Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
1 s2.0 S0891584903005379 Main
Caricato da
mfhfhfTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
1 s2.0 S0891584903005379 Main
Caricato da
mfhfhfCopyright:
Formati disponibili
Free Radical Biology & Medicine, Vol. 35, No. 11, pp.
1448 1452, 2003
Copyright 2003 Elsevier Inc.
Printed in the USA. All rights reserved
0891-5849/03/$see front matter
doi:10.1016/j.freeradbiomed.2003.08.001
Original Contribution
EFFECT OF CIGARETTE SMOKE ON ORAL PEROXIDASE ACTIVITY IN
HUMAN SALIVA: ROLE OF HYDROGEN CYANIDE
IFAT KLEIN,* RAFAEL M. NAGLER,*, RUTH TOFFLER,* ALBERT
VAN
DER VLIET,
and
ABRAHAM Z. REZNICK*
*Department of Anatomy and Cell Biology, Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa,
Israel; Laboratory of Oral Biochemistry, Rambam Medical Center, Haifa, Israel; and Department of Pathology, University of
Vermont, Burlington, VT, USA
(Received 16 May 2003; Revised 12 August 2003; Accepted 14 August 2003)
AbstractPeroxidase activity in human saliva is composed of salivary peroxidase (80%), of salivary glandular origin,
and myeloperoxidase (20%), of leukocyte origin. The term oral peroxidase (OPO) is used here to denote the total
activity of both peroxidase species. Using the 2-nitrobenzoic acid-thiocyanate assay, OPO activity was measured in the
saliva of nonsmokers after exposure to gas-phase cigarette smoke (CS) in an in vitro system using three puffs of CS in
1 h. A marked decrease of 76% of activity was observed following three puffs of CS. In order to elucidate the
mechanism by which CS caused loss of OPO activity, several oxidants and antioxidants were applied to saliva in vitro
in the presence and absence of CS. No protection for CS-induced loss of OPO activity occurred in the presence of
glutathione, N-acetylcysteine, ascorbic acid, or Desferal. Exposure of saliva to purified aldehydes present in CS did not
significantly affect OPO loss of activity. Similarly, ascorbic acid in the presence of FeCl3 and nicotine also had no effect
on OPO activity. Exposure of OPO to cyanate at levels present in CS caused a 6570% loss of OPO activity, which was
reversible after 24 h of dialysis. Moreover, hydroxocobalamin, a known cyanate chelator, could prevent CS- and
potassium cyanide-induced inactivation of OPO by 70 90%. The results show that hydrogen cyanide, known to be
present in microgram amounts per cigarette, is likely to be the species in CS responsible for loss of salivary OPO
activity. The finding of reduced salivary OPO levels after CS exposure may represent a contributory mechanism for
CS-related compromises in antimicrobial defenses in the aerodigestive tract. 2003 Elsevier Inc.
KeywordsCigarette smoke, Hydrogen cyanide, Hydroxocobalamin, Oral peroxidase, Free radicals
INTRODUCTION
MPO, produced mainly by leukocytes, contributes the
remaining 20 40% of OPO activity [3]. SPO has been
shown to decompose the H2O2 produced by oral bacteria,
while the product of SPO activity, hypothiocyanite ion
(OSCN), is instrumental in killing oral bacteria by
inactivating bacterial glycolytic enzymes such as hexokinase, aldolase, and pyruvate kinase [4 7].
In previous studies we showed that exposure of
plasma to CS resulted in protein modifications as measured by increased protein carbonyls [8] and in oxidation
of plasma lipids and antioxidants [9]. The source of the
accumulation of protein carbonyls was attributed mainly
to the volatile aldehydes present in CS [10]. In more
recent studies, we obtained similar results when human
saliva was exposed to CS in vitro [11]. In addition, we
showed that several salivary enzymes such as amylase,
lactate dehydrogenase (LDH), and acid phosphatase
were considerably affected by CS, while CS-based aldehydes, such as acrolein and crotonaldehyde, as well as
Saliva is a unique fluid, as it is the first body fluid to
encounter the exogenous materials, gases, and fluids that
penetrate the human body [1]. Therefore, nature has
endowed saliva with multiple defense systems, the most
important of which are the immunological and antioxidant defense systems. One of the most important components of the salivary antioxidant system, as well as
being an essential ingredient in the antibacterial system,
is the enzyme salivary peroxidase (SPO), which, together
with myeloperoxidase (MPO), comprises oral peroxidase
activity (OPO). SPO, secreted from the major salivary
glands [2], contributes 60 80% of OPO activity, while
Address correspondence to: Abraham Z. Reznick, Department of
Anatomy and Cell Biology, The Bruce Rappaport Faculty of Medicine,
Technion-Israel Institute of Technology, P.O. Box 9649, 31096 Haifa,
Israel; Tel: 972 (4) 829-5388; Fax: 972 (4) 829-5403; E-Mail:
reznick@tx.technion.ac.il.
1448
CS and oral peroxidase activity
oxygen free radicals, were implicated as the causative
agents affecting those enzymes [11,12]. Thiol compounds such as glutathione (GSH), N-acetylcysteine, and
the iron-chelating agent deferoxamine mesylate (Desferal) could provide substantial protection from the CSassociated loss of salivary amylase and LDH activities.
Recently, we showed that exposure of saliva to CS of
one cigarette caused marked decreases of 48% and 68%
of OPO activity in smokers and nonsmokers, respectively, in both in vivo and in vitro studies [13]. In the in
vivo studies, this loss of activity could be reversed within
1 h and returned to presmoking levels. In the in vitro
studies, it could not be reversed, as no new saliva could
be formed [13]. The purpose of the present study was to
elucidate the mechanisms whereby CS constituents
might influence OPO activity.
