Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Test 2
Caricato da
fatevilcowTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Test 2
Caricato da
fatevilcowCopyright:
Formati disponibili
Name: ____________________,_________________
print last
print first
July 28, 2010
Test #2
Chem151-001 Dr. Canelas
Instructions and Information - Read Carefully
Put away all papers, books, calculators, cell phones, and pagers.
You may use a model kit and a ruler; do not share your models with classmates during the test.
The time allowed for this exam is 75 minutes.
This test has 7 pages of questions (6 sheets of paper.)
The last page of your exam is a table of spectroscopy data. Please feel free to detach this for use
1.
2.
3.
4.
5.
I pledge that I have neither given nor received unauthorized aid on this exam:
_____________________________________
(signature)
Periodic Table
1
1.008
1.008
Li
Be
6.941
9.012
10.81
12.01
14.01
16.00
19.00
11
12
Na Mg
22.99
24.31
19
20
21
22
23
24
13
14
Al
Si
26.98
25
26
27
28
29
30
31
28.09
32
15
16
17
Cl
30.97
32.07
33
34
35.45
35
He 1
4.00
10
Ne 2
2 0.18
20.18
18
Ar 3
39.95
36
Ca
Sc Ti
Cr Mn Fe
Co Ni
Cu Zn Ga Ge
As Se Br
Kr 4
39.10
40.08
44.96
47.87
50.94
52.00
54.94
55.85
58.93
58.69
63.55
65.39
69.72
72.61
74.92
74.92
78.96
79.90
83.80
37
38
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
Zr
Nb Mo Tc Ru Rh Pd Ag Cd
In
Sn Sb Te
Xe
91.22
92.91
95.94
98
01.1
101.1
1
102.9
118.7
121.8
127.6
126.9
131.3
75
76
82
83
84
85
Rb Sr
85.47
87.62
88.91
55
56
57
106.4
72
73
74
77
78
Cs Ba La
Hf
Ta
W Re Os Ir
Pt
132.9
178.5
180.9
87
137.3
138.9
88
89
186.2
190.2
192.2
195.1
112.4
114.8
79
80
81
Au Hg Tl
197.0
200.6
204.4
86
Pb Bi
Po At
Rn 6
207.2
209
222
209.0
210
104
Fr Ra
Ac Rf
(223)
227
226
183.8
107.9
261
58
59
60
61
62
63
64
65
66
67
68
69
70
71
Ce Pr
Nd Pm Sm Eu Gd Tb Dy Ho Er
Tm Yb Lu
140.1
140.9
144.2
90
145
152.0
157.3
158.9
162.5
94
95
96
97
98
164.9
167.3
168.9
173.0
175.0
99
100
101
102
103
91
92
Th Pa
Np Pu Am Cm Bk Cf
Es Fm Md No Lr
232.0
238.0
(237)
(252)
(231)
93
150.4
(242)
(243)
(247)
(247)
(251)
(257)
(258)
(259)
(260)
Test #2
Chem151L Canelas
Summer, 2010
Last Name: ___________________
Name:__________________________,_____________________
print last
print first
Score Summary:
Number
Points
/30
/36
/12
/16
/16
/20
/20
Total
/150
Test #2
Chem151L Canelas
Summer, 2010
Last Name: ___________________
1. (30 points)
a) Please used curved arrow notation to write reaction mechanism for the following transformation:
HBr, H2O2,
Br
b) Using the axis below as a guideline, draw the proton NMR spectrum of the major organic
product shown above for the reaction in part a. Please consider the most likely chemical shifts,
multiplicity caused by coupling between protons, and integral values; clearly indicate these on
your drawing.
Test #2
Chem151L Canelas
Last Name: ___________________
Summer, 2010
2. Consider the structures shown in each part and choose the best answer. (36 points)
a) For parts I and II, choose the letter that corresponds to the relationship between each of the pairs listed
below and write it in the answer blank.
B
a) no relationship
b) constitutional isomers
E trans-1,4-dimethylcyclohexane
(most stable chair conformation)
c) different energy conformations of one molecule
F cis-1-ethyl-2-propylcyclobutane
d) stereoisomers
I) C and D ______
e) exact same molecule with the same energy
II) A and E ______
b) Determine whether each molecule is an enantiomer in the R configuration, an enantiomer in the S
configuration, or an achiral molecule (A). Please answer with R, S, or A.
