Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Panchasama Churna
Caricato da
drsa2Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Panchasama Churna
Caricato da
drsa2Copyright:
Formati disponibili
Available online on www.ijppr.
com
International Journal of Pharmacognosy and Phytochemical Research 2010, 2(1); 11-14
ISSN: 0975 4873
Research Article
Standardisation of Ayurvedic Polyherbal Formulation,
Pancasama Churna
Ajay Kr Meena1*, M M Rao1, P Panda, Kiran2, Ajay Yadav2, Uttam Singh2 and B Singh
1
National Institute of Ayurvedic Pharmaceutical Research, Patiala 147001, Punjab, (India)
2
School of Pharmaceutical Sciences, Shobhit University, Meerut, UP
ABSTARCT
Ayurvedic medicine, Pancasama Churna known to be effective mainly on gastrointestinal tract (GIT), has been
standardized by following modern scientific quality control procedures both for the raw material and the finished
product. Pancasama Churna was subjected to macro-microscopic, Physico-chemical, preliminary phytochemical, TLC
and HPTLC to fix the quality standards of this drug. This study results a set of diagnostic characters essential for its
standardisation. TLC and HPTLC fingerprinting were employed to fix standards. The values obtained after
physicochemical parameters study showed that these values should be helpful to develop new pharmacopoeial
standards. This will be helpful to overcome batch to batch variations in traditional preparation of Pancasama churna.
The physicochemical constituents found to be present in raw material used for the preparation of Pancasama churna
possibly facilitate the desirable therapeutic efficacy of the medicinal formulation.
KEYWORDS: Pancasama Churna, Phytochemical studies, Ayurvedic Drug.
INTRODUCTION
Churna is a fine powder of a drug or drugs which is
prepared by mixing clean, finely powdered and sieved
drugs. Pancasama churna shows its effects mainly on
gastrointestinal tract. It increases peristaltic movements
of GI tract. It is used as antiflatulent (admana),
antirheumatic (amavata). It is also used in the treatment
of abdominal disorders (udara roga), pain (sula) and in
treatment of piles (arsa).
Practitioners usually do the identification of different
herbs used in Pancasama churna according to Ayurvedic
parameters. The preparation of Pancasama churna is
based on traditional methods in accordance with the
procedures given in classical texts [1]. Due to lack of
modern pharmacopoeial standards laid down and
followed for processing of Pancasama churna, the
medicine prepared using traditional methods may not
have the desired quality and batch to batch consistency.
Hence this formulation required standardization of
following scientific parameters including organoleptic
characters, chemical analysis, chromatographic pattern
and microbial screening. The work was undertaken in
trust as part of a programme of testing and validation of
traditional practice of using the Ayurvedic medicine
Pancasama churna in management of admana. Some
standards already exist for Pancasama churna. However,
the work deals with the details of following latest
standardization guidlines involving Good Manufacturing
Practices (GMP) for preparation of Ayurvedic
*Corresponding author: Ajay Kumar Meena,
Research Officer, Chemistry, National Institute of
Ayurvedic Pharmaceutical Resesarch, CCRAS, Patiala,
E-mail:ajaysheera@yahoo.com; ajaysheera@gmail.com
medicines. Standardization guidelines to be followed for
herbal products provided by international bodies like
World Health Organization (WHO), European Agency
for the evaluation of Medicinal Products (EMEA) and
United States Pharmacopeia (USP) have also been
considered.
Table 1. Pancasama churna contains following
ingredients:
Sanskrit Scientific
Part Used
Quantity
name
name
Mustha
Cyprus
(Rizomes)
1 part
Rotundus L.
Haritaki Terminalia
(plant)
1part
chebula
Retz.
Pippali
Piper
(Fruit)
1 part
longum
Linn.
Trivart
Operculina
(Root)
1 part
turpethum L.
Sandha
lavana
MATERIALS AND METHODS
Plant material was collected from NIAPR, Patiala. The
authenticity of the species of herbs was checked and
confirmed. For microscopical study, properly washed
plant material was cut in to desirable size. Free hand
sections were taken and stained with phoroglucinol and
HCL. [2] Physico-chemical studies like total ash, water
soluble ash, acid insoluble ash, water and alcohol soluble
extract, loss on drying at 105C, heavy metals and
successive extractive values by soxhlet extraction
method were carried out as per the WHO guide lines [3].
