Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Bronchopulmonary Dysplasia
Caricato da
le0331Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Bronchopulmonary Dysplasia
Caricato da
le0331Copyright:
Formati disponibili
CASE REPORT
Lung volume reduction surgery in preterm infants with
bronchopulmonary dysplasia. A case report
rgen Bauer3, Lars D. Berthold4, Dirk Faas1 &
Jan de Laffolie1, Markus Hirschburger2, Ju
5
Matthias Heckmann
1
Department
Department
3
Department
4
Department
5
Department
2
General Pediatrics and Neonatology, Childrens Hospital, University Giessen, Giessen, Germany
General and Pediatric Surgery, University Hospital Giessen, Giessen, Germany
Pediatric Cardiology, Childrens Hospital, University Giessen, Giessen, Germany
Diagnostic and Interventional Radiology, Medical School, Hannover, Germany
Neonatology and Pediatric Intensive Medicine, University Greifswald,Greifswald, Germany
Correspondence
Jan de Laffolie, Childrens Hospital, University
Giessen, Feulgenstr 14, 35390 Giessen,
Germany. Tel: +49 (0)641 98543400;
Fax: +49 (0)641 985 43; E-mail:
jan.delaffolie@paediat.med.uni-giessen.de
Funding Information
No funding information provided.
Key Clinical Message
A preterm infant at the age of 9 months with severe bronchopulmonary dysplasia (BPD) and large lobar emphysema, compromising ventilation into adjacent
lobes with respiratory failure under maximal conservative treatment and
pulmonary arterial hypertension recovered initially well after bilateral lung volume reduction surgery, but progressed 2 years later into respiratory failure. The
initial imaging with Magnetic-Resonance-Imaging (MRI)-Angiography and
decision-making was difficult and interdisciplinary treatment was essential.
Received: 17 April 2013; Revised: 21 August
2013; Accepted: 21 September 2013
Keywords
Clinical Case Reports 2013; 1(2): 9699
Bronchopulmonary dysplasia, emphysema, prematurity, surgery.
doi: 10.1002/ccr3.35
Introduction
Patient
Some preterm infants with bronchopulmonary dysplasia
(BPD) develop acquired lobar emphysema (ALE) with
high mortality and morbidity [1, 2].
Currently, there is no satisfactory way to improve
lobar emphysema in infants, especially when the
formation of bullae has lead to mechanical problems in
ventilation, pulmonary hypertension, and compresses
adjacent lobes. In severe emphysema in adults, one of
the most promising approaches is lung volume
reduction surgery (LVRS), in which large bullae
or lobar emphysema is resected to allow adjacent lung
tissue to expand and improve respiratory function
[3].
Experiences with children are limited; cases are
reported of successful LVRS for interstitial emphysema
[4] and bullae after congenital diaphragmatic hernia
(CDH) [5].
In addition to a case we reported earlier [6] of a female
patient who was successfully treated with surgical lung
volume reduction, we would like to share a recent case
that was referred to us at 9 months of age, much later
than our previous experiences.
The patient was born at 25 + 1 weeks of gestation with a
birth weight of 790 g after premature rupture of membrane. Initially, he presented with second-degree respiratory distress syndrome and pronounced pulmonary
hypertension. After standard initial treatment including
surfactant twice, he was treated with high-frequency oscillation (HFO) for 12 days, repeated surfactant administration, and inhaled nitric oxide (iNO, max 20 ppm).
Extubation was accomplished on the 37th day of life.
He was discharged at the age of 6 months with home
oxygen 0.21 L/min via nasal insufflation. To quantify
pulmonary hypertension, a catheter investigation was performed at the age of 7 months. A pronounced pulmonary
artery hypertension (pulmonary artery pressure 42/
16 mmHg, 2/3 systemic pressure under FiO2 100%) was
found, which was not improved by NO or Ilomedin (pulmonary vascular resistance was 7.8 WExm).
