Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Mechanical Ventilation - Lessons From The ARDSNet Trial
Caricato da
RDG20202Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Mechanical Ventilation - Lessons From The ARDSNet Trial
Caricato da
RDG20202Copyright:
Formati disponibili
http://respiratory-research.
com/content/1/2/073
Commentary
Mechanical ventilation: lessons from the ARDSNet trial
Arthur S Slutsky and V Marco Ranieri*
Received: 17 July 2000
Revisions requested: 17 August 2000
Revisions received: 18 August 2000
Accepted: 18 August 2000
Published: 31 August 2000
commentary
St Michaels Hospital, University of Toronto, Toronto, Canada, and *Ospedale Sanata Chiara,
Universit di Pisa, Pisa, Italy
Respir Res 2000, 1:7377
The electronic version of this article can be found online at
http://respiratory-research.com/content/1/2/073
Current Science Ltd (Print ISSN 1465-9921; Online ISSN 1465-993X)
Abstract
review
The acute respiratory distress syndrome (ARDS) is an inflammatory disease of the lungs
characterized clinically by bilateral pulmonary infiltrates, decreased pulmonary compliance
and hypoxemia. Although supportive care for ARDS seems to have improved over the past
few decades, few studies have shown that any treatment can decrease mortality for this
deadly syndrome. In the 4 May 2000 issue of New England Journal of Medicine, the results
of an NIH-sponsored trial were presented; they demonstrated that the use of a ventilatory
strategy that minimizes ventilator-induced lung injury leads to a 22% decrease in mortality.
The implications of this study with respect to clinical practice, further ARDS studies and
clinical research in the critical care setting are discussed.
Keywords: acute lung injury, artificial respiration, barotrauma, biotrauma, iatrogenic, respiratory failure
reports
Introduction
As with any therapy, there are side effects of mechanical
ventilation; for decades our understanding of these complications was largely limited to the gross air leaks
induced by the large transpulmonary pressures socalled barotrauma. Over the past decade we have learned
about more subtle detrimental sequelae of mechanical
ventilation, based largely on basic studies on mechanisms
of injury [4]. These studies have demonstrated that
mechanical ventilation can induce injury manifested as
increased alveolar-capillary permeability due to overdistension of the lung (volutrauma) [5], can worsen lung injury by
the stresses produced as lung units collapse and re-open
(atelectrauma) [6,7], and can lead to even more subtle
injury manifested by the release of various mediators
ARDS = acute respiratory distress syndrome; HFV = high-frequency ventilation; ICU = intensive care unit; MSOF = multiple system organ failure;
PBW = predicted body weight; PEEP = positive end-expiratory pressure; Pplat = plateau pressure; VILI = ventilator-induced lung injury; Vt = tidal
volume.
primary research
ARDS is an inflammatory disease of the lungs characterized clinically by bilateral pulmonary infiltrates, decreased
pulmonary compliance and hypoxemia [1,2]. Despite
intense research for decades, the mortality rate in patients
with ARDS remains very high, although there is some evidence that these rates might be decreasing [3]. Interestingly, although the major initial physiological abnormalities
are often pulmonary in origin, patients who go on to die of
their acute illness usually die of multiple system organ
failure (MSOF) rather than a respiratory death (ie hypoxemia). Virtually all patients with ARDS require mechanical
ventilation to provide adequate oxygenation; this therapy is
supportive, providing time for the lungs to heal.
Respiratory Research
Vol 1 No 2
Slutsky and Ranieri
(biotrauma) [8,9]. The latter provides a putative mechanism to explain the high mortality rate in patients with
ARDS: if the mediators released by the lung owing to the
increased pulmonary stresses enter the circulation it could
lead to distal organ dysfunction, and ultimately organ
failure [10]. Ironically, although mechanical ventilation is
life-saving, a logical conclusion of the large body of data
on ventilator-induced lung injury (VILI) is that it might be
causing or perpetuating the pulmonary inflammation, preventing or delaying the recovery process. This reasoning
led to a recommendation to limit end-inspiratory lung
stretch in mechanically ventilated patients [11], and led to
a number of randomized clinical trials of lung protective
strategies. The results of the most recently completed trial
were presented in the 4 May 2000 issue of New England
Journal of Medicine [12]. This landmark paper answers a
key question in relation to the supportive therapy of
patients with ARDS but, as with any exciting research,
raises a number of interesting questions, which will be
addressed in this Commentary.
