Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
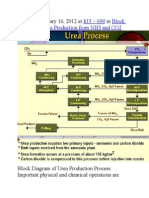
Producing Urea Through Bosch-Meiser Process
Caricato da
pushprajDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Producing Urea Through Bosch-Meiser Process
Caricato da
pushprajCopyright:
Formati disponibili
http://nzic.org.nz/ChemProcesses/production/1A.
http://teacher.buet.ac.bd/mahammad/Urea%206.pdf
process ppt
http://nptel.ac.in/courses/103107086/module2/lecture11/lecture11.pdf
http://en.wikipedia.org/wiki/Urea#Industrial_methods
http://ethesis.nitrkl.ac.in/4237/1/Manufacture_of_Urea.pdf
Laboratory preparation[edit]
Ureas in the more general sense can be accessed in the laboratory by reaction
of phosgene with primary or secondaryamines, proceeding through
an isocyanate intermediate. Non-symmetric ureas can be accessed by reaction of primary
or secondary amines with an isocyanate.
Also, urea is produced when phosgene reacts with ammonia:
COCl2 + 4 NH3 (NH2)2CO + 2 NH4Cl
the BoschMeiser urea process
The basic process, developed in 1922, is also called the BoschMeiser urea process after
its discoverers. The various commercial urea processes are characterized by the conditions
under which urea formation takes place and the way in which unconverted reactants are
further processed. The process consists of two main equilibrium reactions, with incomplete
conversion of the reactants. The first is carbamate formation: the fast exothermic reaction
of liquid ammonia with gaseous carbon dioxide (CO 2) at high temperature and pressure to
form ammonium carbamate (H2N-COONH4):[14]
2NH3 + CO2
H2N-COONH4
The second is urea conversion: the slower endothermic decomposition of ammonium
carbamate into urea and water:
H2N-COONH4
(NH2)2CO + H2O
The overall conversion of NH3 and CO2 to urea is exothermic,
the first reaction of the high temperature (around 19 000
C) needed for the second is
compensated for by conducting the process under high pressure (140175 bar), which
favours the first reaction. Although it is necessary to compress gaseous carbon dioxide to
this pressure, the ammonia is available from the ammonia plant in liquid form, which can be
pumped into the system much more economically. To allow the slow urea formation reaction
time to reach equilibrium a large reaction space is needed, so the synthesis reactor in a
large urea plant tends to be a massive pressure vessel.
Originally, because it was not economic to recompress the ammonia and carbon dioxide for
recycle, the ammonia at least would be used for the manufacture of other products, for
example ammonium nitrate or sulfate. (The carbon dioxide would be wasted, as likely as
not.) Later process schemes were developed to allow recycling of the unused ammonia and
carbon dioxide.-+
Side reaction-:
Biuret is formed when two molecules of urea combine with the loss of a molecule of
ammonia.
2NH2CONH2 H2NCONHCONH2 + NH3
Isocyanic acid results from the thermal decomposition of ammonium cyanate, which is
in chemical equilibrium with urea:
NH2CONH2 NH4NCO HNCO + NH3
Potrebbero piacerti anche
- Production of Ammonia: Department of Chemical Engineering A Report OnDocumento29 pagineProduction of Ammonia: Department of Chemical Engineering A Report OnDr.AhmedNessuna valutazione finora
- Stamicarbon Project PDFDocumento30 pagineStamicarbon Project PDFMir Hasib Ul Latif100% (7)
- AmmoniaDocumento59 pagineAmmoniavcoolkrazy0% (3)
- CPT Lecture Urea ProcessDocumento31 pagineCPT Lecture Urea ProcesssaisounyaNessuna valutazione finora
- Feasibilty For The ProjectDocumento18 pagineFeasibilty For The ProjectRana UzairNessuna valutazione finora
- Ammonia ProductionDocumento7 pagineAmmonia ProductionIkhtiander IkhtianderNessuna valutazione finora
- Production of AmmoniaDocumento29 pagineProduction of AmmoniaBhavna Bajpai83% (6)
- Ammonia Formation ProjectDocumento14 pagineAmmonia Formation ProjectNebyu DanielNessuna valutazione finora