96
Result/Course at Our Hospital
After referral (pCO2 85 mmHg, 45 L O2/min), the
patient was stabilized with diuretics (furosemide,
2013 The Authors. Clinical Case Reports published by John Wiley & Sons Ltd.
This is an open access article under the terms of the Creative Commons Attribution-NonCommercial License, which permits use,
distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
J. de Laffolie et al.
thiazide), sedation, theophylline, inhaled iloprost, inhalative bronchodilators, and systemic glucocorticoids. Chest
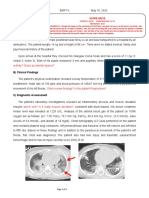
radiographs revealed two massive bullae in both lower
lobes (Fig. 1).
The decision for LVRS on the right side was interdisciplinary, based on computer tomography (CT) and MR
Angiography, because conservative prognosis was limited.
Informed consent was obtained and the situation was
explained in detail to the parents.
Spiral CT showed 50% emphysematous deformations,
20% compressed, and 30% normal parts of the right lung,
and 40% emphysematous deformations, 20% compressed,
and 40% normal parts of the left lung, respectively
(Fig. 2). MR angiography confirmed the CT findings, that
is, an impaired perfusion of the right lower lobe
(Fig. 3A).
As a first step, an atypical lower lobe resection on the
right side was performed via lateral thoracotomy. Intraoperatively, the middle lobe seemed normal. The upper lobe
was compressed, but extended after resection of the bulla
in the lower lobe. Extubation on day 3 was successful
after a short course of iNO.
In between surgeries, we established full enteral feeding
with good weight gain (catch up growth to 10th percentile and improvement of psychomotor development and
minimal oxygen requirement). In the following echocardiography, signs of pulmonary hypertension persisted.
LVRS in preterms with BPD
Four weeks after the first surgery, atypical excision of
the bulla in the left lower lobe was performed without
complications. A postoperative MRI showed good ventilation of all lobes, but a reappearance of bullae in the right
lower lobe (Fig. 3).
We could discharge the patient with minimal oxygen
supplementation (0.10.2 L/min) and good enteral feeding partially via percutaneous gastrostomy catheter leading to weight gain and growth parallel to the 10th
percentile. Neurobehavioral development was assessed by
Griffith Score at age of 14 months: motor skills, personal/
social, auditory/voice 8 months, and general performance
78 months.
(A)
(B)
Figure 1. Initial chest X-ray. Massive deformed thorax with dominant
lower lobe bullae.
2013 The Authors. Clinical Case Reports published by John Wiley & Sons Ltd.
Figure 2. (A/B) Preoperative HRCT. Huge bullae bilaterally in lower
lobes with compressed adjacent lung tissue, atelectasis, and
herniation of left lung toward right.
97
J. de Laffolie et al.
LVRS in preterms with BPD
(A)
(B)
Figure 4. CT on readmission in acute infectious exacerbation.
escalation. The patient died in the arms of his parents
4 weeks after admission to the ICU.
Comment
Figure 3. (A/B) MRI angiography with contrast preoperative/
postoperative. In comparison, better perfusion on left, but recurrent
bulla in right lower lobe.
Eighteen months after discharge from the hospital and
outpatient follow-up, the patient presented again with
acute infectious exacerbation and respiratory failure. On
initial CT, a recurrence of large emphysematous areas in
the right lower lobe was diagnosed with compression of
upper lobe tissue (Fig. 4).
The patients clinical situation deteriorated under maximal conservative therapy and the parents decided together
with the team of our ICU to limit further therapeutic
98
LVRS has posed a therapeutic option for severe ALE
when conservative treatment does not yield satisfactory
results. We reported a case at our hospital that was treated with LVRS successfully 10 years ago and is still well
on follow-up with perfectly normal development [6].
In adults, successful studies were undertaken to define
specific criteria to identify patients who benefit from
LVRS [7, 8]. So far, there are no criteria to identify
infants that benefit from surgery. Due to structural lung
development within the first 2-(4-6) years of life, surgery
should probably not be withheld too long to provide time
for pulmonary adaptation [9].
Initial imaging was difficult; high-resolution CT has
been shown to be weakly predictive for postoperative
outcome [10] and ventilationperfusion scans have also
been used with moderate predictive value in adults and
even neonates [11]. In the pediatric population, MRI has
been discussed as gold-standard imaging of bronchopulmonary dysplasia, providing additional valuable information [12].
