Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
1) Chromatography
Caricato da
Altaf Ur RehmanCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
1) Chromatography
Caricato da
Altaf Ur RehmanCopyright:
Formati disponibili
Dr .
Mehdi Hasan Kazmi
SEPERATION
The validity of results from chemical analysis depends initially upon the quality and integrity of the sample
that has been obtained. In the majority of cases, the sample to be analyzed will contain more than one
component and may require some separation and/or concentration to be carried out prior to analysis.
Even pure single substances used as standards rarely exceed 99.99% purity, which still leaves the
possibility of 10 / of another material being present.
SEPERATION TECHNIQUES
For this purpose, separation techniques can be considered to fall into two main groupings.
Bulk Separation
(Physical Method of Separation)
Large scale separations of one component form another, typically they include,
Filtration
Temperature dependant effects ( distillation, evaporation & drying )
Solubility effects ( solvent extraction, crystallization, and precipitation)
Ion exchange dialysis and lyophilisation.
Instrumental Separation
(Chemical Method of Separation)
The most common instrumental separation includes,
Chromatography (GC, HPLC, TLC, Supercritical Fluid chromatography SFC)
Electrophoretic group of separation techniques (Capillary Electrophoresis).
Chromatography
Chromatography is a Greek word,
Chroma means color & Graphy means to write or to analyze.
Chromatography is a way to separate single chemical compounds from mixed substances that depends on
the speed at which they move through special media, or chemical substances.
It is a powerful separation technique which was discovered by a botanist. In order to get familiar with
chromatography, some chromatography terms must be understand which are defined below.
Sample
It is the matter analyzed in chromatography. It may consist of a single component or may be a
mixture of components. When the sample is analyzed, the phase or phases contains analyte of
interests is/are referred to as sample, whereas everything out of interests separated from the
sample before or during analysis is referred to as waste.
O. A. Ansari
CHROMATOGRAPHY TERMS
Dr . Mehdi Hasan Kazmi
Analyte
The analyte is the substance that has to be removed during Chromatography.
Solute
The solute refers to the sample components in partition chromatography.
Solvent
The solvent refers to any substance capable of solubilizing other substance, and especially the
liquid mobile phase in LC.
Mobile Phase
The mobile phase is the phase which moves in a definite direction. It may be a liquid (LC and CEC),
a gas (GC), or a supercritical fluid (supercritical-fluid chromatography, SFC). A better definition:
The mobile phase consists of the sample being separated/analyzed and the solvent that moves the
sample through the column.
The effluent is the mobile phase leaving the column.
Stationary Phase
The stationary phase is the substance which is fixed in place for the chromatography procedure.
A bonded phase is a stationary phase that is covalently bonded to the support
particles or to the inside wall of the column tubing.
An immobilized phase is a stationary phase which is immobilized on the support
particles, or on the inner wall of the column tubing.
Chromatograph
A chromatograph is equipment that enables a sophisticated separation e.g. gas chromatographic
or liquid chromatographic separation.
EXPLAINATION
Suppose we have a mixture of two solutes, A and B, dissolved in a suitable buffer solution. A column is
filled with beads of an insoluble substance
that is expected to bind differentially and
reversibly to A and B. The column is initially
equilibrated by running a certain volume of
the buffer through it, and the mixture
applied to the top of the column as a
narrow layer. If the stopcock of the column
is carefully opened, the layer of solutes will
O. A. Ansari
Chromatogram
A chromatogram is the visual output of the chromatograph. In the case of an optimal separation,
different peaks or patterns on the chromatogram correspond to different components of the
separated mixture.
Dr . Mehdi Hasan Kazmi
pass into the column, and the solutes can be washed out slowly by pouring more buffer into the column.
This process is called elution, and the solution emerging at the bottom of the column is called the eluate
Let us assume, for the purpose of this illustration, that solute B binds more strongly to the matrix than
solute A. Then, as the mixture travels down the column, the molecules of B will be retarded with respect
to the molecules of A. In time, they will separate into bands, and be eluted at different times.
On the basis of phases, Chromatography can be classified as under
Chromatography
Adsorption
LSC
Partition
GSC
ADSORPTION CHROMATOGRAPHY
Stationary Phase
Mobile Phase
1.
2.
3.
4.
5.
6.
LLC
GLC
Solid
Liquid or Gas
LSC (Liquid-Solid Chromatography) or LCC.
SCC (Simple Column Chromatography).
IEC (Ion Exchange Chromatography).