H3C
Br
H3C
H3C
CH2CH3
HO
CH2CH2OH
HO
CH3
Answers:
c) Complete the Fischer projections by drawing the atoms or groups (H or OH) attached to the
horizontal bonds. Then, compare these structures and circle their relationship below.
Compound X:
H
OH
Compound Y:
CH
O
H
O
H
OH
CH
H OH
CH3
HO H
CH3
Circle Relationship Between X and Y:
Identical
Constitutional isomers
Enantiomers
Diastereomers
Test #2
Chem151L Canelas
Summer, 2010
Last Name: ___________________
3. Draw the following compounds: (12 points)
a) Most stable chair conformation of
H3C
CH3
CH(CH3)2
CH3
b) cis-bicyclo[3.3.0]octane
Test #2
Chem151L Canelas
Summer, 2010
Last Name: ___________________
4. For the reactions shown below, draw the structure of the starting material or major organic
product(s), whichever is missing. Please carefully denote configuration of chiral carbons using linewedge-dash notation, if necessary; if a reaction is stereoselective (syn- or anti- addition) and a racemic
mixture results, then please show both products for this exercise. "D" is deuterium, an isotope of
hydrogen that behaves similarly. Reaction mechanisms do NOT need to be shown. (16 points)
a.
Cl2
b.
1. BD3, THF
2. H2O2, OH-
c.
1. O3
2. (CH3)2S
O
H
O
d.
OH
H-O-O-H, hv
HO
Test #2
Chem151L Canelas
Summer, 2010
Last Name: ___________________
5. 1,2,3-trifluorocyclopentane has two different meso stereoisomers. Complete the exercises or answer
the questions below. (16 points)
a) Starting with the template below for each, complete the structures by filling in the missing
fluorines and hydrogens for each of the two different meso forms of 1,2,3-trifluorocyclopentane.
(Please note: one fluorine is shown to get you started; please fill in the other 2 F and 3 H atoms
on each line-wedge-dash structure.)
b) On your drawings above, draw an asterisk next to every carbon that is a stereocenter.
c) Pick ONE of the stereocenters youve designated and clearly indicate its absolute configuration (R
or S.) Please make sure you clearly indicate which carbon you have assigned.
d) Would a solution of the compound youve completed on the left rotate plane polarized light? Why
or why not?
e) Circle the relationship between the two structures youve completed in part a.
Identical
Constitutional isomers
Enantiomers
Diastereomers
Test #2
Chem151L Canelas
Summer, 2010
Last Name: ___________________
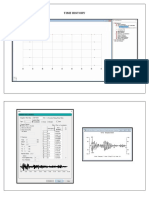
6. Draw the structure of the unknown compound by solving the IR, proton NMR, and mass spectra
provided. Write information about the presence or absence of various functional groups next to
the IR spectrum for full or partial credit. Also, indicate the correlation between the signals on the
NMR and the different protons on your final structure. Please recall that a table of spectral values
is provided as the last page.(20 points)
Functional groups:
Structure of Compound:
Test #2
7.