Due to the small number of patients, it will be hard to
implement good studies to evaluate patients with deterioration under maximal conservative therapy for surgery,
but indications for LVRS in infants need to be defined
and cases like this need to be communicated.
2013 The Authors. Clinical Case Reports published by John Wiley & Sons Ltd.
J. de Laffolie et al.
Conflict of Interest
None declared.
References
1. Azizkhan, R. G., D. L. Grimmer, F. B. Askin, S. R. Lacey,
D. F. Merten, and R. E. Wood. 1992. Acquired lobar
emphysema (overinflation): clinical and pathological
evaluation of infants requiring lobectomy. J. Pediatr. Surg.
27:11451151; discussion 11511152.
2. Wong, P. M., A. N. Lees, J. Louw, F. Y. Lee, N. French,
K. Gain, et al. 2008. Emphysema in young adult survivors
of moderate-to-severe bronchopulmonary dysplasia. Eur.
Respir. J. 32:321328.
3. Criner, G. J., and A. J. Mamary. 2010. Lung volume
reduction surgery and lung volume reduction in advanced
emphysema: who and why? Semin. Respir. Crit. Care Med.
31:348364.
4. Messineo, A., F. Fusaro, G. Mognato, M. Sabatti, E. S.
DAmore, and M. Guglielmi. 2001. Lung volume reduction
surgery in lieu of pneumonectomy in an infant with severe
unilateral pulmonary interstitial emphysema. Pediatr.
Pulmonol. 31:389393.
5. Yonekura, T., S. Hirooka, A. Kubota, M. Hoki, T. Kosumi,
K. Yamauchi, et al. 2000. Surgical intervention for
emphysematous pulmonary regions in a postoperative
infant with congenital diaphragmatic hernia. J. Pediatr.
Surg. 35:18201821.
2013 The Authors. Clinical Case Reports published by John Wiley & Sons Ltd.
LVRS in preterms with BPD
6. Siaplaouras, J., M. Heckmann, I. Reiss, T. Schaible, K. L.
Waag, and L. Gortner. 2003. Lung volume reduction
surgery in bronchopulmonary dysplasia. Acta Paediatr.
92:754756.
7. Ginsburg, M. E., B. M. Thomashow, C. K. Yip, A. M.
Dimango, R. A. Maxfield, M. N. Bartels, et al. 2011. Lung
volume reduction surgery using the NETT selection
criteria. Ann. Thorac. Surg. 91:15561561.
8. Berger, R. L., M. M. Decamp, G. J. Criner, and B. R. Celli.
2010. Lung volume reduction therapies for advanced
emphysema: an update. Chest 138:407417.
9. Burri, P. H.. 2006. Structural aspects of postnatal lung
development alveolar formation and growth. Biol.
Neonate 89:313322.
10. Washko, G. R., F. J. Martinez, E. A. Hoffman, S. H.
Loring, R. S. Estepar, A. A. Diaz, et al. 2010. Physiological
and computed tomographic predictors of outcome from
lung volume reduction surgery. Am. J. Respir. Crit. Care
Med. 181:494500.
11. Leonidas, J. C., F. M. Moylan, P. C. Kahn, and M. L.
Ramenofsky. 1978. Ventilation-perfusion scans in neonatal
regional pulmonary emphysema complicating ventilatory
assistance. AJR Am. J. Roentgenol. 131:243246.
12. Ley-Zaporozhan, J., M. Puderbach, and H. U. Kauczor.
2008. MR for the evaluation of obstructive pulmonary
disease. Magn. Reson. Imaging Clin. N. Am. 16:291308,
ix.