MATERIALS AND METHODS
Cigarettes
The cigarettes used in this study were popular commercial cigarettes (Time cigarettes; Dubek Ltd., Tel
Aviv, Israel) containing 14 mg of tar and 0.9 mg of
nicotine per cigarette.
Exposure of saliva to CS: in vitro study
In vitro study was carried out using Time cigarettes
combined with a vacuum system, as described previously
[11,12]. Saliva (4 5 ml) from consenting volunteers was
placed in 50 ml flasks with a sidearm to which the
cigarettes were attached. A reproducible vacuum was
created in the flask and, upon opening the vacuum, the
CS of the whole lighted cigarette was drawn into the
flask. Saliva samples for OPO analysis were drawn before smoking (zero time), and cigarettes were smoked at
intervals of 20 min. Saliva samples for OPO analysis
were drawn at 20 min intervals, prior to the next CS
exposure.
Peroxidase activity
OPO activity was measured according to the 2-nitrobenzoic acid-thiocyanate assay [2]. Briefly, in this assay
5,5-dithiobis, 2-nitrobenzoic acid (DTNB) is reduced to
2-nitrobenzoic acid (NBS) by the addition of -mercaptoethanol. The disappearance of 2-nitrobenzoic acid
while reacting with OSCN, the product of OPO, was
monitored at 412 nM at pH 5.6, as described previously
[3]. One unit of enzyme activity was defined as the level
of enzyme activity needed to cleave 1 mol of 2-nitrobenzoic acid/min at 22C using a molar extinction coefficient of 12,800 [2].
1449
Fig. 1. Effect of exposure of saliva in vitro to three puffs of cigarette
smoke over a 1 h period. Each point represents the mean SEM of
four different experiments. OPO oral peroxidase.
Addition of antioxidants, inhibitors, and oxidants
to saliva
The various reagents and materials were added to
saliva usually at zero time prior to the first smoking puff,
at concentrations specified in the figure legends. Usually,
between 50 100 l of saliva was added to 1 ml of NBS
solution and 100 l of 1 mM of H202 solution making a
dilution of the original saliva of 1:121:24. Thus, the
compounds effects on the assay conditions were really
negligible. In addition, in all experiments, a control without the compounds was run with no difference in the
assay conditions.
All chemicals were purchased from Sigma Chemical
Co. (St. Louis, MO, USA), unless otherwise specified.
Statistical analysis
Statistical analysis was performed using unpaired Students t-test. To determine statistical significance, the
ranges, means, SD, and SEM were computed. Results are
reported as mean SD or SEM. Statistical significance
was set at p .05.
RESULTS
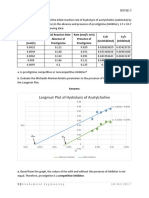
Figure 1 shows the loss of OPO activity after exposure of saliva of four volunteers to three puffs of CS over
a 1 h time period. It can be seen that OPO activity was
decreased over time to about 22% of the control. The
initial mean SEM of the four volunteers was 708 52
mU/ml (n 4).
CS contains a number of constituents (e.g., oxidants,
aldehydes, cyanide) that can cause protein modifications
and influence protein enzyme activity. In order to elucidate which constituents could cause loss of OPO activity,
the saliva of several subjects was exposed to CS in the
presence of 1 mM GSH, Desferal, or ascorbic acid.
1450
I. KLEIN et al.
Fig. 2. Effect of antioxidants on oral peroxidase (OPO) activity exposed to cigarette smoke (CS). exposure to CS only;
exposure to CS 1 mM glutathione; exposure to CS 1 mM
Desferal; exposure to CS 1 mM ascorbate (n 3). Each point
represents the mean SD of saliva from three or four subjects.
Fig. 4. Effect of addition of purified aldehydes present in cigarette
smoke on oral peroxidase activity in saliva of four or five subjects.
control, saliva left in air for 3 h; saliva exposed to acetyl
aldehyde; saliva exposed to crotonaldehyde; saliva exposed
to acrolein.
Figure 2 shows that the rate of loss of OPO activity was
uninfluenced by the presence or absence of all these
antioxidants. Oxidizing the saliva of three volunteers
with 100 M FeCl3 and 1 mM ascorbic acid for 1 or 2 h
did not significantly affect OPO activity and even
slightly increased its level after 2 h (Fig. 3). The addition
of the major aldehydes present at concentrations contained in the CS of one cigarette (2 mM acetyl aldehyde,
80 M acrolein, and 20 M crotonaldehyde) likewise
did not influence salivary OPO activity (Fig. 4).
We also decided to examine the possibility that nicotine at a concentration of 0.9 mg might influence OPO
activity (4 ml of saliva was incubated for 40 min). No
effect on OPO activity was observed (Fig. 5). Next, we
evaluated the degree to which CS-related loss of OPO
activity was reversible by dialysis. After dialysis for 18 h
with no exposure to CS, loss of OPO activity was approximately 20% (Fig. 6, column 2), while saliva exposed to CS with no dialysis lost about 68% of its initial
activity (column 3). Importantly, the activity of saliva
exposed to CS and dialyzed for 18 h returned to 94% of
the level of initial OPO activity, showing that the loss of
OPO activity due to CS exposure was reversible by
dialysis.
Cyanide is a known inhibitor of heme peroxidase, and
the presence of hydrogen cyanide has been reported in
CS [14]. The saliva of four volunteers was incubated in
the presence of 150 M potassium cyanide (KCN) and,
as shown in Fig. 7, caused a drastic loss of OPO activity
after only 2 min of KCN incubation (65%, column 3),
which was reversible by dialysis (column 4), strongly
suggesting that KCN is capable of inhibiting salivary
peroxidase activities (Fig. 7).