Ajay K Meena et.al. / Standardization of Ayurvedic polyherbal formulation, Pancasama Churna
Figure 1. Microscopy of Pancasama Churna
Preliminary phytochemical tests were performed as per
the standard methods [4]. The HPTLC finger print profile
of ethanolic extract of Pancasama churna was taken on
aluminium plate coated with silica gel 60 F254 of 0.2 mm
thickness (E. Merck) as adsorbent and employing
CAMAG Linomat IV applicator [5, 6]. The mobile phase
used was Toluene: Ethyl acetate: Formic acid
(5.0:3.5:1.0 v/v). The plate was dried and visualised
under UV 254 nm and 366 nm. The plate was dipped in
Vanillin- Sulphuric acid and heated at 105 C till the
spots appeared [7, 8].
RESULT AND DISCUSSIONS
Sample of raw material was examined for probable
adulterants such as plant material of similar appearance
which were found to be absent.
IJPPR March- May 2010, Vol.2, Issue1, 11-14
12
Ajay K Meena et.al. / Standardization of Ayurvedic polyherbal formulation, Pancasama Churna
Table 2. Physico-chemical parameters of
Pancasama churna
S.
Parameters
Mean
value
No
(%w/w) n=3
.
1 Loss on drying at 105C
8.30
(%w/w)
2 Total ash (%w/w)
23.50
3 Acid-insoluble ash
3.52
(%w/w)
4 Water soluble extractive
37.62
value (%w/w)
5 Alcohol soluble extractive
21.14
value(%w/w)
6 pH value (10% aqueous
4.65
solution)
7 Sodium
15
8
Total viable aerobic count
1.4x106
9 Total Enterobacteriaceae
Nil
10 Total fungal count
Nil
Macroscopical characters: Pale brown, moderately fine
powder; odour pungent; taste slightly pungent with
tingling sensation.
Microscopic characters: A few mg of powder was
washed with plain water, treated with iodine and
potassium iodide, drop of glycerine was added and
mounted. It showed the characters like orange coloured
Parenchymatous cells, stone cells (6-8), aril tissue,
perisperm cells, vessels with spiral thickening up to 75
in length, spiral thickening up to 75 in length, rosette
and prismatic crystals of calcium oxalate, biseriata and
multiseriate medullary ray, lignified sclereids and orange
coloured particles [9, 10].
Physio-chemical study
Physio-chemical parameters of Pancasama churna are
tabulated in (Table-2). Deterioration time of the plant
material depends upon the amount of water present in
plant material. If the water content is high, the plant can
be easily deteriorated due to fungus. The loss on drying
at 105C in Pancasama churna was found to be 8.30 %.
Total ash value of plant material indicated the amount of
minerals and earthy materials present in the plant
material. Analytical results showed total ash value of
23.50 %. The amount of acid-insoluble siliceous matter
present in the plant was 3.52 %. The water-soluble
extractive value indicated the presence of sugar, acids
and inorganic compounds. The alcohol soluble extractive
values indicated the presence of polar constituents like
phenols, alkaloids, steroids, glycosides, flavonoids and
n-hexane (hot) extractive values indicate the non-polar
secondary metabolites present in the plant.
TLC/ HPTLC Analysis: TLC and HPTLC finger
printing profile of Pancasama Churna were developed in
Toluene: Ethyl acetate: Formic acid (5.0:3.5:1.0 v/v)
solvent system. Under 254 nm, it showed 3 spots with
Rf value of 0.33, 0.41 and 0.78 (all green colour); under
366nm it showed 5 spots with Rf value of 0.33 (blue),
0.41 (blue), 0.69 (pale blue), 0.75 (pale blue), 0.89 (pale
blue). After derivatization with the vanillin and sulphuric
acid it showed 6 spots with Rf value: 0.50 (grey colour),
0.58 (grey colour), 0.69 (blue), 0.72 (grey), 0.83 (bluish
grey) and 0.88 (grey).