- 1صناعاتDocumento15 pagine1صناعاتroaanaseem267Nessuna valutazione finora
- Engineers Guide: The Major Features of These Processes Are Described BelowDocumento2 pagineEngineers Guide: The Major Features of These Processes Are Described BelowzeeshanNessuna valutazione finora
- Bosch-Meiser Urea Process, 1922Documento7 pagineBosch-Meiser Urea Process, 1922Wow WowNessuna valutazione finora
- Hydrogen Production by Steam Reforming Chemical Engineering ProcessingDocumento8 pagineHydrogen Production by Steam Reforming Chemical Engineering ProcessingviettiennguyenNessuna valutazione finora
- Chapter 2Documento45 pagineChapter 2Haiqal AzizNessuna valutazione finora
- Rapid Pressure Swing Adsorption For Small Scale Ammonia Separation A Proof-Of-ConceptDocumento15 pagineRapid Pressure Swing Adsorption For Small Scale Ammonia Separation A Proof-Of-ConceptKhai Q TranNessuna valutazione finora
- Description of Ammonia Manufacturing ProcessesDocumento4 pagineDescription of Ammonia Manufacturing ProcessesSameer PandeyNessuna valutazione finora
- KC28 6 1380Documento6 pagineKC28 6 1380JuavNessuna valutazione finora
- Patente Tratamiento de Condensando de AmonioDocumento6 paginePatente Tratamiento de Condensando de AmonioJesus PerezNessuna valutazione finora
- Fertilizer Technology Ammonia Production ProcessesDocumento29 pagineFertilizer Technology Ammonia Production ProcessesNitin HansaliaNessuna valutazione finora
- ChemistryDocumento1 paginaChemistryequilife.foundationNessuna valutazione finora
- Haber-Bosch Process PaperDocumento15 pagineHaber-Bosch Process Paperapi-529628802Nessuna valutazione finora
- 3.1 - Process and Technologies For Grass-Root Ammonia Plants - EnGDocumento21 pagine3.1 - Process and Technologies For Grass-Root Ammonia Plants - EnGHendriyana St0% (1)
- Obt AmoniacoDocumento16 pagineObt AmoniacoNatalia ParedesNessuna valutazione finora
- Production of Ammonia: Sunny ChawlaDocumento6 pagineProduction of Ammonia: Sunny ChawlapsshnkrNessuna valutazione finora
- RRL ProcessRationale Bello Ver1Documento1 paginaRRL ProcessRationale Bello Ver1Rexel ReedusNessuna valutazione finora
- Haber - Bosch ProcessDocumento12 pagineHaber - Bosch Processapi-487208181Nessuna valutazione finora
- AmmoniaDocumento46 pagineAmmoniabac_nobita7657Nessuna valutazione finora
- The Process of Manufacture of Urea in A Naphtha Based PlantDocumento4 pagineThe Process of Manufacture of Urea in A Naphtha Based PlantahmedkhidryagoubNessuna valutazione finora
- Equilibrium and Kinetic Studies on Urea Hydrolysis for Ammonia GenerationDocumento8 pagineEquilibrium and Kinetic Studies on Urea Hydrolysis for Ammonia Generationtamil vaananNessuna valutazione finora
- The Process of Making Ammonium NitrateDocumento11 pagineThe Process of Making Ammonium NitratelicrankyilNessuna valutazione finora
- New Microsoft Office Word DocumentDocumento5 pagineNew Microsoft Office Word DocumentSangam GuptaNessuna valutazione finora
- Urea - Organic compound used in fertilizersDocumento8 pagineUrea - Organic compound used in fertilizersNour Saad EdweekNessuna valutazione finora
- Remove sulfur from industrial gases using N-alkylated pyrrolidonesDocumento5 pagineRemove sulfur from industrial gases using N-alkylated pyrrolidonesNateek SharmaNessuna valutazione finora
- Production of Ammonia: Haber-Bosch ProcessDocumento3 pagineProduction of Ammonia: Haber-Bosch ProcessSk jahidul IslamNessuna valutazione finora
- 1996 CASALE HEtraysDocumento10 pagine1996 CASALE HEtraysFatima KazmiNessuna valutazione finora
- ChemistryDocumento1 paginaChemistryequilife.foundationNessuna valutazione finora
- Ullmann S Encyclopedia of Industrial Chemistry - 2011 - Appl - Ammonia 2 Production ProcessesDocumento88 pagineUllmann S Encyclopedia of Industrial Chemistry - 2011 - Appl - Ammonia 2 Production ProcessesJulius SuhermanNessuna valutazione finora
