastroDocumento64 pagineClaisen Rearrangement Over The Past Nine Decades: Ana M. Martı N CastroAitor PastorNessuna valutazione finora
- Thermodynamics Lesson 3Documento3 pagineThermodynamics Lesson 3Ruth100% (1)
- H. Zhang Et Al. Separation and Purification Technology 63 (2008) 264-268Documento5 pagineH. Zhang Et Al. Separation and Purification Technology 63 (2008) 264-268ZIA UR REHMANNessuna valutazione finora
- Origins of Color in MineralsDocumento35 pagineOrigins of Color in MineralsMeisam Rasouli100% (2)
- Oc ch17Documento34 pagineOc ch17xavier8491Nessuna valutazione finora