Figure 8 shows that addition of increasing amounts of
hydroxocobalamin (OH-CO), a known chelator of cyanide, could to a significant extent prevent the CS-associated loss of OPO activity after 60 min of exposure to
CS (three puffs) (columns 4, 5, and 6). Finally, a similar
Fig. 3. Influence of oxidation on oral peroxidase (OPO) activity at
incubation times of 1 and 2 h. Each value represents the mean SD of
three subjects.
Fig. 5. Effect of incubation of 0.9 mg nicotine/4 ml of saliva for 40 min
in the absence of cigarette smoke. Each value represents the mean
SD of five different experiments. OPO oral peroxidase.
CS and oral peroxidase activity
Fig. 6. Effect of cigarette smoke (CS) on oral peroxidase (OPO)
activity after exposure for 1 h and after dialysis for 18 h. Column 1
control OPO activity at zero time; column 2 control OPO activity
after dialysis for 18 h without exposure to CS; column 3 OPO
activity after exposure to CS for 2 h with no dialysis; column 4 OPO
activity of saliva exposed to CS and dialysis for 18 h; column 5
saliva exposed to CS after 18 h without dialysis. Results represent the
mean SD of three different experiments. *p .01; **NS not
significant.
study of exposure of saliva to KCN for 2 min showed
that the addition of OH-CO completely prevented KCNassociated loss of OPO activity (Fig. 9, columns 5 and 6).
1451
Fig. 8. Effect of cigarette smoke (CS) on oral peroxidase (OPO)
activity incubated for 60 min (three puffs) in the absence or presence of
various concentrations of OH-CO. Column 1 control zero time
activity; column 2 control 1 mM OH-CO without exposure to CS;
column 3 saliva CS exposure without addition of OH-CO; column
4 saliva CS 0.5 mM OH-CO; column 5 saliva CS 1 mM
OH-CO (n 3); column 6 saliva CS 2 mM OH-CO. Each
value represents the mean SD of three or four experiments with
saliva of three or four different subjects. *p .01; **p .05 vs. loss
at 60 min.
The major aim of the present study was to try to
understand the underlying causes behind the phenomenon that gas-phase CS can rapidly inactivate a large
percentage of OPO activity in both in vitro and in vivo
systems. Because CS contains an abundance of oxidants,
our initial approach was to determine whether or not CS
oxidants were responsible for the loss of OPO activity.
Ascorbic acid, Desferal, and GSH were shown not to
influence the rate of CS-associated loss of OPO activity
(Figs. 2 and 3). The fact that oxidants in CS were
unlikely to influence loss of OPO activity was buttressed
by the fact that the oxidizing FeCl3/ascorbic acid system
also failed to influence salivary OPO activity (Fig. 3).
A second approach was to try to examine the effect of
known aldehydes present in CS, knowing that reactive
aldehydes are able to influence structure and functions of
Fig. 7. Effect of addition of potassium cyanide (KCN) on oral peroxidase (OPO) activity and recovery after dialysis. Column 1 control
OPO activity at zero time; column 2 control OPO activity after
dialysis for 18 h without exposure to KCN; column 3 OPO activity
after exposure to 150 M KCN for 2 min with no dialysis; column 4
OPO activity of saliva exposed to KCN and dialysis for 18 h; column
5 saliva exposed to KCN after 18 h without dialysis. Results
represent the mean SD of four different experiments. *p .02;
**NS not significant.
Fig. 9. Effect of potassium cyanide (KCN) on oral peroxidase (OPO)
activity incubated for 2 min in the absence or presence of various
concentrations of OH-CO. Column 1 control zero time activity of
OPO; column 2 control 1 mM OH-CO; column 3 saliva 0.5
mM KCN; column 4 saliva 1 mM KCN; column 5 saliva CS
0.5 mM KCN 1 mM OH-CO; column 6 saliva CS 1 mM
KCN 1 mM OH-CO. Each value represents the mean SD of three
or four experiments with saliva of three or four different subjects. *p
.01.
DISCUSSION
1452
I. KLEIN et al.
many proteins. This was attempted in light of previous
studies in which aldehydes were shown to cause accumulation of protein carbonyls in plasma [8] and GSH and
N-acetylcysteine were capable of ameliorating this accumulation in both plasma and salivary proteins [8,11].
However, the results of the current study clearly demonstrated that neither thiols nor exposure to aldehydes
could affect the activity of OPO (Figs. 3 and 4). After
realizing that most common known compounds in CS
that can possibly effect OPO activity failed to do so, we
hypothesized that it could be a small stable molecule that
binds reversibly to OPO active site. Dialysis is one way
to prove this hypothesis because, upon using kinetics of
osmotic concentration where there is no cyanide outside
the dialysis bag, the cyanide inside the bag will move
outside and disturb the equilibrium between bound and
free cyanide, where eventually the bound cyanide will
also dissociate from OPO active site and OPO activity
will be restored.
Thus, using this third approach, investigating whether
loss of OPO activity would be reversible after dialysis,
indicated that indeed a small, stable molecule present in
CS was responsible for the observed effect. Figures 6 and
7 demonstrate that the loss of activity was reversible by
dialysis and that the most potent inhibitor of heme peroxidases, the cyanide ion, was most probably the culprit
underlying this inactivation. As was mentioned in the
introduction, OPO is composed of two distinct enzymes,
SPO and MPO. The fact that KCN did not inhibit OPO
completely may hint that cyanide can influence the two
enzymes differently. However, in a previous publication
[13], it was shown that using a specific inhibitor of SPO,
dapsone, the kinetics of SPO and MPO inactivation by
CS was quite similar, hinting that cyanide inhibits both
peroxidases in a similar way. Nevertheless, one can
assume that there can be other factors that may influence
OPO activity besides cyanide. However, preincubation
of saliva with OH-CO could prevent the CS- and KCNassociated loss of OPO activity, indicating again that
cyanide was most probably responsible for the major loss
of OPO activity. Indeed, it has been reported that hydrogen cyanide is present in 70 350 mol in gas-phase CS
of various cigarette brands [14].