- Control of Evaporator in The Production of UreaDocumento15 pagineControl of Evaporator in The Production of Ureatariq fareedNessuna valutazione finora
- Controlling the evaporator in urea productionDocumento15 pagineControlling the evaporator in urea productiontariq fareedNessuna valutazione finora
- Urea Prilling Tower DustDocumento14 pagineUrea Prilling Tower DustRajeshkumar ElangoNessuna valutazione finora
- Wet Air OxidationDocumento16 pagineWet Air OxidationcharlesNessuna valutazione finora
- Nitrogen IndustriesDocumento22 pagineNitrogen IndustriesKarla Joy P. SucgangNessuna valutazione finora
- Process DescriptionDocumento9 pagineProcess DescriptionnoelNessuna valutazione finora
- Chemical Reaction Engineering: INTRODUCTION TO COMPANY (Pak American Fertilizers LTD.)Documento24 pagineChemical Reaction Engineering: INTRODUCTION TO COMPANY (Pak American Fertilizers LTD.)Badar RasheedNessuna valutazione finora
- Urea ProjectDocumento17 pagineUrea ProjectAbdo Shaaban100% (2)
- Urea Production Block Diagram ExplainedDocumento9 pagineUrea Production Block Diagram Explainedhimanshuchawla654Nessuna valutazione finora
- Ullmanns Enclopedia ChemistryDocumento36 pagineUllmanns Enclopedia ChemistryLutfiNessuna valutazione finora
- AmmoniaDocumento7 pagineAmmoniaAkshay SharmaNessuna valutazione finora
- Ammonia and Derivatives - Trans & Gas UsageDocumento17 pagineAmmonia and Derivatives - Trans & Gas UsageragilpriyantoNessuna valutazione finora
- Us Patent Process For Production of Urea, 1970Documento4 pagineUs Patent Process For Production of Urea, 197025A Syifa Salsabila AlfianiNessuna valutazione finora
- Chapter 3: Synthesis Gas Production and Derived ChemicalsDocumento78 pagineChapter 3: Synthesis Gas Production and Derived ChemicalsFadhli JapryNessuna valutazione finora
- Simulation Ammonia Plant On PRO IIDocumento58 pagineSimulation Ammonia Plant On PRO IIFabrizio Dugo100% (1)
- Fertilizer: Methods of Production: Anhydrous Ammonia (NH Raw Materials: HDocumento8 pagineFertilizer: Methods of Production: Anhydrous Ammonia (NH Raw Materials: HindumathijayakaranNessuna valutazione finora
- Simulation of Processes For Efficient Methanol Production Using Co and Solar EnergyDocumento6 pagineSimulation of Processes For Efficient Methanol Production Using Co and Solar EnergyaitormrNessuna valutazione finora
- Amoníaco A Partir de H2 VerdeDocumento10 pagineAmoníaco A Partir de H2 VerdeMariangel RicciardiNessuna valutazione finora
- The Catalytic Amination of AlcoholsDocumento12 pagineThe Catalytic Amination of AlcoholsClement CharlesNessuna valutazione finora
- Electrochemical Synthesis of Ammonia As ADocumento4 pagineElectrochemical Synthesis of Ammonia As ADung Phan Thị ThùyNessuna valutazione finora
- Elena Petricci and Maurizio Taddei - Microwave Assisted Reactions With Gas ReagentsDocumento5 pagineElena Petricci and Maurizio Taddei - Microwave Assisted Reactions With Gas ReagentsnnnnjwNessuna valutazione finora
- Urea HMDDocumento106 pagineUrea HMDAlmahadi AliasNessuna valutazione finora
- SS304 304L Data SheetDocumento2 pagineSS304 304L Data SheetPrakash KumarNessuna valutazione finora
- Flow Diagram of Urea Production Process From Ammonia and CarbonDocumento10 pagineFlow Diagram of Urea Production Process From Ammonia and CarbonGilang RamadhanNessuna valutazione finora
- ProgrammingKnowledge2 - YouTube PDFDocumento2 pagineProgrammingKnowledge2 - YouTube PDFpushprajNessuna valutazione finora
- UreaDocumento86 pagineUreaAdi Ahmad100% (1)
- Lab Sheet 1Documento2 pagineLab Sheet 1pushprajNessuna valutazione finora
- Basf Masterprotect 1812 Tds PDFDocumento2 pagineBasf Masterprotect 1812 Tds PDFHassan Ahmed Syed0% (1)
- Ie 12 Lec Act IiiDocumento2 pagineIe 12 Lec Act IiiLance JayomaNessuna valutazione finora
- M-DS31 Filcor Eps Data Sheet Cordek v1Documento2 pagineM-DS31 Filcor Eps Data Sheet Cordek v1ShamaNessuna valutazione finora
- H1 SteeringDocumento225 pagineH1 SteeringVinay Kumar NeelamNessuna valutazione finora