Chem151L Canelas
Summer, 2010
Last Name: ___________________
Draw the structure of the unknown compound by solving the IR, proton NMR, and mass spectra
provided. Write information about the presence or absence of various functional groups next to
the IR spectrum for full or partial credit. Also, indicate the correlation between the signals on the
NMR and the different protons on your final structure. Please recall that a table of spectral values
is provided as the last page.(20 points)
Functional groups:
Structure of Compound:
Test #2
Chem151L Canelas
Summer, 2010
Last Name: ___________________
Typical Values of IR Stretching Frequencies:
Approximate Chemical Shifts of Carbons in
13
Approximate Chemical Shifts of
1
Hydrogens in H-NMR Spectra:
C-NMR Spectra
Potrebbero piacerti anche
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- Atomic Absorption & EmissionDocumento80 pagineAtomic Absorption & EmissionAkshay Patil100% (1)
- DME ProcessDocumento5 pagineDME ProcessAndres FragosoNessuna valutazione finora
- Ap Calculus Course DescriptionDocumento60 pagineAp Calculus Course DescriptionDavid WeiNessuna valutazione finora
- Self Heall PPT EditedDocumento34 pagineSelf Heall PPT EditedYASHAS K CNessuna valutazione finora
- Adobe - 2023 MBA University Graduate - Customer Experience Strategy & OpsDocumento3 pagineAdobe - 2023 MBA University Graduate - Customer Experience Strategy & OpsfatevilcowNessuna valutazione finora
- Adobe - 2023 MBA University Graduate - Strategy & Ops ManagerDocumento3 pagineAdobe - 2023 MBA University Graduate - Strategy & Ops ManagerfatevilcowNessuna valutazione finora
- Bridgeton QuestionsDocumento1 paginaBridgeton QuestionsfatevilcowNessuna valutazione finora
- Adobe - 2023 MBA University Graduate - Engagement Strategy ManagerDocumento3 pagineAdobe - 2023 MBA University Graduate - Engagement Strategy ManagerfatevilcowNessuna valutazione finora
- Activision Blizzard - Rotation Manager Job in Santa Monica, California, USADocumento7 pagineActivision Blizzard - Rotation Manager Job in Santa Monica, California, USAfatevilcowNessuna valutazione finora
- USA CumulativepdfDocumento1 paginaUSA CumulativepdffatevilcowNessuna valutazione finora
- Bridgeton DataDocumento3 pagineBridgeton DatafatevilcowNessuna valutazione finora
- Calendar August 2016 - July 2017Documento12 pagineCalendar August 2016 - July 2017fatevilcowNessuna valutazione finora
- Qual Trics Survey SoftwareDocumento209 pagineQual Trics Survey SoftwareDaniel Miranda100% (1)
- Test 3 KeyDocumento6 pagineTest 3 KeyfatevilcowNessuna valutazione finora
- Mycology 11-3 NotesDocumento3 pagineMycology 11-3 NotesfatevilcowNessuna valutazione finora
- 2014 Employer Health Benefits Survey Full ReportDocumento244 pagine2014 Employer Health Benefits Survey Full ReportfatevilcowNessuna valutazione finora
- Lab 3 - Integument and Connective TissuesDocumento13 pagineLab 3 - Integument and Connective TissuesfatevilcowNessuna valutazione finora
- Appetizers: All Burgers Are Served On Fresh Buns With Lettuce, Tomato, Onion, and A Side of FriesDocumento1 paginaAppetizers: All Burgers Are Served On Fresh Buns With Lettuce, Tomato, Onion, and A Side of FriesfatevilcowNessuna valutazione finora
- Wwe 2k14 Ps3 Extended ManualDocumento34 pagineWwe 2k14 Ps3 Extended ManualfatevilcowNessuna valutazione finora
- 7 ColumnChromDocumento7 pagine7 ColumnChromfatevilcowNessuna valutazione finora
- Evanth S15 OutlineDocumento5 pagineEvanth S15 OutlinefatevilcowNessuna valutazione finora
- Diels Alder LabDocumento8 pagineDiels Alder Labfatevilcow0% (1)
- Hernandez 15 TipsDocumento10 pagineHernandez 15 TipsfatevilcowNessuna valutazione finora
- Diels Alder LabDocumento8 pagineDiels Alder Labfatevilcow0% (1)
- University Education in Italy PDFDocumento29 pagineUniversity Education in Italy PDFfatevilcowNessuna valutazione finora