Brief review of ARDSNet study
The study was a multi-centered randomized controlled
trial performed by a group called the ARDSNet who
were funded by the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health (NIH) to
conduct clinical trials in patients with ARDS. Ten academic centers with 75 intensive care units (ICUs) enrolled
patients with acute lung injury or ARDS into the trial,
which compared a control ventilatory strategy with a tidal
volume (Vt) of 12 ml/kg [based on predicted body weight
(PBW)] to a lung protective strategy using a Vt of
6 ml/kg PBW [12]. The study set out to enroll up to
1000 patients but accrual was stopped at 861 patients
when an interim analysis revealed that the mortality rate
in the lung protective group was 22% lower than in the
control group. These beneficial results seemed to hold
across a wide spectrum of patients, including septic and
non-septic patients, and also those with different
degrees of lung dysfunction as assessed by respiratory
system compliances. The study is very important from a
clinical perspective, but also raises a large number of
questions on the mechanisms underlying the decreased
mortality, on the optimal way to ventilate patients with
ARDS, and more broadly on the conduct of clinical trials
in the critical care setting.
Why was this trial positive when three
previous trials were negative?
This was not the first trial to assess a lung protective strategy in patients with acute lung injury or ARDS; in fact
there were three previous negative trials [1315], but this
was the first large trial that showed a decrease in mortality
by simply addressing the injury imposed by overstretching
the lung. Why was this trial positive when other similar
trials were negative?
One possible reason could be the relative power of the
various studies; the ARDSNet trial enrolled 861 patients
compared with the 288 patients enrolled in the three previous studies. This seems unlikely to be the major factor
for the difference in results, because in each of the three
negative trials the trend was for the high-Vt groups to
have a lower mortality rate than the protective arms; combining all three studies the mortality rate was 44% in the
control arm and 48% in the lung protective arm. Another
possible explanation for the lack of efficacy in the previous trials might be related to the different approaches
used to control respiratory acidosis. All the trials applied
the concept of permissive hypercapnia [16], ie allowing
the PaCO2 to increase if necessary, rather than increasing Vt (or pressure). However, the approach to increases
in PaCO2 differed substantially between studies. Specifically, the ARDSNet study was the most aggressive in
terms of trying to maintain PaCO2 relatively close to the
normal range, employing higher respiratory rates as well
as more liberal use of bicarbonate than the other studies.
There are reasons to believe that hypercapnia might actually be beneficial in the context of VILI [17,18]; for
example, acidosis attenuates a number of inflammatory
processes, inhibits xanthine oxidase (a key component in
reperfusion injury), and attenuates the production of free
radicals [18]. However, there are also potential detrimental effects such as increased catecholamine release [19]
that might mitigate the potential beneficial effects of
hypercapnia on lung injury.
The higher respiratory rate that was used in the low-Vt arm
of the ARDSNet study to minimize hypercapnia might have
had a fortuitous benefit, by leading to the development of
auto-positive end-expiratory pressure (auto-PEEP).
Increased end-expiratory lung volume has been shown to
be protective in terms of VILI by minimizing the injury due to
recruitment and de-recruitment of lung units (atelectrauma).
No results have yet been presented on the degree of autoPEEP in the ARDSNet patients, but minute ventilation was
virtually identical between the low-Vt and high-Vt groups,
making this explanation less likely because, for any given
respiratory mechanics, minute ventilation is the major determinant of auto-PEEP. Furthermore, one could argue that
the low-Vt group might have been subject to more atelectrauma because the smaller Vt would probably lead to
reduced recruitment with each tidal cycle.
Another explanation for the positive ARDSNet trial might
be related to the greater spread in Vt and plateau pressure
(Pplat) between the control arm and the protective strategy.
For example, the difference between the Pplat (on day 1) in
this study was 8 cmH2O, compared with 4.5 [13], 5.7
[15] and 6.0 [14] ml/kg in other studies; similarly there
was a greater difference in Vt between the control and
intervention arms in the ARDSNet trial. Clearly, the greater
the difference in the independent variable, the greater the
http://respiratory-research.com/content/1/2/073
signal:noise ratio, and the greater the likelihood of a positive finding (if the therapy is efficacious).
review
reports
This latter possibility brings up the issue of whether the
intervention arm was really protective or whether the
control arm was injurious because the Vt used was too
large. The implication of this question is that the
ARDSNet used a Vt in the control arm that would not be
considered to be conventional (at the time). In
addressing this issue it is important to point out that the
ARDSNet calculated Vt on PBW, not measured body
weight. The latter was approx. 20% greater in the study
than PBW. Thus, on the basis of measured body weight,
the Vt used in the control arm was approx. 10 ml/kg.
This is certainly a value that would have been considered conventional.