CONCLUSION
Morphology as well as various phamacognostic aspects
of the sample was studied and along with phytochemical,
physio-chemical, TLC and HPTLC studies. Pancasama
churna exhibits a set of diagnostic characters, which may
Figure. 2. HPTLC Fingur print of Pancasama Churna at 254 nm
IJPPR March- May 2010, Vol.2, Issue1, 11-14
13
Ajay K Meena et.al. / Standardization of Ayurvedic polyherbal formulation, Pancasama Churna
254nm
366nm
After
Derivatisation
Authors are thankful to the Director of Central Council
for Research in Ayurveda and Siddha (CCRAS), New
Delhi for providing facilities.
REFERENCES
Anonymous. The Ayurvedic Formulary of India. Part I, 2nd Edn.
Government of India, Ministry of Health and Family Welfare,
New Delhi, 2003,113.
2.
Lala PK. Lab Manuals of Pharmacognosy (5th Edition). CSI
Publishers and Distributors, Calcutta, 1993.
3.
Ananymous. Quality Control Methods for Medicinal Plant
Materials, World Health Organisation, Geneva, 1998, 25-28.
4.
Harborne JB. Phytochemical Methods. Jackman H. (Ed.),
London, 1973, 70.
5.
Saraswathy A. HPTLC finger printing of some Ayurveda and
Siddha drugs and their substitutes/adulterants. Indian Drugs.
2003; 40: 462-466.
6.
Sethi PD. High Performance Thin Layer Chromatography (1st
Edition). CBS Publishers and Distributors, New Delhi, 1996;
Vol X: 1-56.
7.
\Stahl I. Thin Layer Chromatography, A Laboratory Hand Book
(Student Edition). Springer-Verlag, Berlin, 1969, 52-86.
8.
Wagner H. and Bladt S, Plant Drug Analysis, IInd Ed., 1996.
9.
Kirtikar KR, and Basu BD. Indian Medicinal Plants. Allahabad
1989; 2:1237.
10. Trease GE, Evans WC. Pharmacognosy. 13th Edn.
11. Bailliere
Tuidal,
London,
1989;
799-803
1.
Figure 3. Thin layer chromatography of Pancasama
Churna (Toluene: Ethyl acetate : Formic acid :: 5.0 : 3.5 :
1.0 v/v)
help in identifying the drug in dried condition.
ACKNOWLEDGEMENT
IJPPR March- May 2010, Vol.2, Issue1, 11-14
14
Potrebbero piacerti anche
- Shalkya TantraDocumento3 pagineShalkya Tantratejpat2k7Nessuna valutazione finora
- Muniyal Institute Panchakarma CertificateDocumento13 pagineMuniyal Institute Panchakarma CertificateAswathy KumaranNessuna valutazione finora
- Aushadhi Sukumara GhritaDocumento27 pagineAushadhi Sukumara GhritaDharitri PurohitNessuna valutazione finora
- Free 1 Year Varmam-Marma Online Retreat Program From VKRCDocumento6 pagineFree 1 Year Varmam-Marma Online Retreat Program From VKRCVarmam Academy SriNessuna valutazione finora
- Yava Barley A Dietary Solution in ObesityDocumento5 pagineYava Barley A Dietary Solution in ObesityDigito DunkeyNessuna valutazione finora
- Asava and ArishtaDocumento7 pagineAsava and Arishtadrsa2Nessuna valutazione finora
- Diabetes An Ayurvedic Perspective PDFDocumento8 pagineDiabetes An Ayurvedic Perspective PDFVaibhav KavathekarNessuna valutazione finora
- Guna Vikalpa in AyurvedaDocumento22 pagineGuna Vikalpa in AyurvedaManoj SankaranarayanaNessuna valutazione finora
- Hridroga Samprapti and Modern Pathology of IhdDocumento11 pagineHridroga Samprapti and Modern Pathology of IhdGAURAVNessuna valutazione finora
- Bhaishajya KalpanaDocumento10 pagineBhaishajya KalpanavkasatiNessuna valutazione finora
- Pharmaceutical Standardization of Ichhabhedi RasaDocumento4 paginePharmaceutical Standardization of Ichhabhedi RasaMujahid KhanNessuna valutazione finora