- BS en 10052-94Documento35 pagineBS en 10052-94HosseinNessuna valutazione finora
- Residue HpalDocumento2 pagineResidue HpalsyafirarkNessuna valutazione finora
- EPA Method - 200 0 Metals Analysis by Atomic AbsorptionDocumento18 pagineEPA Method - 200 0 Metals Analysis by Atomic AbsorptionAnggun Teh PamegetNessuna valutazione finora
- Std9-Geog-latitude and Longitude (2013)Documento8 pagineStd9-Geog-latitude and Longitude (2013)LuciaNessuna valutazione finora
- 4PH1 2PR MSC 20210304Documento13 pagine4PH1 2PR MSC 20210304Dazy ChowdhuryNessuna valutazione finora
- Brochure Transcutol P For Efficient Skin PenetrationDocumento24 pagineBrochure Transcutol P For Efficient Skin PenetrationJoaozinhoMéndez100% (1)
- Interpretations and DFT Calculations For Polypropylene/Cupper Oxide NanosphereDocumento14 pagineInterpretations and DFT Calculations For Polypropylene/Cupper Oxide Nanosphereyousif husseinNessuna valutazione finora
- Gravitation Past Paper 9702Documento14 pagineGravitation Past Paper 9702Salman AsimNessuna valutazione finora
- Radicals PDFDocumento34 pagineRadicals PDFadelNessuna valutazione finora
- Grade 6 DLL SCIENCE 6 Q3 Week 6Documento5 pagineGrade 6 DLL SCIENCE 6 Q3 Week 6Mark neil a. GalutNessuna valutazione finora
- HURDCO International School: Subject-Biology Chapter 3: Diffusion, Osmosis and Surface Area: Volume RatioDocumento26 pagineHURDCO International School: Subject-Biology Chapter 3: Diffusion, Osmosis and Surface Area: Volume RatioMahin IslamNessuna valutazione finora
- CpiDocumento7 pagineCpiBenzeneNessuna valutazione finora
- Học phần: EE3033E - NGUYÊN LÝ TRƯỜNG Điện Từ Ngày thi: 18/02/2022 Thời gian làm bài (duration) : 60 phút (minutes) Ký duyệtDocumento1 paginaHọc phần: EE3033E - NGUYÊN LÝ TRƯỜNG Điện Từ Ngày thi: 18/02/2022 Thời gian làm bài (duration) : 60 phút (minutes) Ký duyệtThảo Nguyễn ThếNessuna valutazione finora
- 01 Numerical Prediction of Dynamic Performance of PeltonDocumento9 pagine01 Numerical Prediction of Dynamic Performance of PeltonSebastián RibadeneiraNessuna valutazione finora
- Liquid Nitrogen As A Non Polluting FuelDocumento30 pagineLiquid Nitrogen As A Non Polluting FuelShubham Raghuvanshi100% (2)
- Cambridge International AS & A Level: CHEMISTRY 9701/23Documento20 pagineCambridge International AS & A Level: CHEMISTRY 9701/23Aadista BhattaNessuna valutazione finora
- Guide On Designing A Solar Photovoltaic Powered DC Water PumpDocumento6 pagineGuide On Designing A Solar Photovoltaic Powered DC Water PumpDesmondNessuna valutazione finora
- Mma 070921 Endress Liquidlevelpart1Documento4 pagineMma 070921 Endress Liquidlevelpart1sarsureshNessuna valutazione finora
- DDDocumento11 pagineDDjamesdigolNessuna valutazione finora
- ESA-TECMSP-TN-007384 Rev2 - Process Verification of Altenative Chemical Conv Coating - SurtecDocumento6 pagineESA-TECMSP-TN-007384 Rev2 - Process Verification of Altenative Chemical Conv Coating - Surtecgoooga299Nessuna valutazione finora
- Modern Steelmaking Processes: Topics To DiscussDocumento10 pagineModern Steelmaking Processes: Topics To DiscussMir RafsanNessuna valutazione finora
- Navarro, Juan Miguel v. - Plan413 - Asynchronous Activity 2Documento4 pagineNavarro, Juan Miguel v. - Plan413 - Asynchronous Activity 2Juan Miguel NavarroNessuna valutazione finora
- (UPDATED) Time HistoryDocumento10 pagine(UPDATED) Time HistoryShaina Mariz PanaliganNessuna valutazione finora
- Labsheet 3 - F2FDocumento9 pagineLabsheet 3 - F2FMuhammad ZahidNessuna valutazione finora
- Atoms and Molecules Grade 9Documento2 pagineAtoms and Molecules Grade 9Pooja DebnathNessuna valutazione finora
- Lab Manual Bio1Documento31 pagineLab Manual Bio1deltaserrapapa0% (2)
- Prob Extras f502 PDFDocumento7 pagineProb Extras f502 PDFLuis Daniel RuizNessuna valutazione finora