The other important issue not addressed in the published
ARDSNet trial is the following: What is the importance of
recruitment maneuvers, and how does one set the appropriate PEEP level in patients with ARDS? This question is
a central one because preventing recruitment and derecruitment seems to be crucial in animal studies of VILI.
The ARDSNet is currently evaluating this question in a
large trial in which they are using recruitment maneuvers
and higher PEEP levels than in their previous study. Similarly, the large body of literature on VILI suggests that
high-frequency ventilation (HFV) may be an ideal way of
ventilating patents with ARDS because it can provide adequate gas exchange, while minimizing both overdistension
and the recruitment and de-recruitment of the lung. A
number of studies are currently re-evaluating this
approach in the context of VILI.
commentary
Finally, there might be a threshold in Pplat (as a surrogate
for overdistension) above which injury due to mechanical
ventilation might increase markedly. This was one of the
explanations put forward in the New England Journal of
Medicine editorial by Dr MJ Tobin that accompanied the
ARDSNet publication [20]. On the basis of the data from
the trials, he suggested that values of Pplat less than 32
cmH2O would be fairly protective; because the three negative trials had had average Pplat values (in both groups)
that were less than 32 cmH2O, one would not expect any
change in mortality between groups because both were
receiving protective strategies. In contrast, the
ARDSNet trial had an average Pplat in the control arm of
33 cmH2O, a value greater than the threshold value. This
suggestion could also explain the results of Amato et al
[21] in which the Pplat over the first 36 h averaged
46.0 cmH2O in the control arm, and 30.5 in the protective arm. We do not know what the shape of a lung injury
versus Vt curve would be and whether there is some
magical Vt or Pplat above which ventilation is unsafe and
below which ventilation is safe. It seems highly unlikely
that there is a specific break point for every patient, especially when one considers the spatial heterogeneity in
injury and the difficulty in interpreting a high Pplat in the
context of a stiff chest wall.
Must one use volume controlled ventilation with a Vt of 6
ml/kg (as was used in the ARDSNet trial), or can one use
pressure controlled ventilation (PCV) with relatively low
pressures that are in the range of those found in the lung
protective arm (ie less than 30 cmH2O)? This question is
difficult to answer given the results available. From a physiological standpoint, it seems reasonable to suggest that
PCV with relatively low values of pressure is acceptable;
however, from an evidence-based medicine perspective
one could argue that this is not the strategy that the
ARDSNet investigators used and thus PCV might not be
appropriate. There are cogent arguments on both sides.
Physiologically, lung distension is minimized if Pplat is kept
reasonably low arguing that a pressure limited strategy
should be as good as a volume limited strategy. However,
we have to acknowledge that there might be something
specific to the ARDSNet strategy not incorporated by
using pressure limitation. Although this suggestion is
somewhat unappealing, it might have some merit; for
example, in a patient with a very stiff chest wall, limiting the
Pplat to 30 cmH2O might limit Vt more than is necessary to
minimize overdistension, and in fact might lead to underrecruitment of the lung, poor oxygenation and further derecruitment. This might not have occurred if the
hypothetical patient had been treated exactly as in the
ARDSNet protocol.
Implications of the ARDSNet trial
The study also raises broader questions with regard to
clinical trials in the context of the ICU setting. For many
years there has been an uneasy feeling in the critical care
community that perhaps it would not be possible to prove
that any therapy is beneficial in patients with ARDS or
sepsis. This pessimism was based on the large number of
negative phase III type (randomized, large n, multicentered) clinical trials in the treatment of these diseases.
There are a number of possible reasons for the large
number of negative trials, including of course the possibility that the tested therapy was indeed not effective.
However, the major concern was that we might never
primary research
What are the messages from this landmark paper? From
an ARDS research perspective, there is no question that a
Vt of 6 ml/kg as implemented in the ARDSNet trial is, for
now, the gold standard against which all other ventilation
studies in ARDS will be judged. From a clinical perspective there are a number of issues and still many unanswered questions. In applying the results of this study at
the bedside, it is important to re-emphasize the fact that Vt
was calculated on the basis of predicted body weight; this
must also be borne in mind when comparing the Vt values
used in the various ventilation trials, which used different
definitions for calculating Vt.
Respiratory Research
Vol 1 No 2
Slutsky and Ranieri
obtain a positive trial even if a therapy was effective,
because of the tremendous heterogeneity in the patient
population, multiple co-morbidities, widely differing underlying diseases, difficulty in controlling co-interventions, and
so on. The ARDSNet trial has partially put these concerns
to rest: it is the first large-scale trial to show that a particular therapy is effective in patients with ARDS, and in some
sense it can be considered a proof of concept that the
obstacles to successful trials in patients with ARDS and, it
is hoped, in patients with sepsis are surmountable.