The significance of CS-induced reductions in salivary
peroxidase activities is unknown. Our findings suggest
that it might be one of the mechanisms involved in
CS-related mouth morbidity. Further studies are needed
to elucidate the role of CS-related inhibition of saliva
peroxidase activities in oral pathobiology.
Acknowledgement This work was supported by a grant from the
Technion Vice-President for Research and a grant from the Krol
Foundation of Lakewood, NJ, USA.
REFERENCES
[1] Mandel, I. D. The role of saliva in maintaining oral homeostasis.
J. Am. Dent. Assoc. 119:298 304; 1989.
[2] Nagler, R. M.; Klein, I.; Zarzhevsky, N.; Drigues, N.; Reznick,
A. Z. Characterization of the differentiated antioxidant profile of
human saliva. Free Radic. Biol. Med. 32:268 277; 2002.
[3] Pruitt, K. M.; Kamau, D. N.; Miller, K.; Mansson-Rahemtulla, B.;
Rahemtulla, F. Quantitative, standardized assays for determining
the concentrations of bovine lactoperoxidase, human salivary
peroxidase, and human myeloperoxidase. Anal. Biochem. 191:
278 286; 1990.
[4] Toljanic, J. A.; Siddiqui, A. A.; Patterson, G. L.; Irwin, M. E. An
evaluation of a dentifrice containing salivary peroxidase elements
for the control of gingival disease in patients with irradiated head
and neck cancer. J. Prosthet. Dent. 76:292296; 1996.
[5] Grisham, M. B.; Ryan, E. M. Cytotoxic properties of salivary
oxidants. Am. J. Physiol. Cell Physiol. 258:C115C121; 1990.
[6] Guven, Y.; Satman, I.; Dinccag, N.; Alptekin, S. Salivary peroxidase activity in whole saliva of patients with insulin-dependent
(type-1) diabetes mellitus. J. Clin. Periodontol. 23:879 881;
1996.
[7] Aune, T. M.; Thomas, E. L. Accumulation of hypothiocyanite ion
during peroxidase-catalyzed oxidation of thiocyanate ion. Eur.
J. Biochem. 80:209 214; 1977.
[8] Reznick, A. Z.; Cross, C. E.; Hu, M.-L.; Suzuki, Y. J.; Khwaja, S.;
Safadi, A.; Motchnik, P. A.; Packer, L.; Halliwell, B. Modification of plasma proteins by cigarette smoke as measured by protein
carbonyl formation. Biochem. J. 286:607 611; 1992.
[9] Reznick, A. Z.; Han, D.; Packer, L. Cigarette smoke induced
oxidation of human plasma proteins, lipids, and antioxidants;
selective protection by the biothiols dihydrolipoic acid and glutathione. Redox Rep. 3:169 174; 1997.
[10] ONeill, C. A.; Halliwell, B.; van der Vliet, A.; Davis, P. A.;
Packer, L.; Tritschler, H.; Strohman, W. J.; Rieland, T.; Cross,
C. E.; Reznick, A. Z. Aldehyde-induced protein modifications in
human plasma: protection by glutathione and dihydrolipoic acid.
J. Lab. Clin. Med. 124:359 370; 1994.
[11] Nagler, R.; Lischinsky, S.; Diamond, E.; Drigues, N.; Klein, I.;
Reznick, A. Z. Effect of cigarette smoke on salivary proteins and
enzyme activities. Arch. Biochem. Biophys. 379:229 236; 2000.
[12] Nagler, R. M.; Lischinsky, S.; Diamond, E.; Klein, I.; Reznick,
A. Z. New insights into salivary lactate dehydrogenase of human
subjects. J. Lab. Clin. Med. 137:363369; 2001.
[13] Reznick, A. Z.; Klein, I.; Eiserich, J. P.; Cross, C. E.; Nagler,
R. M. Inhibition of oral peroxidase activity by cigarette smoke: in
vivo and in vitro studies. Free Radic. Biol. Med. 34:377384;
2003.
[14] Fowles, J.; Bates, M. The chemical constituents in cigarettes and
cigarette smoke: priorities for harm reduction. A report to the
New Zealand Ministry of Health; March, 2000:16.