- Virechana KarmaDocumento28 pagineVirechana KarmaSurendra DavanagereNessuna valutazione finora
- KSR RS BZAp1Documento41 pagineKSR RS BZAp1ksr prasadNessuna valutazione finora
- Keraleeya Pnachakarma BrochureDocumento8 pagineKeraleeya Pnachakarma BrochureVatasal SinghNessuna valutazione finora
- MAHATIKTAKAM GHRITAM - in Different ClassicDocumento5 pagineMAHATIKTAKAM GHRITAM - in Different ClassicAyurvistaNessuna valutazione finora
- Dravyaguna part 3 by Prof. Dr. R.R. DeshpandeDocumento84 pagineDravyaguna part 3 by Prof. Dr. R.R. DeshpandeRajeshKizziNessuna valutazione finora
- Virechana KarmaDocumento76 pagineVirechana KarmaHariNessuna valutazione finora
- Clinical Application of Shadvidhopkrama A CriticalDocumento6 pagineClinical Application of Shadvidhopkrama A CriticalBhonsle AmayNessuna valutazione finora
- Avarana in VyadhiDocumento4 pagineAvarana in VyadhiSamhitha Ayurvedic ChennaiNessuna valutazione finora
- Management of Meniere's Disease - An Analytical Ayurveda PerspectiveDocumento3 pagineManagement of Meniere's Disease - An Analytical Ayurveda PerspectiveAdvanced Research PublicationsNessuna valutazione finora
- Ritucharya (ऋतुचर्या) - Ayurveda Recommended Seasonal Habits - Part 1Documento18 pagineRitucharya (ऋतुचर्या) - Ayurveda Recommended Seasonal Habits - Part 1aarogya100% (1)
- Physiological Understanding of Medovaha Srotas in The Current PerspectiveDocumento3 paginePhysiological Understanding of Medovaha Srotas in The Current PerspectiveAdvanced Research PublicationsNessuna valutazione finora
- 05 - Chapter 1 Heavy MetalDocumento57 pagine05 - Chapter 1 Heavy MetalMonica KuhonNessuna valutazione finora
- Final PPT of GauravDocumento24 pagineFinal PPT of Gauravkirti pawar100% (1)
- Amritottaram Kashaya: Ayurvedic Medicine for FeverDocumento11 pagineAmritottaram Kashaya: Ayurvedic Medicine for FeverPrajeesh nathNessuna valutazione finora
- Vāta Vyādhi: Types, Subtypes, and Symptoms of Vata DisordersDocumento49 pagineVāta Vyādhi: Types, Subtypes, and Symptoms of Vata DisordersAjay IyerNessuna valutazione finora
- Conceptual Study On The State of Swastha'Documento4 pagineConceptual Study On The State of Swastha'Advanced Research Publications100% (1)
- Vishamajwaram Kc029gdg 121226100307 Phpapp02Documento238 pagineVishamajwaram Kc029gdg 121226100307 Phpapp02Samhitha Ayurvedic Chennai100% (2)
- Role of AnupanaDocumento9 pagineRole of AnupanaBrad YantzerNessuna valutazione finora
- Importance of Ayurveda SharirDocumento7 pagineImportance of Ayurveda SharirHitesh LonareNessuna valutazione finora
- Importance of Paryayas (Synonyms) in Plant IdentificationDocumento22 pagineImportance of Paryayas (Synonyms) in Plant IdentificationSatish GuptaNessuna valutazione finora
- Physico-Chemical Evaluation of RasakarpuraDocumento14 paginePhysico-Chemical Evaluation of RasakarpuraDrVikasNessuna valutazione finora
- Amavata KCDocumento45 pagineAmavata KCBarsha Meher100% (1)
- Malas Are The MetabolicDocumento34 pagineMalas Are The MetabolicallenNessuna valutazione finora
- Pancha Karma For Diifferent Rogas - VangasenaDocumento7 paginePancha Karma For Diifferent Rogas - VangasenaManikandan NarayananNessuna valutazione finora
- Kshara PDFDocumento8 pagineKshara PDFVineet NavrangNessuna valutazione finora