The trial is a role model of the way in which clinical trials
should be conducted in the ICU; however, it required a
large number of patients, took a long time to complete, and
was extremely expensive. If studies this large, long, and
costly are to be performed to evaluate all changes in management of our patients with or without ARDS, it will be
extremely difficult to prove almost anything definitively in
the ICU setting, other than interventions that are extremely
effective. How, then, will it be possible to evaluate the use
of inhaled nitric oxide, HFV, the prone position, less restrictive Vt values, optimal PEEP levels and a whole host of
changes in management? We do not have any definitive
answers to these questions; ideally other networks such as
the ARDSNet should be set up to answer some of these
questions with large-scale trials. In addition, it would be
wonderful if a reasonably robust, yet less expensive (both
in monetary terms and in the numbers of patients required)
study designs could be developed. Perhaps for some
questions we should accept less stringent P values when
assessing a mortality endpoint. After all, a P value of less
than 0.05 is arbitrary, and for studies that make physiological sense and have other physiological endpoints that
seem to be improving, a less stringent statistical hurdle
might be appropriate. This is particularly true for therapies
for which there is no physiological or biological concern a
priori concerning the toxicity of the intervention.
In this regard, it has been argued that physiological (also
called intermediate) endpoints might be useless, and even
grossly misleading. For example, in the ARDSNet study
the PaO2 : inspired fractional concentration of O2 (FIO2)
ratio was higher in the 12 ml/kg group for the first couple
of days and yet mortality also ended being higher in this
group. Results such as this have been used to suggest
that studies that use physiological endpoints should not
be used to change clinical practice. We would argue that
physiological endpoints might be useful but should be
used advisedly. Intermediate endpoints that are immediately downstream from a specific intervention might not
be useful. By downstream we mean physiological events
that are a direct consequence of the intervention. For
example, we know that higher mean airway pressures, as
would be observed with higher Vt values, usually lead
directly to higher PaO2 values; the use of inhaled nitric
oxide also leads directly to increases in PaO2. Because
these endpoints are a direct consequence of the intervention, they might not give us clues to potential detrimental
effects of the interventions and hence might not be ideal
endpoints for outcome studies. However, endpoints that
are further downstream and are correlated with mortality
might be suitable; an example of such an endpoint within
the context of ventilation trials might be changes in inflammatory cytokines with different ventilatory strategies.
Finally, what are the mechanisms that led to the lower
mortality in the 6 ml/kg group? It was certainly not due to a
decrease in barotrauma, as the incidence of barotrauma
was virtually identical in the two groups (10% versus
11%). It is tempting to speculate that it might have been
related to the greater decrease in serum cytokines (interleukin-6 was measured in the present study). As discussed above, it had previously been suggested that
injurious forms of mechanical ventilation could lead to an
increase in various mediators in the lung (biotrauma) and,
owing to the increased alveolar-capillary permeability, that
these mediators might enter the circulation and cause
organ dysfunction. This hypothesis is attractive and has
some indirect experimental support data [22], but it is
extremely difficult to prove at the moment all we have is
tantalizing correlative results, but a definitive answer to
this question might require a study that specifically targets
these mediators and examines changes in outcome.
Indeed, if this hypothesis is correct, it would suggest possible novel approaches to the assessment and treatment of
patients at risk for VILI. Ideally, one should apply ventilatory
strategies that are relatively non-injurious, but in patients
with severe ARDS this might be extremely difficult, if not
impossible, because of the spatial heterogeneity of their
lung disease [23]. A strategy that maintains a given lung
unit open might lead to the overdistension of other units. In
situations such as this, anti-inflammatory therapies (such as
anti-cytokine therapies) might prove to be useful adjuncts
to lung protective strategies [24,25], possibly by preventing distal organ injury. Admittedly this approach is purely
conjectural at the moment, but if it turns out to be correct,
how might we decide which patients would benefit from
these therapies? A number of studies have now shown that
septic patients who are homozygous for a specific polymorphism in the tumor necrosis factor- (TNF-) gene
have increased TNF- levels and have an increased risk of
death. If similar polymorphisms turn out to be important in
the context of the biotrauma induced during mechanical
ventilation, then a ventilated patients therapy in future
might depend on their particular genotype, an approach
that might be known as ventilogenomics, indicating the
interplay between the patients genetic predisposition to
biotrauma and ventilatory strategy. Perhaps patients with a
genetic predisposition to the development of high levels of
pro-inflammatory mediators would be those who require
these novel adjunctive anti-inflammatory therapies.
http://respiratory-research.com/content/1/2/073
Summary
Acknowledgement
References
1.