- Ductile-Brittle Transition Temperature and Impact Energy Tests - Yena EngineeringDocumento7 pagineDuctile-Brittle Transition Temperature and Impact Energy Tests - Yena EngineeringKASHFI UDDINNessuna valutazione finora
- 08 SpreaderDocumento58 pagine08 SpreaderMartin Vargas Pedro100% (1)
- MCP 101 Product Realization Lab ManualDocumento75 pagineMCP 101 Product Realization Lab ManualjasvindersinghsagguNessuna valutazione finora
- Dwnload Full Operations Management Sustainability and Supply Chain Management 12th Edition Heizer Test Bank PDFDocumento36 pagineDwnload Full Operations Management Sustainability and Supply Chain Management 12th Edition Heizer Test Bank PDFardellazusman100% (13)
- 12.4 Phase DiagramsDocumento6 pagine12.4 Phase DiagramsDr-SabaJamilNessuna valutazione finora
- DOE Guidance WBSDocumento20 pagineDOE Guidance WBSShowki WaniNessuna valutazione finora
- Advances in Thermochemical Conversion of Woody Biomass To Energy, Fuels and ChemicalsDocumento9 pagineAdvances in Thermochemical Conversion of Woody Biomass To Energy, Fuels and ChemicalsAsem AmairyhNessuna valutazione finora
- 6001 FluorescentLightFittings EK00 III en PDFDocumento9 pagine6001 FluorescentLightFittings EK00 III en PDFarturonc100% (1)
- Colt ShadovoltaicLouvreDocumento3 pagineColt ShadovoltaicLouvrePutri Astri NafisaNessuna valutazione finora
- Drying Shrinkage of ConcreteDocumento6 pagineDrying Shrinkage of Concretecrownguard100% (1)
- Blanco Ku 0099M 13094 DATA 1 PDFDocumento96 pagineBlanco Ku 0099M 13094 DATA 1 PDFCm EtcmNessuna valutazione finora
- Checklist of Material Submission (Concrete)Documento10 pagineChecklist of Material Submission (Concrete)Yau Ka Ki Jacky0% (1)
- MIDEL 7131 Technical Information Pack USDocumento15 pagineMIDEL 7131 Technical Information Pack USkatherine100% (1)
- SAB Flexible Cables C1Documento20 pagineSAB Flexible Cables C1Carlos OzaetaNessuna valutazione finora
- Fisherpaykel 4517554260 dh9060p 428210c Heat Pump Clothes Dryer User GuideDocumento40 pagineFisherpaykel 4517554260 dh9060p 428210c Heat Pump Clothes Dryer User Guidemember1000Nessuna valutazione finora
- Plastic Mixed Reinforced Concrete - BehaviourDocumento4 paginePlastic Mixed Reinforced Concrete - BehaviourThiaga RajanNessuna valutazione finora
- Vulcan LIBS Analyser For QA QC - 2Documento6 pagineVulcan LIBS Analyser For QA QC - 2Trần Văn LộcNessuna valutazione finora
- Rotho Peristaltic Pumps PDFDocumento40 pagineRotho Peristaltic Pumps PDFxxxxxxxxxxxxNessuna valutazione finora
- Lubrizol 219Documento2 pagineLubrizol 219BobNessuna valutazione finora
- Mini ProjectDocumento7 pagineMini ProjectSyakirin SpearsNessuna valutazione finora
- Manual 3 Full BrickDocumento25 pagineManual 3 Full BrickkeithjonathanNessuna valutazione finora
- Defogging rear window with thin heating elementDocumento3 pagineDefogging rear window with thin heating elementMuhammad MoollaNessuna valutazione finora
- Milling Concept MILL 450 enDocumento6 pagineMilling Concept MILL 450 enHeineken Ya PraneetpongrungNessuna valutazione finora
- Olive Oil InfrastructuresDocumento5 pagineOlive Oil InfrastructuresShahzad ShameemNessuna valutazione finora
- Brochure Steam AccumulatorDocumento2 pagineBrochure Steam AccumulatorFrank HuNessuna valutazione finora
- Technical Data Sheet Baltoflake EcolifeDocumento5 pagineTechnical Data Sheet Baltoflake EcolifeAkram AlhaddadNessuna valutazione finora
- The Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaDa EverandThe Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaNessuna valutazione finora
- Einstein's Fridge: How the Difference Between Hot and Cold Explains the UniverseDa EverandEinstein's Fridge: How the Difference Between Hot and Cold Explains the UniverseValutazione: 4.5 su 5 stelle4.5/5 (50)
- The Fabric of Civilization: How Textiles Made the WorldDa EverandThe Fabric of Civilization: How Textiles Made the WorldValutazione: 4.5 su 5 stelle4.5/5 (57)