- Medorog Visheshank March 2008Documento8 pagineMedorog Visheshank March 2008Mitra PathakNessuna valutazione finora
- Gunas As Principle Karana PDFDocumento16 pagineGunas As Principle Karana PDFडा. सत्यदेव त्यागी आर्य0% (1)
- Charaka Samhita: Handbook On AyurvedaDocumento100 pagineCharaka Samhita: Handbook On AyurvedaRaj GuptaNessuna valutazione finora
- Samhitokta Conceptual Review of Marma and PranayatanaDocumento4 pagineSamhitokta Conceptual Review of Marma and PranayatanaAnonymous sAVN1eN5NuNessuna valutazione finora
- The Three Pillars of Health and Dietary Factors in AyurvedaDocumento34 pagineThe Three Pillars of Health and Dietary Factors in AyurvedaDhanwantari JhaNessuna valutazione finora
- EFFICACY OF GANDHARVAHASTADI KASHAYAM IN HYPERTENSIONDocumento12 pagineEFFICACY OF GANDHARVAHASTADI KASHAYAM IN HYPERTENSIONPradeep SambandamNessuna valutazione finora
- Making Herbarium GuideDocumento7 pagineMaking Herbarium GuideFaisal HafizNessuna valutazione finora
- Literature Review On Garbhini Chardi Emesis GravidumDocumento3 pagineLiterature Review On Garbhini Chardi Emesis GravidumResearch ParkNessuna valutazione finora
- Practical Apporoach To UttarbastiDocumento19 paginePractical Apporoach To UttarbastiMadhura PhadtareNessuna valutazione finora
- Application of Panchakarma in Shalya Tantra A ReviewDocumento5 pagineApplication of Panchakarma in Shalya Tantra A ReviewEditor IJTSRDNessuna valutazione finora
- Ajara-Rasayana KSRPrasadDocumento56 pagineAjara-Rasayana KSRPrasadksr prasad100% (1)
- A Critical Conceptual Study of Applied Anatomy in Charaka SamhitaDocumento3 pagineA Critical Conceptual Study of Applied Anatomy in Charaka SamhitaIJARP PublicationsNessuna valutazione finora
- Anorectal Disease Presentatione 2Documento91 pagineAnorectal Disease Presentatione 2kavindukarunarathnaNessuna valutazione finora
- Clinico-Pathological Review On Pravahika Vis-A-Vis AmoebiasisDocumento3 pagineClinico-Pathological Review On Pravahika Vis-A-Vis AmoebiasisAdvanced Research PublicationsNessuna valutazione finora
- Asava and Aristha An Ayurvedic Medicine - An OvervDocumento8 pagineAsava and Aristha An Ayurvedic Medicine - An OvervGarima KhandelwalNessuna valutazione finora
- Understanding Ayurvedic Inhalers W.S.R. To Tamaka SwasaDocumento15 pagineUnderstanding Ayurvedic Inhalers W.S.R. To Tamaka SwasaDrVikasNessuna valutazione finora
- A Prospective Study On Parpati Kalpana W.S.R To Panchamrut ParpatiDocumento11 pagineA Prospective Study On Parpati Kalpana W.S.R To Panchamrut ParpatiDrHassan Ahmed ShaikhNessuna valutazione finora
- Assessment of Vata DoshaDocumento11 pagineAssessment of Vata DoshaRajendra DeshpandeNessuna valutazione finora
- An Ayurvedic Perspective of Bhallataka S PDFDocumento16 pagineAn Ayurvedic Perspective of Bhallataka S PDFBhavana GangurdeNessuna valutazione finora
- Ayurvedic Concept of Vishada (Depression) and Its Healing Through Traditional Regimen A Review ArticleDocumento5 pagineAyurvedic Concept of Vishada (Depression) and Its Healing Through Traditional Regimen A Review ArticleInternational Journal of Innovative Science and Research TechnologyNessuna valutazione finora
- Panchakarma CourseDocumento2 paginePanchakarma CourseshripathiNessuna valutazione finora