2.
3.
4.
5.
7.
8.
9.
10.
11.
12.
14.
Correspondence: Arthur Slutsky, MD, St Michaels Hospital, Queen
Street Wing, Room 4-042, 30 Bond Street, Toronto, Ontario M5B
1W8, Canada. Tel: +1 416 864 5637; fax: +1 416 864 5117;
e-mail: arthur.slutsky@utoronto.ca
primary research
13.
Authors affiliations: Arthur S Slutsky (St Michaels Hospital,
University of Toronto, Toronto, Canada) and V Marco Ranieri
(Dipartimento di Chirurgia, Anestesiologia, Rianimazione,
Universit di Pisa, Pisa, Italy)
reports
6.
Ashbaugh DG, Bigelow DB, Petty TL, Levine BE: Acute respiratory
distress in adults. Lancet 1967, ii:319323.
Ware LB, Matthay MA: The acute respiratory distress syndrome. N
Engl J Med 2000, 342:13341349.
Milberg JA, Davis DR, Steinberg KP, Hudson LD: Improved survival
of patients with acute respiratory distress syndrome (ARDS):
19831993. J Am Med Ass 1995, 273:306309.
Dreyfuss D, Saumon G: Ventilator-induced lung injury: lessons
from experimental studies. Am J Respir Crit Care Med 1998, 157:
294323.
Dreyfuss D, Soler P, Basset G, Saumon G: High inflation pressure
pulmonary edema. Respective effects of high airway pressure,
high tidal volume, and positive end-expiratory pressure. Am Rev
Respir Dis 1988, 137:11591164.
Muscedere JG, Mullen JB, Gan K, Slutsky AS: Tidal ventilation at low
airway pressures can augment lung injury. Am J Respir Crit Care
Med 1994, 149:13271334.
Slutsky AS: Lung injury caused by mechanical ventilation. Chest
1999, 116(Suppl 1):9S15S.
Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS: Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in
an isolated rat lung model. J Clin Invest 1997, 99:944952.
Tremblay LN, Slutsky AS: Ventilator-induced injury: from barotrauma to biotrauma. Proc Ass Am Physicians 1998, 110:482488.
Slutsky AS, Tremblay LN: Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med
1998, 157:17211725.
Slutsky AS: Mechanical ventilation. Chest 1993, 10:18331859.
[Published erratum appears in Chest 1994, 106:656.]
The Acute Respiratory Distress Syndrome Network: Ventilation with
lower tidal volumes as compared with traditional tidal volumes for
acute lung injury and the acute respiratory distress syndrome. N
Engl J Med 2000, 342:13011308.
Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky
SE, Mazer CD, McLean RF, Rogovein TS, Schouten BD, Todd TR,
Slutsky AS: Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. N Engl J Med 1998, 338:355361.
Brochard L, Roudot-Thoraval F, Roupie E, Delclaux C, Chastre J, Fernandez-Mondejar E, Clementi E, Mancebo J, Factor P, Matamis D,
Ranieri M, Blanch L, Rodi G, Mentec H, Dreyfuss D, Ferrer M, BrunBuisson C, Tobin M, Lemaire F: Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress
syndrome. The Multicenter Trail Group on Tidal Volume Reduction
in ARDS. Am J Respir Crit Care Med 1998, 158:18311838.
review
This work was supported in part by the Medical Research Council of
Canada (grant no. 8558).
15. Brower RG, Shanholtz CB, Fessler HE, Shade DM, White P, Jr,
Wiener CM, Teeter JG, Dodd-o JM, Almog Y, Piantadosi S: Prospective, randomized, controlled clinical trial comparing traditional
versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med 1999, 27:14921498.
16. Hickling KG, Henderson SJ, Jackson R: Low mortality associated
with low volume pressure limited ventilation with permissive
hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med 1990, 16:372377.
17. Shibata K, Cregg N, Engelberts D, Takeuchi A, Fedorko L, Kavanagh
BP: Hypercapnic acidosis may attenuate acute lung injury by inhibition of endogenous xanthine oxidase. Am J Respir Crit Care Med
1998, 158:15781584.
18. Laffey JG, Kavanagh BP: Carbon dioxide and the critically ill too
little of a good thing? Lancet 1999, 354:12831286.
19. Feihl F, Perret C: Permissive hypercapnia. How permissive should
we be? Am J Respir Crit Care Med 1994, 150:17221737.
20. Tobin MJ: Culmination of an era in research on the acute respiratory distress syndrome [editorial; comment]. N Engl J Med 2000,
342:13601361.
21. Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP,
Lorenzi-Filho G, kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR: Effect of a protective-ventilation strategy on
mortality in the acute respiratory distress syndrome. N Engl J Med
1998, 338:347354.
22. Ranieri VM, Giunta F, Suter PM, Slutsky AS: Mechanical ventilation
as a mediator of multisystem organ failure in acute respiratory
distress syndrome. J Am Med Ass 2000, 284:4344.
23. Gattinoni L, DAndrea L, Pelosi P, Vitale G, Pesenti A, Fumagalli R:
Regional effects and mechanism of positive end-expiratory pressure in early adult respiratory distress syndrome. J Am Med Ass
1993, 269:21222127. [Published erratum appears in J Am Med Ass
1993, 270:1814].
24. Narimanbekov IO, Rozycki HJ: Effect of IL-1 blockade on inflammatory manifestations of acute ventilator-induced lung injury in a
rabbit model. Exp Lung Res 1995, 21:239254.
25. Imai Y, Kawano T, Iwamoto S, Nakagawa S, Takata M, Miyasaka K:
Intratracheal anti-tumor necrosis factor-alpha antibody attenuates
ventilator-induced lung injury in rabbits. J Appl Physiol 1999, 87:
510515.
commentary
These are exciting times for basic scientists, clinical
researchers and physicians caring for patients with ARDS.
Basic discoveries in the laboratory have been translated
into randomized controlled trials, demonstrating
decreases in mortality in patients with ARDS by changes
in ventilatory strategy that are relatively easy to implement
in all ICUs. Furthermore, there is now the hope that a
number of other ventilatory and non-ventilatory interventions that are currently under intense study (recruitment
maneuvers, higher PEEP levels, prone positioning, highfrequency ventilation, liquid ventilation) will be found to
decrease mortality further in ARDS patients. Finally, as our
understanding of the molecular consequences of VILI
increases, and as our understanding of genetic DNAsequence variants increases, novel approaches to antiinflammatory therapies of VILI will certainly emerge.
Potrebbero piacerti anche
- Close Lung 2018Documento8 pagineClose Lung 2018Sergio CastellanosNessuna valutazione finora
- Ventilation During Lung Resection and Critical Care ComparativeDocumento11 pagineVentilation During Lung Resection and Critical Care Comparativeema moralesNessuna valutazione finora
- Ventilator Induced Lung Injury PreventionDocumento8 pagineVentilator Induced Lung Injury Preventionapi-602219685Nessuna valutazione finora
- Pharmacologictreatments Foracuterespiratory DistresssyndromeDocumento17 paginePharmacologictreatments Foracuterespiratory DistresssyndromeLuis HernándezNessuna valutazione finora
- 9mechanical Ventilation in Sepsis-Induced Acute LungDocumento6 pagine9mechanical Ventilation in Sepsis-Induced Acute LungTeodora ZamfirescuNessuna valutazione finora
- Newer Modes of Ventilation2Documento9 pagineNewer Modes of Ventilation2Saradha PellatiNessuna valutazione finora
- Acute Respiratory Distress SyndromeDocumento43 pagineAcute Respiratory Distress SyndromeAgnes Pritama Fahmi100% (1)
- Acuterespiratorydistress Syndrome: Ventilator Management and Rescue TherapiesDocumento16 pagineAcuterespiratorydistress Syndrome: Ventilator Management and Rescue TherapiessamuelNessuna valutazione finora
- Minmizar El VILIDocumento9 pagineMinmizar El VILIgiseladelarosa2006Nessuna valutazione finora
- Ventilación Del Pulmón Sano CC Current 20Documento5 pagineVentilación Del Pulmón Sano CC Current 20Silvia Lorena Mireles GonzálezNessuna valutazione finora
- The Acute Respiratory Distress Syndrome in 2013: Review Open AccessDocumento6 pagineThe Acute Respiratory Distress Syndrome in 2013: Review Open Accessindriyanti natasya ayu utami kottenNessuna valutazione finora
- Acute Respiratory Distress SyndromeDocumento6 pagineAcute Respiratory Distress SyndromeNove Mae EscabarteNessuna valutazione finora
- Tidal Volume in Patients With Normal Lungs During General AnesthesiaDocumento9 pagineTidal Volume in Patients With Normal Lungs During General Anesthesiariri fitrisariNessuna valutazione finora