99
Potrebbero piacerti anche
- 500 FullDocumento4 pagine500 FullAina NurlailaNessuna valutazione finora
- Crit Care Med.: Krause@kkl200.ukl - Uni-Freiburg - de Back To TopDocumento11 pagineCrit Care Med.: Krause@kkl200.ukl - Uni-Freiburg - de Back To TopTharshini_Indr_6713Nessuna valutazione finora
- Recurrent Pneumonia Caused by Rare Granular Cell TumorDocumento7 pagineRecurrent Pneumonia Caused by Rare Granular Cell TumorJoshua MendozaNessuna valutazione finora
- Flail Chest Part I: Case Report Review A) Patient InformationDocumento5 pagineFlail Chest Part I: Case Report Review A) Patient InformationAllan Castro100% (1)
- Chest Physiotherapy in Mechanically Ventilated Children A ReviewDocumento10 pagineChest Physiotherapy in Mechanically Ventilated Children A Reviewmrizki_1Nessuna valutazione finora
- Bronko Dislasia RadiologiDocumento4 pagineBronko Dislasia RadiologiAndi Wulandari DjoyosoemartoNessuna valutazione finora
- Case 25-2020: A 47-Year-Old Woman With A Lung Mass: Case Records Massachusetts General HospitalDocumento10 pagineCase 25-2020: A 47-Year-Old Woman With A Lung Mass: Case Records Massachusetts General HospitalClinica CorominasNessuna valutazione finora
- Dyspnea in PregnancyDocumento5 pagineDyspnea in PregnancyMelati Dwi PutriNessuna valutazione finora
- Foreign Body Aspiration in Children-A Diagnostic ChallengeDocumento3 pagineForeign Body Aspiration in Children-A Diagnostic ChallengeViany RehansyahNessuna valutazione finora
- Seear 2017Documento8 pagineSeear 2017Lucia NiculaeNessuna valutazione finora
- Anesthetic Management of A Case of Congenital Diaphragmatic Hernia Delayed DiagnosisDocumento3 pagineAnesthetic Management of A Case of Congenital Diaphragmatic Hernia Delayed DiagnosisFhomensNessuna valutazione finora
- 09-1434 FinallDocumento2 pagine09-1434 FinallRemy SummersNessuna valutazione finora
- Rare Bilateral Diaphragmatic Eventration Anomaly with Complex CareDocumento13 pagineRare Bilateral Diaphragmatic Eventration Anomaly with Complex CareTommyNessuna valutazione finora
- Neu Motor AxDocumento6 pagineNeu Motor AxZay SiagNessuna valutazione finora
- Breathless: Clinical Problem-SolvingDocumento7 pagineBreathless: Clinical Problem-SolvingLarisa ZamfirNessuna valutazione finora
- Indian J Radiol Imaging Word 1Documento14 pagineIndian J Radiol Imaging Word 1Adie BrianNessuna valutazione finora
- Pulmonary Tuberculosis Literature ReviewDocumento5 paginePulmonary Tuberculosis Literature Reviewafdtvwccj100% (1)
- Clearing The Air - A Conservative Option For Spontaneous PneumothoraxDocumento2 pagineClearing The Air - A Conservative Option For Spontaneous PneumothoraxDivara SyautaNessuna valutazione finora
- Congenital Lobar EmphysemaDocumento4 pagineCongenital Lobar EmphysemaIjahss JournalNessuna valutazione finora
- Case Report: Hydropneumothorax in Children: A Rare Complication of A Bacterial PneumoniaDocumento5 pagineCase Report: Hydropneumothorax in Children: A Rare Complication of A Bacterial PneumoniaNadya NovianiNessuna valutazione finora
- 1 LaryngoDocumento5 pagine1 LaryngoRanjum ChaudharyNessuna valutazione finora
- Early Intervention in AIP Improves OutcomesDocumento9 pagineEarly Intervention in AIP Improves OutcomesHerbert Baquerizo VargasNessuna valutazione finora
- Non Small-Cell Lung Cancer in A 15-Year-Old NonsmokerDocumento2 pagineNon Small-Cell Lung Cancer in A 15-Year-Old Nonsmokertonirian99Nessuna valutazione finora