- Yoga Fiction: Yoga Truth, Ebook 2: Flowing Prana, Sparks Your Creative VolitionDa EverandYoga Fiction: Yoga Truth, Ebook 2: Flowing Prana, Sparks Your Creative VolitionNessuna valutazione finora
- The Science of Ayurveda: The Ancient System to Unleash Your Body's Natural Healing PowerDa EverandThe Science of Ayurveda: The Ancient System to Unleash Your Body's Natural Healing PowerNessuna valutazione finora
- BhaishajyaDocumento28 pagineBhaishajyadrsa2Nessuna valutazione finora
- Varatika BhasmaDocumento5 pagineVaratika Bhasmadrsa2Nessuna valutazione finora
- Standardization of Herbal Vasa Candy LozengesDocumento6 pagineStandardization of Herbal Vasa Candy Lozengesdrsa2Nessuna valutazione finora
- Standardisation of Sudharshana Churna-A Polyherbal FormulationDocumento5 pagineStandardisation of Sudharshana Churna-A Polyherbal Formulationdrsa2Nessuna valutazione finora
- Standardization of An Ayurvedic Drug: Trivanga BhasmaDocumento2 pagineStandardization of An Ayurvedic Drug: Trivanga Bhasmadrsa2Nessuna valutazione finora
- Vanga BhasmaDocumento1 paginaVanga Bhasmadrsa2Nessuna valutazione finora
- Standardization of Sitopaladi Churna: A Poly-Herbal FormulationDocumento12 pagineStandardization of Sitopaladi Churna: A Poly-Herbal Formulationdrsa2Nessuna valutazione finora
- Mahasudarshan ChurnaDocumento6 pagineMahasudarshan Churnadrsa2100% (1)
- Aswagandadi LehyaDocumento5 pagineAswagandadi Lehyadrsa2Nessuna valutazione finora
- Standardization of Ayurvedic formulationDocumento5 pagineStandardization of Ayurvedic formulationdrsa2Nessuna valutazione finora
- Metal FormulationDocumento18 pagineMetal FormulationDoyel RaiNessuna valutazione finora
- Asoka RishtaDocumento6 pagineAsoka Rishtadrsa2Nessuna valutazione finora
- BilvadilehaDocumento5 pagineBilvadilehadrsa2Nessuna valutazione finora
- Standardisation of Ayurvedic Medicines-Dasamulam KasayamDocumento7 pagineStandardisation of Ayurvedic Medicines-Dasamulam Kasayamdrsa2Nessuna valutazione finora
- AswagandharishtaDocumento4 pagineAswagandharishtadrsa2Nessuna valutazione finora
- AGASTYA HaritakiDocumento7 pagineAGASTYA Haritakidrsa2Nessuna valutazione finora
- Asava and ArishtaDocumento7 pagineAsava and Arishtadrsa2Nessuna valutazione finora
- StandardizationDocumento1 paginaStandardizationdrsa2Nessuna valutazione finora
- ArjunarishtaDocumento3 pagineArjunarishtadrsa2Nessuna valutazione finora
- Traditionally Fermented Biomedicines, Arishtas and Asavas From AyurvedaDocumento9 pagineTraditionally Fermented Biomedicines, Arishtas and Asavas From Ayurvedadrsa2Nessuna valutazione finora
- Architecture of Drug Regulation in IndiaDocumento20 pagineArchitecture of Drug Regulation in Indiadrsa2100% (1)
- Abhraka BhasmaDocumento7 pagineAbhraka Bhasmadrsa2Nessuna valutazione finora
- SITXWHS001 Student Assessment v2.1Documento16 pagineSITXWHS001 Student Assessment v2.1Sukhpreet KaurNessuna valutazione finora
- Fragrance ReportDocumento44 pagineFragrance ReportIani MarinescuNessuna valutazione finora
- Installation and Preoperative TestDocumento27 pagineInstallation and Preoperative TestLeonardo BaiaoNessuna valutazione finora
- Good Will Hunting Report AnalysisDocumento8 pagineGood Will Hunting Report AnalysislylavanesNessuna valutazione finora
- Among The Laws Affecting The Practice of NursingDocumento3 pagineAmong The Laws Affecting The Practice of Nursingmarie100% (35)