HPLC (High performance Liquid Chromatography).
GC (Gel Chromatography) or Protein Analysis.
GSC (Gas-Solid Chromatography).
PARTITION CHROMATOGRAPHY
Stationary Phase
Liquid
1.
2.
3.
4.
5.
6.
7.
8.
9.
Liquid or Gas
LLC (Liquid-Liquid Chromatography) or Plane Chromatography.
PC (Paper Chromatography).
TLC (Thin-Layer Chromatography).
Continuous Zinc Electrophoresis.
GLC (Gas-Liquid Chromatography).
HPLC (High pressure/performance Liquid Chromatography).
Reverse Phase Chromatography
BPLC / BPC (Back Pressure Liquid Chromatography).
IPC (Ion-Pair Chromatography).
O. A. Ansari
Mobile Phase
Dr . Mehdi Hasan Kazmi
Stationary Phase
The stationary phase is the substance which is fixed in place for the chromatography procedure. It can
either be solid or liquid.
SOLID STATIONARY PHASE
Solid stationary phase is usually an adsorbent. The adsorbent may be classified with respect to two
different aspects.
ADSORBENTS
1
Inorganic
2
Organic
Column
Thin
Layer
Simple Column
HPLC
GPLC
Gas Plate Coated
with chemical
GSC
Difference between adsorbents used for Column and Thin Layer Chromatography
Simple Column
Adsorbent for column does not require a
binder
Thin Layer
Adsorbent for thin layer must have a
binder
It must be of large mesh size
It must be of small mesh size
Inorganic Adsorbent
Silica Gel (consists of fluorescent indicator)
Alumina
Silicates (Ca, Mg, Phosphates)
Class
Mesh Size (mm)
G40
0.04-0.
G60
0.025-0.04
G100
0.04-0.063
0.063-0.2
0.02-0.5
G60F254
0.63-0.2
O. A. Ansari
Silica Gel
Dr . Mehdi Hasan Kazmi
Alimina (Aluminium Oxide)
Activity according to Brokman Scale
Class
Moisture (%age)
I
0
II
3
III
6
IV
10
V
15
Note: Brokmnan Scale is only for Alumina not for Silica.
Medium
pH
Basic
Neutral
Acidic
Class (mesh mm)
9.5 0.2
60 (0.083-0.2)
90 (0.063-0.2)
7 0.2
4.5 0.2
90 (0.063-0.2)
90 (0.063-0.2)
Powder
Ferric & Chromic Oxides
Zinc Carbonate & Ferro Cyanide
Active Carbon
Zirconium [ phosphate, Hydrous]
Lenthanum Oxides
Combination
LIQUID STATIONARY PHASE
Acetonyl Acetone
Apiezon L, M
Bentone 34
Benzyl Cyanide + AgNO3
Polyethylene Glycol
DOP
Silicone OV-17
Silicone OV-1
Silicone OV-101
Normal Cellulose Powder.
Acetylated Cellulose Powder.
Cellulose Ion-Exchange Powder
Starch
Sucrose
Mannitol
Dextron Gells
O. A. Ansari
Organic Adsorbent
Dr . Mehdi Hasan Kazmi
Mobile Phase
The mobile phase is the phase which moves in a definite direction. On the basis of polarity and di-electric
constant we can grade mobile phase as under,
2 > > > > > > >
> > > > >
> > > > 4 > >
Simple Column Chromatography
INTRODUCTION & WORKING MECHANISM
Column chromatography separates compounds using many chemical actions between the chemical being
tested and the chromatography column (a rod with a blending of special chemicals). The column is run
using either gravity or a pump.
Column chromatography is advantageous over most other
chromatographic techniques because it can be used in both
analytical and preparative applications. Not only can column
chromatography be used to determine the number of
components of a mixture, but it can also be used to separate
and purify substantial quantities of those components for
subsequent analysis.
With all its advantages and preparative power, column chromatography does have its complications.
Properly setting up the column (something that will be done for you prior to experiment) requires some
technical skill, and takes some time. Column chromatography is less foolproof than paper chromatography
and requires constant attention while the experiment is being performed: collection vessels must be
frequently switched and solvent levels need to be topped up.
In short, running a column is time-consuming and tedious, especially for large samples. If it is unnecessary
to preparatively separate large quantities of sample, analytical methods such as paper chromatography
may be more suitable and easier to perform.
First of all select the material of column. Usually Glass or Stainless Steel is used as column
material.
Que: Why we use glass or stainless steel as the material of column?