- RCT of Aprv Vs LPV in Ards PtsDocumento11 pagineRCT of Aprv Vs LPV in Ards PtsOldriana Prawiro HapsariNessuna valutazione finora
- Intraoperative Mechanical Ventilation Strategies in Patients Undergoing One-Lung Ventilation: A Meta-AnalysisDocumento12 pagineIntraoperative Mechanical Ventilation Strategies in Patients Undergoing One-Lung Ventilation: A Meta-AnalysisdrvmkamathNessuna valutazione finora
- Driving Pressure Study OLVDocumento13 pagineDriving Pressure Study OLVlucasNessuna valutazione finora
- The ABC of Weaning Failure - A Structured ApproachDocumento9 pagineThe ABC of Weaning Failure - A Structured ApproachArul ShanmugamNessuna valutazione finora
- Applsci 09 02842 PDFDocumento13 pagineApplsci 09 02842 PDFFelicia SutarliNessuna valutazione finora
- BMJ Open 2015 ZhangDocumento10 pagineBMJ Open 2015 ZhangLuis CordovaNessuna valutazione finora
- Acute Respiratory Distress Syndrome: Clinical ReviewDocumento6 pagineAcute Respiratory Distress Syndrome: Clinical ReviewVijeyachandhar DorairajNessuna valutazione finora
- The Acute Respiratory Distress Syndrome: Review SeriesDocumento10 pagineThe Acute Respiratory Distress Syndrome: Review Seriesadek07Nessuna valutazione finora
- Low-Tidal-Volume Ventilation in The Acute Respiratory Distress SyndromeDocumento8 pagineLow-Tidal-Volume Ventilation in The Acute Respiratory Distress SyndromeJimmy Christianto SuryoNessuna valutazione finora
- Invasive Mechanical Ventilation PDFDocumento4 pagineInvasive Mechanical Ventilation PDFRaquel AguilarNessuna valutazione finora
- CHEST Nov 2005Documento183 pagineCHEST Nov 2005rizjkuramaNessuna valutazione finora
- Unraveling The Pathophysiology of The Asthma-COPD Overlap SyndromeDocumento8 pagineUnraveling The Pathophysiology of The Asthma-COPD Overlap SyndromeRadhianie DjanNessuna valutazione finora
- Perioperative Lung ProtectionDocumento6 paginePerioperative Lung ProtectionWilliamRayCassidyNessuna valutazione finora
- Ards 2Documento7 pagineArds 2LUCIBELLOT1Nessuna valutazione finora
- Roberts 2019 Year in Review Adult Invasive MechDocumento6 pagineRoberts 2019 Year in Review Adult Invasive MechJose Luis Escobar zabaleguiNessuna valutazione finora
- Essentials in Lung TransplantationDa EverandEssentials in Lung TransplantationAllan R. GlanvilleNessuna valutazione finora
- AsmathDocumento6 pagineAsmathRido SaputraNessuna valutazione finora
- Ventilation and ARDS: by George WileyDocumento5 pagineVentilation and ARDS: by George Wileyfandi dwi setyokoNessuna valutazione finora
- Fisiopatologia de SDRADocumento19 pagineFisiopatologia de SDRARuben HerediaNessuna valutazione finora
- 40Documento9 pagine40Eduardo SoaresNessuna valutazione finora
- Acute Respiratory Distress Syndrome: Jesse B. Hall, MD, FCCPDocumento12 pagineAcute Respiratory Distress Syndrome: Jesse B. Hall, MD, FCCPMayun CacoellNessuna valutazione finora
- Ajustesiniciales VNIDocumento3 pagineAjustesiniciales VNIDamian Torres SperanzaNessuna valutazione finora
- Editorial: Acute Respiratory Failure: Pathophysiological Basis From A Multidisciplinary Clinical ApproachDocumento2 pagineEditorial: Acute Respiratory Failure: Pathophysiological Basis From A Multidisciplinary Clinical ApproachAgil Rumboko SumitroNessuna valutazione finora
- CcventilacioninicialbuenoDocumento3 pagineCcventilacioninicialbuenoARELHI ROSARIO GARCIA MONROYNessuna valutazione finora
- Ventilatorymanagement Ofthenoninjuredlung: David L. Bowton,, Louis Keith ScottDocumento10 pagineVentilatorymanagement Ofthenoninjuredlung: David L. Bowton,, Louis Keith ScottGhost11mNessuna valutazione finora
- PDF PDFDocumento8 paginePDF PDFIcha Chaphedech hildantraNessuna valutazione finora
- Hfo 2Documento9 pagineHfo 2markus_danusantosoNessuna valutazione finora
- Dasenbrook higherPEEP 11 PrintedDocumento8 pagineDasenbrook higherPEEP 11 PrintedОлег СатишурNessuna valutazione finora