- Sully: The Untold Story Behind the Miracle on the HudsonDa EverandSully: The Untold Story Behind the Miracle on the HudsonValutazione: 4 su 5 stelle4/5 (103)
- The Technology Trap: Capital, Labor, and Power in the Age of AutomationDa EverandThe Technology Trap: Capital, Labor, and Power in the Age of AutomationValutazione: 4.5 su 5 stelle4.5/5 (46)
- A Place of My Own: The Architecture of DaydreamsDa EverandA Place of My Own: The Architecture of DaydreamsValutazione: 4 su 5 stelle4/5 (241)
- Pale Blue Dot: A Vision of the Human Future in SpaceDa EverandPale Blue Dot: A Vision of the Human Future in SpaceValutazione: 4.5 su 5 stelle4.5/5 (586)
- Highest Duty: My Search for What Really MattersDa EverandHighest Duty: My Search for What Really MattersNessuna valutazione finora
- The Weather Machine: A Journey Inside the ForecastDa EverandThe Weather Machine: A Journey Inside the ForecastValutazione: 3.5 su 5 stelle3.5/5 (31)
- Transformed: Moving to the Product Operating ModelDa EverandTransformed: Moving to the Product Operating ModelValutazione: 4 su 5 stelle4/5 (1)
- Faster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestDa EverandFaster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestValutazione: 4 su 5 stelle4/5 (28)
- 35 Miles From Shore: The Ditching and Rescue of ALM Flight 980Da Everand35 Miles From Shore: The Ditching and Rescue of ALM Flight 980Valutazione: 4 su 5 stelle4/5 (21)
- The Future of Geography: How the Competition in Space Will Change Our WorldDa EverandThe Future of Geography: How the Competition in Space Will Change Our WorldValutazione: 4.5 su 5 stelle4.5/5 (4)
- A Garden of Marvels: How We Discovered that Flowers Have Sex, Leaves Eat Air, and Other Secrets of PlantsDa EverandA Garden of Marvels: How We Discovered that Flowers Have Sex, Leaves Eat Air, and Other Secrets of PlantsNessuna valutazione finora
- Recording Unhinged: Creative and Unconventional Music Recording TechniquesDa EverandRecording Unhinged: Creative and Unconventional Music Recording TechniquesNessuna valutazione finora
- Data-ism: The Revolution Transforming Decision Making, Consumer Behavior, and Almost Everything ElseDa EverandData-ism: The Revolution Transforming Decision Making, Consumer Behavior, and Almost Everything ElseValutazione: 3.5 su 5 stelle3.5/5 (12)
- The End of Craving: Recovering the Lost Wisdom of Eating WellDa EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellValutazione: 4.5 su 5 stelle4.5/5 (80)
- Packing for Mars: The Curious Science of Life in the VoidDa EverandPacking for Mars: The Curious Science of Life in the VoidValutazione: 4 su 5 stelle4/5 (1395)
- Dirt to Soil: One Family’s Journey into Regenerative AgricultureDa EverandDirt to Soil: One Family’s Journey into Regenerative AgricultureValutazione: 5 su 5 stelle5/5 (124)
- Across the Airless Wilds: The Lunar Rover and the Triumph of the Final Moon LandingsDa EverandAcross the Airless Wilds: The Lunar Rover and the Triumph of the Final Moon LandingsNessuna valutazione finora
- ChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindDa EverandChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindNessuna valutazione finora
- The Things We Make: The Unknown History of Invention from Cathedrals to Soda CansDa EverandThe Things We Make: The Unknown History of Invention from Cathedrals to Soda CansNessuna valutazione finora
- Reality+: Virtual Worlds and the Problems of PhilosophyDa EverandReality+: Virtual Worlds and the Problems of PhilosophyValutazione: 4 su 5 stelle4/5 (24)
- Artificial Intelligence: A Guide for Thinking HumansDa EverandArtificial Intelligence: A Guide for Thinking HumansValutazione: 4.5 su 5 stelle4.5/5 (30)
- Broken Money: Why Our Financial System is Failing Us and How We Can Make it BetterDa EverandBroken Money: Why Our Financial System is Failing Us and How We Can Make it BetterValutazione: 5 su 5 stelle5/5 (3)