Ans: We use glass or stainless steel because both of these materials
do not react with the solid or mobile phase.
Column is packed.
Washing is carried out.
Selection of adsorbent is done. It depends upon the sample.
For e.g. Silica with fluorescent or without fluorescent.
Before using the adsorbent, it should be first activated and different adsorbent require different
temperatures for activation.
For e.g. Silica 100oC / hr
O. A. Ansari
EXPERIMENTAL PROCEDURE
Dr . Mehdi Hasan Kazmi
Alumina 320oC / hr
The adsorbent is activated in the oven.
Adsorbent is then cooled in the desiccators.
Close the inner side of column by
Select a non-polar solvent system. For e.g. Hexane
Que: Why we use non-polar solvent i.e. hexane?
Ans: We use hexane because the particles soluble in hexane will
come out.
Fill the column with solvent to the required length, then add adsorbent.
When the column is filled half with the adsorbent, release the solvent drop wise.
The column should be packed carefully there should be no air bubbles. To remove air bubbles
completely, we pass the solvent two times.
Now introduce the sample.
Before adding the sample the surface is covered with cotton, asbestos or glass wool.
Prepare the sample in an organic or inorganic solvent system.
If the sample is organic in nature, organic solvent system can be used. For e.g. Chloroform.
The sample is prepared in a petridish & chloroform is added to the sample.
We form layers
Que: Why we form layers?
Ans: Because particles of sample must not be disturbed.
We pass non-polar solvent i.e. hexane because the particles soluble in hexane will come out.
Then we take 98% Hexane and 2% Chloroform and the particles soluble in 2% Chloroform will
come out.
and so on..
For organic sample, we use non-polar solvent.
For inorganic sample, we use polar solvent.
TERMINOLOGIES
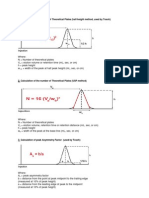
RETENTION TIME (tR)
O. A. Ansari
The maximum time required to separate a particular constituent completely from the column is called
retention time.
Dr . Mehdi Hasan Kazmi
RETENTION VOLUME (VR)
The total volume of a particular constituent (solute) obtained from the column is called retention volume.
O. A. Ansari
Additional Information
The retention time of a solute is taken as the elapsed time between the time of
injection of a solute and the time of elution of the peak maximum of that solute. It is a
unique characteristic of the solute and can be used for identification purposes.
The corrected retention time of a solute is the retention time minus the retention time
of a completely unretained solute. By multiplying the corrected retention time of a
solute by the exit flow rate then the corrected retention volume can be obtained. If the
mobile phase is compressible (i.e. the mobile phase is a gas) a pressure correction must
be applied which is a function of the column inlet-outlet pressure ratio. Values of the
corrected retention volume per ml of stationary phase for a solute measured over a
range of temperatures can provide the standard energy of distribution, the standard
enthalpy of distribution and the standard entropy of distribution for the solute
concerned.
Potrebbero piacerti anche
- LC Presentation QICTDocumento22 pagineLC Presentation QICTAltaf Ur RehmanNessuna valutazione finora
- Advt No 7-2015Documento3 pagineAdvt No 7-2015Waqas TayyabNessuna valutazione finora
- Deputy Assistant DirectorDocumento5 pagineDeputy Assistant DirectorDanish RazaNessuna valutazione finora
- Adc Invoice 400Documento2 pagineAdc Invoice 400Altaf Ur RehmanNessuna valutazione finora
- The Drugs Act, 1976Documento32 pagineThe Drugs Act, 1976Dr-Usman KhanNessuna valutazione finora
- Tariff ComparisonDocumento2 pagineTariff ComparisonAltaf Ur RehmanNessuna valutazione finora
- Application Form Instructions To Candidates - Latest (After Adv. No. 4) 153rd Approved 30.4.2015Documento8 pagineApplication Form Instructions To Candidates - Latest (After Adv. No. 4) 153rd Approved 30.4.2015knight_riderrNessuna valutazione finora