ABBREVIATIONS
CN cyanide
CS cigarette smoke
GSH glutathione
KCNpotassium cyanide
LDHlactate dehydrogenase
MPOmyeloperoxidase
OH-CO hydroxocobalamin
OPO oral peroxidase
OSCN hypothiocyanite ion
SPOsalivary peroxidase
Potrebbero piacerti anche
- Biochemical Factors Concerned in the Functional Activity of the Nervous System: First International Meeting of the International Society for Neurochemistry, Strasbourg, 1967Da EverandBiochemical Factors Concerned in the Functional Activity of the Nervous System: First International Meeting of the International Society for Neurochemistry, Strasbourg, 1967D. RichterNessuna valutazione finora
- Diagnosis of Aluminum Phosphide Poisoning Using A New Analytical Approach: Forensic Application To A Lethal IntoxicationDocumento7 pagineDiagnosis of Aluminum Phosphide Poisoning Using A New Analytical Approach: Forensic Application To A Lethal IntoxicationIsmael Pacheco vaqueroNessuna valutazione finora
- Environmental Forensics for Persistent Organic PollutantsDa EverandEnvironmental Forensics for Persistent Organic PollutantsGwen O'SullivanNessuna valutazione finora
- Ozone As A Modulator of The Immune SystemDocumento10 pagineOzone As A Modulator of The Immune Systemweb3351Nessuna valutazione finora
- The Handbook of Histopathological Practices in Aquatic Environments: Guide to Histology for Environmental ToxicologyDa EverandThe Handbook of Histopathological Practices in Aquatic Environments: Guide to Histology for Environmental ToxicologyValutazione: 4.5 su 5 stelle4.5/5 (2)
- All Products MSDSDocumento143 pagineAll Products MSDSkuldeepchahal1987Nessuna valutazione finora
- Bioorganic & Medicinal Chemistry: Article InfoDocumento8 pagineBioorganic & Medicinal Chemistry: Article InfoLeo RuilovaNessuna valutazione finora
- OrganophosphatesDocumento12 pagineOrganophosphatesapi-237290361Nessuna valutazione finora
- Mitigation of Aluminium Phosphide-Induced Hematotoxicity and Ovarian Oxidative Damage in Wistar Rats by HesperidinDocumento10 pagineMitigation of Aluminium Phosphide-Induced Hematotoxicity and Ovarian Oxidative Damage in Wistar Rats by HesperidinBiochem M. JulyNessuna valutazione finora
- Forensic Science InternationalDocumento5 pagineForensic Science InternationalnonawitaNessuna valutazione finora
- Aplicacao Do Ozonio Na MedicinaDocumento12 pagineAplicacao Do Ozonio Na MedicinaJoão Jacques Legatti100% (1)
- Pintilie o 2 16Documento3 paginePintilie o 2 16Anonymous p52JDZOdNessuna valutazione finora
- AminofostineDocumento5 pagineAminofostinePedro JuarezNessuna valutazione finora
- 3 AminoPropylTriEthoxySilaneDocumento123 pagine3 AminoPropylTriEthoxySilanethuronNessuna valutazione finora
- Suratman 2015Documento15 pagineSuratman 2015PatySA2Nessuna valutazione finora
- Labinvest 2014147 ADocumento15 pagineLabinvest 2014147 AKatira PazNessuna valutazione finora
- Ethylbenzene: Hazard SummaryDocumento4 pagineEthylbenzene: Hazard SummaryTrường Tùng LýNessuna valutazione finora
- Antioxidant From PeelsDocumento5 pagineAntioxidant From PeelsEliana Otero PerezNessuna valutazione finora
- Hydrogen Sulfide: CAS Registry Number: 7783-06-4Documento6 pagineHydrogen Sulfide: CAS Registry Number: 7783-06-4Yasir ShafiqueNessuna valutazione finora
- 10 1 1 232 716 PDFDocumento17 pagine10 1 1 232 716 PDFSounak BoseNessuna valutazione finora
- Etilen OksidaDocumento7 pagineEtilen OksidaugegkusumaNessuna valutazione finora
- 2002 Abused Inhalants and Central Reward PathwaysDocumento12 pagine2002 Abused Inhalants and Central Reward PathwaysEm ManuelNessuna valutazione finora
- Propo XurDocumento4 paginePropo XurIntan Alwaystry TobehappyNessuna valutazione finora
- Risk Assessment of Triclosan, A Cosmetic Preservative: Invited ReviewDocumento18 pagineRisk Assessment of Triclosan, A Cosmetic Preservative: Invited ReviewMaja TastanoskaNessuna valutazione finora
- Per SulfatesDocumento192 paginePer Sulfatesmarvin.antonie455Nessuna valutazione finora
- 2004 Clinica Chimica ActaDocumento15 pagine2004 Clinica Chimica ActaDina MansourNessuna valutazione finora
- Journal of Agricultural and Food Chemistry Volume 49 Issue 6 2001 (Doi 10.1021 - jf001413m) Burda, Stanislaw Oleszek, Wieslaw - Antioxidant and Antiradical Activities of FlavonoidsDocumento6 pagineJournal of Agricultural and Food Chemistry Volume 49 Issue 6 2001 (Doi 10.1021 - jf001413m) Burda, Stanislaw Oleszek, Wieslaw - Antioxidant and Antiradical Activities of FlavonoidsnaelaniesaNessuna valutazione finora
- SulfuricDocumento10 pagineSulfuricSamantha FarahNessuna valutazione finora
- Punya FiiillaahDocumento9 paginePunya FiiillaahFillahMutySyahidahNessuna valutazione finora
- Acephate SourceDocumento83 pagineAcephate Source0001fidaNessuna valutazione finora
- Morbidity, Mortality and Management of Wheat Pill Poisoning: Background: MethodsDocumento5 pagineMorbidity, Mortality and Management of Wheat Pill Poisoning: Background: MethodsAamir ShahzadNessuna valutazione finora
- Peroxidative Index: A New Marker in Kidney Toxicity Induced by MercuryDocumento8 paginePeroxidative Index: A New Marker in Kidney Toxicity Induced by MercuryIfiq Budiyan NazarNessuna valutazione finora
- Artikel 2Documento6 pagineArtikel 2emnovericiNessuna valutazione finora
- Kem Ind 67 2018Documento8 pagineKem Ind 67 2018BrunoNessuna valutazione finora