- Running Head: Client Case Study 1Documento14 pagineRunning Head: Client Case Study 1api-283774863Nessuna valutazione finora
- Giant Pulmonary Bullae in Children: Journal of Pediatric Surgery Case Reports July 2020Documento7 pagineGiant Pulmonary Bullae in Children: Journal of Pediatric Surgery Case Reports July 2020SOPHIASTIA KUSBIANTI MHS 2017Nessuna valutazione finora
- BPJ Vol 10 No 3 P 1369-1377Documento9 pagineBPJ Vol 10 No 3 P 1369-1377citra annisa fitriNessuna valutazione finora
- Simultaneous Bilateral Primary Spontaneous Pneumothorax (2019)Documento7 pagineSimultaneous Bilateral Primary Spontaneous Pneumothorax (2019)sandi_prawira_yudhaNessuna valutazione finora
- Large Pulmonary Solitary Mass Caused by Mycobacterium Tuberculosis Mimicking A Malignant Tumor in A ChildDocumento4 pagineLarge Pulmonary Solitary Mass Caused by Mycobacterium Tuberculosis Mimicking A Malignant Tumor in A Childadipspain981Nessuna valutazione finora
- Case ARDSDocumento16 pagineCase ARDSAida SiregarNessuna valutazione finora
- Nej M CPC 1109276Documento11 pagineNej M CPC 1109276Connie Raina CarissaNessuna valutazione finora
- Paclitaxel-Induced Pneumonitis in PatientsDocumento5 paginePaclitaxel-Induced Pneumonitis in PatientsAndiie ResminNessuna valutazione finora
- Diaphragmatic Eventration Misdiagnosed As Diaphragmatic Hernia in A Preterm Infant With Respiratory Distress: A Case Report and Review of Diagnosis and ManagementDocumento4 pagineDiaphragmatic Eventration Misdiagnosed As Diaphragmatic Hernia in A Preterm Infant With Respiratory Distress: A Case Report and Review of Diagnosis and ManagementPeertechz Publications Inc.Nessuna valutazione finora
- Dyke 2023 Ped Radiol PREFUL Lung MRI Ventilation NICUDocumento9 pagineDyke 2023 Ped Radiol PREFUL Lung MRI Ventilation NICUJonathan DykeNessuna valutazione finora
- Case Report: Systemic Steroid Treatment For Severe Expanding Pneumococcal PneumoniaDocumento4 pagineCase Report: Systemic Steroid Treatment For Severe Expanding Pneumococcal PneumoniaadhiniNessuna valutazione finora
- Sep 1, 2011) Journal: Medscape Radiology: Radiological Society of North AmericaDocumento19 pagineSep 1, 2011) Journal: Medscape Radiology: Radiological Society of North AmericaMohamad Rizki DwikaneNessuna valutazione finora
- Conflicts of Interest: Spontaneous Pneumomediastinum in Pregnancy: A Case ReportDocumento3 pagineConflicts of Interest: Spontaneous Pneumomediastinum in Pregnancy: A Case ReportRika PutriNessuna valutazione finora
- Radiological Findings of Pulmonary Tuberculosis in Infants and Young ChildrenDocumento4 pagineRadiological Findings of Pulmonary Tuberculosis in Infants and Young ChildrenEva ZheichuaNessuna valutazione finora
- P ('t':3) Var B Location Settimeout (Function (If (Typeof Window - Iframe 'Undefined') (B.href B.href ) ), 2000)Documento9 pagineP ('t':3) Var B Location Settimeout (Function (If (Typeof Window - Iframe 'Undefined') (B.href B.href ) ), 2000)zano_adamNessuna valutazione finora
- A 56 Year Old Man With Chronic Cough, HemoptysisDocumento4 pagineA 56 Year Old Man With Chronic Cough, HemoptysisagamerocallejasNessuna valutazione finora
- 1 PBDocumento9 pagine1 PBMariiaNessuna valutazione finora
- MedicinaDocumento8 pagineMedicinaJose Juan Gómez RamosNessuna valutazione finora
- Therapeutic Hypothermia For Acute Air Embolic Stroke: ASE EportDocumento3 pagineTherapeutic Hypothermia For Acute Air Embolic Stroke: ASE EportTeuku M. FebriansyahNessuna valutazione finora