- CE-2016 Public NoticeDocumento1 paginaCE-2016 Public NoticeKenneth MillerNessuna valutazione finora
- Custom RulesdsdsDocumento279 pagineCustom RulesdsdsAltaf Ur RehmanNessuna valutazione finora
- Import Policy Order 2013 PakistanDocumento132 pagineImport Policy Order 2013 PakistanSher Zaman BhuttoNessuna valutazione finora
- Enquiry ListDocumento12 pagineEnquiry ListAltaf Ur RehmanNessuna valutazione finora
- Experiment # 04: ObjectDocumento8 pagineExperiment # 04: ObjectAltaf Ur RehmanNessuna valutazione finora
- File IndexDocumento113 pagineFile IndexAltaf Ur RehmanNessuna valutazione finora
- 2) Ana DiscussionDocumento3 pagine2) Ana DiscussionAltaf Ur RehmanNessuna valutazione finora
- EXPERIMENT#2 (A) : Analtical Techniques COURSE#605Documento4 pagineEXPERIMENT#2 (A) : Analtical Techniques COURSE#605Altaf Ur RehmanNessuna valutazione finora
- Syllabus PDFDocumento150 pagineSyllabus PDFAsma SethiNessuna valutazione finora
- SCM in PharmaDocumento20 pagineSCM in PharmaAbhiNessuna valutazione finora
- Assignment of DesizingDocumento8 pagineAssignment of DesizingAltaf Ur RehmanNessuna valutazione finora
- 06 Introduction To Oceanography. TomczakDocumento123 pagine06 Introduction To Oceanography. TomczakmishazujevNessuna valutazione finora
- Salient Feature CustomsDocumento3 pagineSalient Feature CustomsAarif ShahNessuna valutazione finora
- Bill of Entry Bill of Export Baggage Declaration Transshipment PermitDocumento2 pagineBill of Entry Bill of Export Baggage Declaration Transshipment PermitAltaf Ur RehmanNessuna valutazione finora
- Documentation Description: For Import & Export GoodsDocumento8 pagineDocumentation Description: For Import & Export GoodsAltaf Ur RehmanNessuna valutazione finora
- Weboc 1Documento30 pagineWeboc 1Altaf Ur RehmanNessuna valutazione finora
- LC Presentation QICTDocumento22 pagineLC Presentation QICTAltaf Ur RehmanNessuna valutazione finora
- 1) Sales Contract 1) Sales Contract Importer or Buyer Importer or BuyerDocumento5 pagine1) Sales Contract 1) Sales Contract Importer or Buyer Importer or BuyerAltaf Ur RehmanNessuna valutazione finora
- Bill of Entry Bill of Export Baggage Declaration Transshipment PermitDocumento2 pagineBill of Entry Bill of Export Baggage Declaration Transshipment PermitAltaf Ur RehmanNessuna valutazione finora
- Advt No 7-2015Documento3 pagineAdvt No 7-2015Waqas TayyabNessuna valutazione finora
- CE-2016 Public NoticeDocumento1 paginaCE-2016 Public NoticeKenneth MillerNessuna valutazione finora
- Syllabus PDFDocumento150 pagineSyllabus PDFAsma SethiNessuna valutazione finora
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- H19F26B en Tosoh-GX Analyser Tech-Specification-sheetDocumento2 pagineH19F26B en Tosoh-GX Analyser Tech-Specification-sheetRadu IchimNessuna valutazione finora
- Supercritical Fluid Extraction Chromatography: Presented byDocumento28 pagineSupercritical Fluid Extraction Chromatography: Presented bygovind ashokraoNessuna valutazione finora
- Cen Iso TS 15011-5 (E)Documento28 pagineCen Iso TS 15011-5 (E)mustafa gözüaçıkNessuna valutazione finora
- Estimate and Validation Cefixime and Azithromycin Simultaneoslyy in Tablt Dosage Froms by RP-HPLC MethodDocumento93 pagineEstimate and Validation Cefixime and Azithromycin Simultaneoslyy in Tablt Dosage Froms by RP-HPLC MethodVamsi SakhamuriNessuna valutazione finora
- Rapid Detection of Histamine in Foods: Kikkoman Biochemifa CompanyDocumento6 pagineRapid Detection of Histamine in Foods: Kikkoman Biochemifa CompanyPedro PedroNessuna valutazione finora
- Isolation and Standardization of Gingerol From Ginger Rhizome by Using TLC, HPLC, and Identification TestsDocumento4 pagineIsolation and Standardization of Gingerol From Ginger Rhizome by Using TLC, HPLC, and Identification Testsronahaniifah11Nessuna valutazione finora