- Lal Test An Alternative Method For Detection of Bacterial EndotoxinsDocumento6 pagineLal Test An Alternative Method For Detection of Bacterial EndotoxinsStanislaw YohanNessuna valutazione finora
- Limulus Amoebocyte Lysate (Lal) Test - An Alternative Method For Detection of Bacterial EndotoxinsDocumento6 pagineLimulus Amoebocyte Lysate (Lal) Test - An Alternative Method For Detection of Bacterial EndotoxinsOula HatahetNessuna valutazione finora
- An Experiment in Enzyme Characterization-Banana PolyphenoloxidaseDocumento3 pagineAn Experiment in Enzyme Characterization-Banana PolyphenoloxidaseKristiani SuhermanNessuna valutazione finora
- Toxicology and Applied Pharmacology: Kevin R. Smith, David Leonard, Jacob D. Mcdonald, Yohannes TesfaigziDocumento8 pagineToxicology and Applied Pharmacology: Kevin R. Smith, David Leonard, Jacob D. Mcdonald, Yohannes TesfaigziCárdenas EfrainNessuna valutazione finora
- Research Open Access: Functional Foods in Health and Disease 2011 7:232-244Documento13 pagineResearch Open Access: Functional Foods in Health and Disease 2011 7:232-244Anonymous 5NXc6NuNessuna valutazione finora
- LIPASEDocumento4 pagineLIPASEKresna Bondan FathoniNessuna valutazione finora
- CL MethaneDocumento135 pagineCL MethaneMufita RamadhinaNessuna valutazione finora
- DEHP Induced Oxidative Stress Suppress Sperm Quality and Serum Androgen Concentration in Wistar RatsDocumento8 pagineDEHP Induced Oxidative Stress Suppress Sperm Quality and Serum Androgen Concentration in Wistar RatsInternational Journal of Innovative Science and Research TechnologyNessuna valutazione finora
- Salivary Markers of Oxidative Stress and Antioxidant Status: Influence of External FactorsDocumento9 pagineSalivary Markers of Oxidative Stress and Antioxidant Status: Influence of External FactorsAndrew NdewNessuna valutazione finora
- Femael Infertility PDFDocumento9 pagineFemael Infertility PDFAyadPalaniNessuna valutazione finora
- Acetophenone: Hazard SummaryDocumento4 pagineAcetophenone: Hazard Summaryalejandra rojasNessuna valutazione finora
- Report Meb Project 1Documento15 pagineReport Meb Project 1Nurul IzzatiNessuna valutazione finora
- 885 12 16 ArticleDocumento10 pagine885 12 16 ArticleMohamed ElshounyNessuna valutazione finora
- Total PhenolicsDocumento3 pagineTotal PhenolicsHajime HikariNessuna valutazione finora
- Practice Essentials: Organophosphate ToxicityDocumento12 paginePractice Essentials: Organophosphate ToxicityDaniel ChristiantoNessuna valutazione finora
- Fisher Et Al-2009-Journal of Applied MicrobiologyDocumento7 pagineFisher Et Al-2009-Journal of Applied MicrobiologydigdouwNessuna valutazione finora
- The Effect of Dexpanthenol On Ototoxicity Induced by CisplatinDocumento7 pagineThe Effect of Dexpanthenol On Ototoxicity Induced by CisplatinNoval ArdianNessuna valutazione finora
- Activation of MMP-9 Activity by Acrolein in Saliva From Patients With Primary Sjo ̈gren's Syndrome and Its MechanismDocumento28 pagineActivation of MMP-9 Activity by Acrolein in Saliva From Patients With Primary Sjo ̈gren's Syndrome and Its MechanismMaite Fernanda CuturrufoNessuna valutazione finora
- Major Intermediates in Organophosphate Synthesis (PCL, Pocl, PSCL, and Their Diethyl Esters) Are Anticholinesterase Agents Directly or On ActivationDocumento7 pagineMajor Intermediates in Organophosphate Synthesis (PCL, Pocl, PSCL, and Their Diethyl Esters) Are Anticholinesterase Agents Directly or On ActivationYancy JuanNessuna valutazione finora
- Validation Endotoxine TestDocumento20 pagineValidation Endotoxine Testsimon escobarNessuna valutazione finora
- Raştirma (Research) : Ebru Olgun Erdemir DDS, PHD, İsmet Duran DDS, PHD, Seyfullah Haliloğlu DVM, PHDDocumento8 pagineRaştirma (Research) : Ebru Olgun Erdemir DDS, PHD, İsmet Duran DDS, PHD, Seyfullah Haliloğlu DVM, PHDAmna AliNessuna valutazione finora
- Inhibition of Ache by Malathion and Some Structurally Similar CompoundsDocumento13 pagineInhibition of Ache by Malathion and Some Structurally Similar CompoundsJavier AntonioNessuna valutazione finora
- Mechanism of Hydrogen Gas Promoted Apoptosis of Lung Adenocarcinoma A549 Cells Through XIAP and BIRC3Documento12 pagineMechanism of Hydrogen Gas Promoted Apoptosis of Lung Adenocarcinoma A549 Cells Through XIAP and BIRC3FananiNessuna valutazione finora
- Ref, Reducing PowerDocumento7 pagineRef, Reducing PowerRumana DishaNessuna valutazione finora
- 7783202Documento231 pagine7783202jjdottaNessuna valutazione finora
- Biomonitoring of Oxidative DNA Damage in Tra C Policemen Exposed To Urban Air PollutionDocumento5 pagineBiomonitoring of Oxidative DNA Damage in Tra C Policemen Exposed To Urban Air PollutionMauro Porcel de PeraltaNessuna valutazione finora
- Genetic PDFDocumento11 pagineGenetic PDFmfhfhfNessuna valutazione finora
- Transportation Security Through Inter Vehicular Ad-Hoc Networks (Vanets) Handovers Using RF Trans ReceiverDocumento6 pagineTransportation Security Through Inter Vehicular Ad-Hoc Networks (Vanets) Handovers Using RF Trans ReceivermfhfhfNessuna valutazione finora
- Vehicular Ad Hoc NetworkDocumento9 pagineVehicular Ad Hoc NetworkmfhfhfNessuna valutazione finora
- Providing Ubiquitous Communication Using: Road-Side Units in VANET Systems: Unveiling The ChallengesDocumento6 pagineProviding Ubiquitous Communication Using: Road-Side Units in VANET Systems: Unveiling The ChallengesmfhfhfNessuna valutazione finora