- Measuring Tools For Management (LCM-Rubina)Documento14 pagineMeasuring Tools For Management (LCM-Rubina)Ruel BariaNessuna valutazione finora
- Technical Specification: Iso/Ts 22002-5Documento8 pagineTechnical Specification: Iso/Ts 22002-5Santi PrihartiniNessuna valutazione finora
- SOP Health Services Management MSCDocumento2 pagineSOP Health Services Management MSCHafiz Mohammad Shuputra50% (2)
- FM 999-3 Counter-Zombie Operations at The Fireteam Level v1.1Documento16 pagineFM 999-3 Counter-Zombie Operations at The Fireteam Level v1.1Arthur BarieNessuna valutazione finora
- Nurses Role Fire and DisasterDocumento25 pagineNurses Role Fire and DisasterchellczyNessuna valutazione finora
- GINA 2021 Guidelines for Asthma Diagnosis and TreatmentDocumento24 pagineGINA 2021 Guidelines for Asthma Diagnosis and TreatmentBianca Watanabe - RatillaNessuna valutazione finora
- SUBASHINIDocumento3 pagineSUBASHINIKB_mitNessuna valutazione finora
- Ca LidahDocumento27 pagineCa LidahArnaz AdisaputraNessuna valutazione finora
- TG 6 Refractive ErrorDocumento11 pagineTG 6 Refractive ErrorNovi AdriNessuna valutazione finora
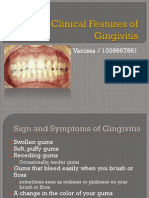
- Clinical Features of GingivitisDocumento26 pagineClinical Features of GingivitisVanissa KarisNessuna valutazione finora
- Mtendere Community Cholera Prevention OutreachDocumento2 pagineMtendere Community Cholera Prevention OutreachReal KezeeNessuna valutazione finora
- End Indifference - Pope: Stray Bullet Cases Hit 36 Firecrackers Injure 459Documento56 pagineEnd Indifference - Pope: Stray Bullet Cases Hit 36 Firecrackers Injure 459Art JasmeNessuna valutazione finora
- Providing Public Area ServicesDocumento48 pagineProviding Public Area ServicesFONCY BUYANNessuna valutazione finora
- Isak 1 2 ViennaDocumento6 pagineIsak 1 2 Viennanifej15595Nessuna valutazione finora
- Hse Pja BtsguideDocumento2 pagineHse Pja BtsguideMohamed Ismail ShehabNessuna valutazione finora
- Patient Complaints About General PracticeDocumento9 paginePatient Complaints About General PracticesanaaNessuna valutazione finora
- Interpreting PDX Dot BlotsDocumento2 pagineInterpreting PDX Dot BlotsSpy CameraNessuna valutazione finora
- Lakshmanarishtam - YogamruthamDocumento6 pagineLakshmanarishtam - YogamruthamRahul KirkNessuna valutazione finora
- Mita Das (Universityof CalcuttaDocumento52 pagineMita Das (Universityof CalcuttaBishal Roy100% (1)
- 2015 EHS Challanges Enablon Reportv9Documento15 pagine2015 EHS Challanges Enablon Reportv9Anonymous sfY8T3q0100% (1)
- India Automotive Component Post Covid 19Documento27 pagineIndia Automotive Component Post Covid 19Anurag MishraNessuna valutazione finora
- Malmstrom Et Al - 1999 - Oral Montelukast, Inhaled Beclomethasone, and Placebo For Chronic AsthmaDocumento10 pagineMalmstrom Et Al - 1999 - Oral Montelukast, Inhaled Beclomethasone, and Placebo For Chronic AsthmaRutvik ShahNessuna valutazione finora
- Burket's Oral Medicine 13th Ed Michael GlickDocumento1.139 pagineBurket's Oral Medicine 13th Ed Michael GlickMahima GoodwaniNessuna valutazione finora
- Intelligent Dosing of RonozymeDocumento25 pagineIntelligent Dosing of RonozymeRaymundus Genty Laras100% (1)
- Lecture No 03 Functional Foods NutraceuticalDocumento15 pagineLecture No 03 Functional Foods Nutraceuticalmaryam khanNessuna valutazione finora