- Tips and Tricks of HPLC Separation PDFDocumento47 pagineTips and Tricks of HPLC Separation PDFFls Fernando Fls LopesNessuna valutazione finora
- Lectut BTN-302 PDF BTN302-Extraction and Chromatography PDFDocumento51 pagineLectut BTN-302 PDF BTN302-Extraction and Chromatography PDFqwertNessuna valutazione finora
- Ashraf Ali: PHD (Analytical Chemistry/Separation Science)Documento5 pagineAshraf Ali: PHD (Analytical Chemistry/Separation Science)UmairNessuna valutazione finora
- Optimization of Lycopene Extraction From Tomato Peels Industrial By-Product Using Maceration in Refined Olive OilDocumento8 pagineOptimization of Lycopene Extraction From Tomato Peels Industrial By-Product Using Maceration in Refined Olive OilDevita AmeliaNessuna valutazione finora
- Kamarudin 2016Documento8 pagineKamarudin 2016Jose perezNessuna valutazione finora
- Application of HPLC-FTIRDocumento0 pagineApplication of HPLC-FTIRWaleed El-azabNessuna valutazione finora
- Chemical 2012Documento5 pagineChemical 2012Bagus BaskoroNessuna valutazione finora
- Hasler1997 OkDocumento10 pagineHasler1997 OkRamiro Manzano NúñezNessuna valutazione finora
- (2019) Synthesis and Characterization of Nanodiamond-Anticancer Drug ConjugatesDocumento14 pagine(2019) Synthesis and Characterization of Nanodiamond-Anticancer Drug Conjugatesس-ح- رNessuna valutazione finora
- Acetogenins From Annonaceae Recent Progress in Isolation Synthesisand Mechanisms of ActionDocumento35 pagineAcetogenins From Annonaceae Recent Progress in Isolation Synthesisand Mechanisms of ActionPanda MndzNessuna valutazione finora
- Antioxidantes Por HidrolisisDocumento5 pagineAntioxidantes Por HidrolisisDiana GarciaNessuna valutazione finora
- Determination of Thiomersal Lidocaine and Phenylepherine in Their Ternary Mixture.2157 7064.1000199 PDFDocumento6 pagineDetermination of Thiomersal Lidocaine and Phenylepherine in Their Ternary Mixture.2157 7064.1000199 PDFiabureid7460Nessuna valutazione finora
- AN 360 Rapid Determination of Azo Dyes in Textiles Using Dionex ASE and UltiMate 3000 HPLC SystemsDocumento6 pagineAN 360 Rapid Determination of Azo Dyes in Textiles Using Dionex ASE and UltiMate 3000 HPLC SystemsDewi WulandhariNessuna valutazione finora
- Elixir Dosage Form in Pharmaceutical PracticeDocumento4 pagineElixir Dosage Form in Pharmaceutical PracticeAri DewiyantiNessuna valutazione finora
- Application of Direct Crystallization For Racemic Compound KetoprofenDocumento47 pagineApplication of Direct Crystallization For Racemic Compound KetoprofenVinay BhayaNessuna valutazione finora
- 10 1021@acsfoodscitech 1c00098Documento8 pagine10 1021@acsfoodscitech 1c00098Huy Hoàng Lê ĐứcNessuna valutazione finora
- Csio-Aist Brochure (25 Aug 09)Documento69 pagineCsio-Aist Brochure (25 Aug 09)Prashant BansalNessuna valutazione finora
- Part 1 The Ruggedness Test in HPLCDocumento11 paginePart 1 The Ruggedness Test in HPLCNestor Armando Marin SolanoNessuna valutazione finora
- Downstream ProcessingDocumento30 pagineDownstream ProcessingResa100% (1)
- A Spectrophotometric Assay For Allicin, Alliin, and Alliinase (Alliin Lyase) With A Chromogenic Thiol: Reaction of 4-Mercaptopyridine With ThiosulfinatesDocumento8 pagineA Spectrophotometric Assay For Allicin, Alliin, and Alliinase (Alliin Lyase) With A Chromogenic Thiol: Reaction of 4-Mercaptopyridine With ThiosulfinatesZachary McCoyNessuna valutazione finora
- Determination of Chloride and Sulfate in Water and Soil: Key WordsDocumento11 pagineDetermination of Chloride and Sulfate in Water and Soil: Key WordsEr RutvikNessuna valutazione finora
- Ultra Performance Liquid Chromatography (Uplc)Documento12 pagineUltra Performance Liquid Chromatography (Uplc)dheeksha puvvadaNessuna valutazione finora
- Column Efficiency TestingDocumento3 pagineColumn Efficiency TestingvamsiNessuna valutazione finora
- Equipment Cleaning ValidationDocumento11 pagineEquipment Cleaning Validationhabibshaikh0973% (11)