- A Qos-Based Scheduling Algorithm in Vanets: Mohammad Shahverdy, Maryam Asgari, Mahmood FathyDocumento10 pagineA Qos-Based Scheduling Algorithm in Vanets: Mohammad Shahverdy, Maryam Asgari, Mahmood FathymfhfhfNessuna valutazione finora
- Pits Ill Ides 2007Documento22 paginePits Ill Ides 2007mfhfhfNessuna valutazione finora
- FTPartial StepValDocumento7 pagineFTPartial StepValmfhfhfNessuna valutazione finora
- Response of The Sorghum Bicolor Crop To The Addition of Potassium and Nitrogen FertilizerDocumento8 pagineResponse of The Sorghum Bicolor Crop To The Addition of Potassium and Nitrogen FertilizermfhfhfNessuna valutazione finora
- 10.18081/MJAS/2019-7/184-192: AbstractDocumento9 pagine10.18081/MJAS/2019-7/184-192: AbstractmfhfhfNessuna valutazione finora
- 20436Documento17 pagine20436mfhfhfNessuna valutazione finora
- Sureepong Phothongsunan Full PaperDocumento13 pagineSureepong Phothongsunan Full PapermfhfhfNessuna valutazione finora
- 1737 RRR 28Documento7 pagine1737 RRR 28mfhfhfNessuna valutazione finora
- Contaminant Transport and BiodegradationDocumento8 pagineContaminant Transport and BiodegradationmfhfhfNessuna valutazione finora
- 1 s2.0 S2090123211000348 MainDocumento9 pagine1 s2.0 S2090123211000348 MainmfhfhfNessuna valutazione finora
- An Improved Numerical Solution of Burgers' Equation by Cubic B-Spline Quasi-Interpolation ?Documento9 pagineAn Improved Numerical Solution of Burgers' Equation by Cubic B-Spline Quasi-Interpolation ?mfhfhfNessuna valutazione finora
- Computing in Applied Science: William J. ThompsonDocumento11 pagineComputing in Applied Science: William J. ThompsonmfhfhfNessuna valutazione finora
- Chromium (III) Ion Selective Electrode Based On Glyoxal Bis (2-Hydroxyanil)Documento7 pagineChromium (III) Ion Selective Electrode Based On Glyoxal Bis (2-Hydroxyanil)mfhfhfNessuna valutazione finora
- Pani Snwaso4Documento14 paginePani Snwaso4mfhfhfNessuna valutazione finora
- Spectrophotometric Determination of Ascorbic Acid and Dehydroascorbic AcidDocumento5 pagineSpectrophotometric Determination of Ascorbic Acid and Dehydroascorbic AcidmfhfhfNessuna valutazione finora
- A New Ion Selective Electrode Method For Determination of Oseltamivir Phosphate (Tami U) and Its Pharmaceutical ApplicationsDocumento8 pagineA New Ion Selective Electrode Method For Determination of Oseltamivir Phosphate (Tami U) and Its Pharmaceutical ApplicationsmfhfhfNessuna valutazione finora
- Application of Ionic Liquid To The Construction of Cu (II) Ion-Selective Electrode With Solid ContactDocumento4 pagineApplication of Ionic Liquid To The Construction of Cu (II) Ion-Selective Electrode With Solid ContactmfhfhfNessuna valutazione finora
- Ubc - 1986 - A1 H36 - 6Documento304 pagineUbc - 1986 - A1 H36 - 6mfhfhfNessuna valutazione finora
- A Mercury (II) Ion-Selective Electrode Based On Neutral Salicylaldehyde ThiosemicarbazoneDocumento5 pagineA Mercury (II) Ion-Selective Electrode Based On Neutral Salicylaldehyde ThiosemicarbazonemfhfhfNessuna valutazione finora
- Journal of Advanced Research: Cairo UniversityDocumento10 pagineJournal of Advanced Research: Cairo UniversitymfhfhfNessuna valutazione finora
- Resumo - 2014 - Iiwdf - Predictors of Reading AbilityDocumento2 pagineResumo - 2014 - Iiwdf - Predictors of Reading AbilitymfhfhfNessuna valutazione finora
- Between Affect and Cognition: Proving at University LevelDocumento8 pagineBetween Affect and Cognition: Proving at University LevelmfhfhfNessuna valutazione finora
- Natale 2009Documento18 pagineNatale 2009mfhfhfNessuna valutazione finora
- Pub CauseDocumento17 paginePub CausemfhfhfNessuna valutazione finora
- A Comparison of Attribution Style, Perceived Stress and Difficulties in Emotion Regulation Between Single-Parent and Two-Parent AdolescentsDocumento4 pagineA Comparison of Attribution Style, Perceived Stress and Difficulties in Emotion Regulation Between Single-Parent and Two-Parent AdolescentsmfhfhfNessuna valutazione finora
- Gate FTDocumento51 pagineGate FTjayavardhanaNessuna valutazione finora
- 9701 w13 QP 43 2 PDFDocumento20 pagine9701 w13 QP 43 2 PDFNeural Spark Physics CieNessuna valutazione finora
- 1 s2.0 S004101010800113X MainDocumento6 pagine1 s2.0 S004101010800113X MainLeena John MathaiNessuna valutazione finora
- GCE Biology-Human Biology-Teachers' Guide Revised 18-02-14Documento93 pagineGCE Biology-Human Biology-Teachers' Guide Revised 18-02-14mariumrana40Nessuna valutazione finora
- 9701 w08 QP 4Documento20 pagine9701 w08 QP 4Hubbak KhanNessuna valutazione finora
- 1st Year Biochemistry Guidance BookletDocumento67 pagine1st Year Biochemistry Guidance Bookletusama HafeezNessuna valutazione finora
- Enzyme InhibitionDocumento49 pagineEnzyme InhibitionJaden StanislausNessuna valutazione finora
- 7 Kinetika Enzim InhibitorDocumento69 pagine7 Kinetika Enzim InhibitorHernanda WidipasuryaNessuna valutazione finora
- AB - Addition Cure HCR Impact of Inhibitors On The Elastomer Cure Profile PDFDocumento7 pagineAB - Addition Cure HCR Impact of Inhibitors On The Elastomer Cure Profile PDFLin NiuNessuna valutazione finora
- Biochem Enzyme NotesDocumento8 pagineBiochem Enzyme NotesBeatriz NideaNessuna valutazione finora
- Reflections On Medicinal Chemistry at Merck, West Point: Chapter OneDocumento9 pagineReflections On Medicinal Chemistry at Merck, West Point: Chapter OneWalid EbaiedNessuna valutazione finora