- Emergencias Respiratorias Pediatricas PDFDocumento20 pagineEmergencias Respiratorias Pediatricas PDFMarco Antonio Mendoza OjedaNessuna valutazione finora
- Jurnal 7 BronkiolitisDocumento7 pagineJurnal 7 BronkiolitisIrsanti sasmitaNessuna valutazione finora
- Phrenic Nerve Palsy: A Rare Cause of Respiratory Distress in NewbornDocumento5 paginePhrenic Nerve Palsy: A Rare Cause of Respiratory Distress in NewbornIJAR JOURNALNessuna valutazione finora
- Inadvertent Chest Tube Insertion in Congential Cystic Adenomatoid Malformation and Congential Lobar Emphysema Highlighting An Important ProblemDocumento0 pagineInadvertent Chest Tube Insertion in Congential Cystic Adenomatoid Malformation and Congential Lobar Emphysema Highlighting An Important ProblemWenny EudensiaNessuna valutazione finora
- Chest Radiography For Children With Pneumonia: A Century of Folly?Documento2 pagineChest Radiography For Children With Pneumonia: A Century of Folly?Ardi WidiatmikaNessuna valutazione finora
- Tuberculosis Masquerading As Recurrent Metastatic Carcinoma of The CervixDocumento2 pagineTuberculosis Masquerading As Recurrent Metastatic Carcinoma of The Cervixpaijosuseno2242Nessuna valutazione finora
- J CLLC 2020 06 021Documento16 pagineJ CLLC 2020 06 021dr. Amori Amar LakcandraNessuna valutazione finora
- Fibrinolytic Treatment of Complicated Pediatric TDocumento2 pagineFibrinolytic Treatment of Complicated Pediatric TDR GURDEEP SINGHNessuna valutazione finora
- Pyothorax-Associated Lymphoma: A Case Report and ReviewDocumento10 paginePyothorax-Associated Lymphoma: A Case Report and ReviewSoukaina SoukainaNessuna valutazione finora
- JACOB 1998_Long-term pulmonary sequelae of severe bronchopulmonary dysplasiaDocumento8 pagineJACOB 1998_Long-term pulmonary sequelae of severe bronchopulmonary dysplasiaRafael JustinoNessuna valutazione finora
- Mediastinal Tuberculous Lymphadenitis Presenting With Insidious Back Pain in A Male Adult: A Case Report and Review of The LiteratureDocumento7 pagineMediastinal Tuberculous Lymphadenitis Presenting With Insidious Back Pain in A Male Adult: A Case Report and Review of The Literaturez9tpwgb5nwNessuna valutazione finora
- ICU JournalDocumento4 pagineICU Journal2080315Nessuna valutazione finora
- Chest Radiographic Findings in Primary Pulmonary Tuberculosis: Observations From High School OutbreaksDocumento6 pagineChest Radiographic Findings in Primary Pulmonary Tuberculosis: Observations From High School OutbreaksDeeny PukidNessuna valutazione finora
- Pneumothorax and PregnancyDocumento5 paginePneumothorax and PregnancyFormeds AzkasepyasNessuna valutazione finora
- Acute Hypotonia in An Infant (2017)Documento3 pagineAcute Hypotonia in An Infant (2017)nikos.alexandrNessuna valutazione finora
- Jurnal Bronchitis Dengan AsmaDocumento5 pagineJurnal Bronchitis Dengan AsmaMauLan SaputraNessuna valutazione finora
- Absceso Esplenico 1Documento2 pagineAbsceso Esplenico 1Luis Alfredo LucioNessuna valutazione finora
- Laryngopharyngeal Reflux Disease: Integrative ApproachesDa EverandLaryngopharyngeal Reflux Disease: Integrative ApproachesNausheen JamalNessuna valutazione finora
- Introduction To Pharmacy ManagementDocumento28 pagineIntroduction To Pharmacy ManagementSirry HidayaniNessuna valutazione finora
- Netscaler 10 With Citrix Triscale™ Technology: Download This SlideDocumento40 pagineNetscaler 10 With Citrix Triscale™ Technology: Download This SlidePhong TrầnNessuna valutazione finora