- Pharmaceutical Therapeutic Categories Outlook: March 2002Documento333 paginePharmaceutical Therapeutic Categories Outlook: March 2002nergaliusNessuna valutazione finora
- ###Tutorial Work 217 Questions and Answers Mcqs PDFDocumento32 pagine###Tutorial Work 217 Questions and Answers Mcqs PDFDave DMNessuna valutazione finora
- Enzyme InhibitionDocumento7 pagineEnzyme InhibitionJane Docdoc100% (1)
- PharmacologyDocumento74 paginePharmacologyKiara Denise Tamayo100% (1)
- BIOENHANCERSDocumento5 pagineBIOENHANCERSbhanu pratapNessuna valutazione finora
- Antimicrobial Drugs: Prof DR Thamer Mutlag JasimDocumento51 pagineAntimicrobial Drugs: Prof DR Thamer Mutlag JasimFlorinda PllanaNessuna valutazione finora
- JrrysnDocumento4 pagineJrrysnEureca Parra100% (2)
- Lesson 6 InhibitorsDocumento12 pagineLesson 6 Inhibitorstbrook2017Nessuna valutazione finora
- Magyar The Pharmacology of SelegilineDocumento20 pagineMagyar The Pharmacology of SelegilineAs DvgNessuna valutazione finora
- Sprout Inhibitors SectionDocumento4 pagineSprout Inhibitors SectionHelton SilvaNessuna valutazione finora
- 731-Article Text-3619-2-10-20210121Documento3 pagine731-Article Text-3619-2-10-20210121Αβραξας ΓαβριήλNessuna valutazione finora
- Taro 2 PDFDocumento7 pagineTaro 2 PDFNatasya PermataNessuna valutazione finora
- Pharmacology Question Bank BPTDocumento5 paginePharmacology Question Bank BPTJoHN SamueLNessuna valutazione finora
- Clinical Chemistry 2 First GradingDocumento25 pagineClinical Chemistry 2 First GradingMHEKAELLA SAMSONNessuna valutazione finora
- A-Level Biology CIE Paper 5 TipsDocumento14 pagineA-Level Biology CIE Paper 5 TipsSara Uyen Tran100% (1)
- CHE599 - 99 - 3313 Research Project University of MaiduguriDocumento64 pagineCHE599 - 99 - 3313 Research Project University of MaiduguriJoseph NwosuNessuna valutazione finora
- 09 - Chapter 1 PDFDocumento59 pagine09 - Chapter 1 PDFchantayya bNessuna valutazione finora
- Han, Levenspiel - 1988 - Extended Monod Kinetics For Substrate, Product, and Cell Inhibition-AnnotatedDocumento8 pagineHan, Levenspiel - 1988 - Extended Monod Kinetics For Substrate, Product, and Cell Inhibition-AnnotatedMarisol Muñoz PonceNessuna valutazione finora
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolDa EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNessuna valutazione finora
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincDa EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincValutazione: 3.5 su 5 stelle3.5/5 (137)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsDa EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsValutazione: 4 su 5 stelle4/5 (146)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeDa EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeValutazione: 5 su 5 stelle5/5 (4)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeDa EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeValutazione: 4 su 5 stelle4/5 (1)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsDa EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsValutazione: 5 su 5 stelle5/5 (3)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableDa EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableValutazione: 3.5 su 5 stelle3.5/5 (22)
- The Periodic Table: A Very Short IntroductionDa EverandThe Periodic Table: A Very Short IntroductionValutazione: 4.5 su 5 stelle4.5/5 (3)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactDa EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactValutazione: 5 su 5 stelle5/5 (5)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeDa EverandChemistry for Breakfast: The Amazing Science of Everyday LifeValutazione: 4.5 su 5 stelle4.5/5 (90)
- Introduction to Strategies for Organic SynthesisDa EverandIntroduction to Strategies for Organic SynthesisNessuna valutazione finora
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideDa EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNessuna valutazione finora
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireDa EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireValutazione: 4 su 5 stelle4/5 (129)
- Water-Based Paint Formulations, Vol. 3Da EverandWater-Based Paint Formulations, Vol. 3Valutazione: 4.5 su 5 stelle4.5/5 (6)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilDa EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilValutazione: 5 su 5 stelle5/5 (1)
- Bioplastics: A Home Inventors HandbookDa EverandBioplastics: A Home Inventors HandbookValutazione: 4 su 5 stelle4/5 (2)
- Chemistry: a QuickStudy Laminated Reference GuideDa EverandChemistry: a QuickStudy Laminated Reference GuideValutazione: 5 su 5 stelle5/5 (1)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsDa EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNessuna valutazione finora
- Guidelines for Integrating Process Safety into Engineering ProjectsDa EverandGuidelines for Integrating Process Safety into Engineering ProjectsNessuna valutazione finora
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookDa EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookNessuna valutazione finora
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookDa EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNessuna valutazione finora