- How To Love The LORD With All Your Heart, Soul, and StrengthDocumento5 pagineHow To Love The LORD With All Your Heart, Soul, and StrengthGodmadeMusic100% (1)
- 1st Part PALIAL CasesDocumento255 pagine1st Part PALIAL CasesAnonymous 4WA9UcnU2XNessuna valutazione finora
- DC-DC Converter Reliability Design and TestingDocumento16 pagineDC-DC Converter Reliability Design and TestinggirisanaNessuna valutazione finora
- Making Hand Sanitizer from Carambola FruitDocumento5 pagineMaking Hand Sanitizer from Carambola FruitMary grace LlagasNessuna valutazione finora
- GUINNESS F13 Full Year BriefingDocumento27 pagineGUINNESS F13 Full Year BriefingImoUstino ImoNessuna valutazione finora
- Current Communist CountriesDocumento4 pagineCurrent Communist CountriesJJ MGNessuna valutazione finora
- VA anesthesia handbook establishes guidelinesDocumento11 pagineVA anesthesia handbook establishes guidelinesapierwolaNessuna valutazione finora
- Physics 5th Edition Walker Test BankDocumento24 paginePhysics 5th Edition Walker Test BankKathyHernandeznobt100% (31)
- Coursework of Signals and Systems: Moh. Kamalul Wafi December 6, 2018Documento2 pagineCoursework of Signals and Systems: Moh. Kamalul Wafi December 6, 2018kartiniNessuna valutazione finora
- Sigram Schindler Beteiligungsgesellschaft PetitionDocumento190 pagineSigram Schindler Beteiligungsgesellschaft PetitionjoshblackmanNessuna valutazione finora
- Outlook Business The Boss July 2015Documento14 pagineOutlook Business The Boss July 2015Nibedita MahatoNessuna valutazione finora
- The Bible in Picture and Story (1889)Documento250 pagineThe Bible in Picture and Story (1889)serjutoNessuna valutazione finora
- 9 Oet Reading Summary 2.0-195-213Documento19 pagine9 Oet Reading Summary 2.0-195-213Vijayalakshmi Narayanaswami0% (1)
- Paradine V Jane - (1646) 82 ER 897Documento2 pagineParadine V Jane - (1646) 82 ER 897TimishaNessuna valutazione finora
- Dialnet AnalysingCommonMistakesInTranslationsOfTouristText 4419765 PDFDocumento16 pagineDialnet AnalysingCommonMistakesInTranslationsOfTouristText 4419765 PDFEquipo CuatroNessuna valutazione finora
- Fourth Edition Hungarian WordlistDocumento12 pagineFourth Edition Hungarian WordlistMarton HorvathNessuna valutazione finora
- Scada Programable Logic Control MCQDocumento2 pagineScada Programable Logic Control MCQAbhigya Bhatnagar85% (13)
- DTF - Houses of The FallenDocumento226 pagineDTF - Houses of The FallenShuang Song100% (1)
- MOH Formulary Drug List 2014Documento115 pagineMOH Formulary Drug List 2014mahmud000Nessuna valutazione finora
- AffirmativedefensemotorvehicleDocumento3 pagineAffirmativedefensemotorvehicleKevinNessuna valutazione finora
- Topic 5 English Sound SystemDocumento7 pagineTopic 5 English Sound SystemAnonymous G91XTp9Nessuna valutazione finora
- Karst RomaniaDocumento7 pagineKarst Romaniaproconstruct0% (1)
- Crafting Your Keto Diet MindsetDocumento3 pagineCrafting Your Keto Diet MindsetoculosoakleyNessuna valutazione finora
- Allosteric EnzymeDocumento22 pagineAllosteric EnzymeAhmed ImranNessuna valutazione finora
- Conquest of The Americas (Eakin-2002)Documento81 pagineConquest of The Americas (Eakin-2002)GregNessuna valutazione finora
- Reserts en MatDocumento20 pagineReserts en MatJohn Gabriel AlmeroNessuna valutazione finora
- New Technology To Reduce Yarn WastageDocumento3 pagineNew Technology To Reduce Yarn WastageDwi Fitria ApriliantiNessuna valutazione finora
- Number System ConversionDocumento26 pagineNumber System ConversionMahesh Chandra UpadhyayNessuna valutazione finora