- Lung and Diaphragm Protective Ventilation Guided by The EsophagealDocumento3 pagineLung and Diaphragm Protective Ventilation Guided by The Esophagealema moralesNessuna valutazione finora
- Manajemen VentiDocumento2 pagineManajemen VentiBos AseNessuna valutazione finora
- Respiratory - ARDS Vent Revised - QuestionsDocumento6 pagineRespiratory - ARDS Vent Revised - QuestionsRyan ReNessuna valutazione finora
- Current Concepts of Protective Ventilation During GA - 2015Documento8 pagineCurrent Concepts of Protective Ventilation During GA - 2015Angy KarakostaNessuna valutazione finora
- PHYS THER-1981-Kigin-1724-36Documento15 paginePHYS THER-1981-Kigin-1724-36Bobeică S MihailNessuna valutazione finora
- Physiology of Mechanical Ventilation Critical Care ClinicsDocumento218 paginePhysiology of Mechanical Ventilation Critical Care ClinicsQuelamiaNessuna valutazione finora
- Effects of Lung Expansion Therapy On Lung Function in Patients With Prolonged Mechanical VentilationDocumento7 pagineEffects of Lung Expansion Therapy On Lung Function in Patients With Prolonged Mechanical Ventilationcard breatheNessuna valutazione finora
- CF Ventilation ManagementDocumento10 pagineCF Ventilation ManagementSherry SaidNessuna valutazione finora
- Author's Accepted Manuscript: Journal of Cardiothoracic and Vascular AnesthesiaDocumento23 pagineAuthor's Accepted Manuscript: Journal of Cardiothoracic and Vascular Anesthesiaricardo villaNessuna valutazione finora
- Acute Lung InjuryDocumento18 pagineAcute Lung InjuryM Rizal IsburhanNessuna valutazione finora
- Definisi MVDocumento7 pagineDefinisi MVArmi ZakaNessuna valutazione finora
- Zampieri 2019Documento8 pagineZampieri 2019Peter AgabaNessuna valutazione finora
- Intraoperative Protective Mechanical Ventilation Fact or FictionDocumento3 pagineIntraoperative Protective Mechanical Ventilation Fact or Fictionema moralesNessuna valutazione finora
- Mechanical Ventilation To Minimize Progression of Lung Injury in Acute Respiratory FailureDocumento5 pagineMechanical Ventilation To Minimize Progression of Lung Injury in Acute Respiratory FailureGenoveva Navarro SolorzaNessuna valutazione finora
- Evidence-Based Management of Acute Lung Injury and Acute Respiratory Distress SyndromeDocumento17 pagineEvidence-Based Management of Acute Lung Injury and Acute Respiratory Distress SyndromeretNessuna valutazione finora
- Asynchrony Consequences and ManagementDocumento17 pagineAsynchrony Consequences and ManagementAna MariaNessuna valutazione finora
- Pertanyaan 7Documento14 paginePertanyaan 7WicakNessuna valutazione finora
- Acute Respiratory Distress Syndrome Ventilator Management Strategies For AdultsDocumento36 pagineAcute Respiratory Distress Syndrome Ventilator Management Strategies For Adultsleokadiobarroso.rjNessuna valutazione finora
- Lung Protective Mechanical Ventilation StrategiesDocumento4 pagineLung Protective Mechanical Ventilation StrategiesAnne Julia AgustinNessuna valutazione finora
- Journal of Hematology & Oncology: A Journal Open To AllDocumento2 pagineJournal of Hematology & Oncology: A Journal Open To AllKay BristolNessuna valutazione finora
- Med Safety WHO HIS SDS 2019.4 EngDocumento16 pagineMed Safety WHO HIS SDS 2019.4 Engdzipraz dziprazNessuna valutazione finora
- El Descubrimiento Científico de Las Islas CanariasDocumento301 pagineEl Descubrimiento Científico de Las Islas CanariasCognosferaNessuna valutazione finora
- Final FlyerDocumento3 pagineFinal FlyerPony ParadiseNessuna valutazione finora
- Dental Asia JulDocumento96 pagineDental Asia JulAnonymous A8l9OjNessuna valutazione finora
- Jipmer Result 2015 UnreservedDocumento1.704 pagineJipmer Result 2015 UnreservedMota Chashma100% (1)
- JURDING Critical AppraisalDocumento4 pagineJURDING Critical AppraisalHafizhan MuhammadNessuna valutazione finora
- "Poison" "Poison": Substances Listed in Poison List "Poison List"Documento1 pagina"Poison" "Poison": Substances Listed in Poison List "Poison List"nizam_ghaniNessuna valutazione finora
- Annie's ResumeDocumento2 pagineAnnie's ResumeAnnie LuNessuna valutazione finora
- PHARMA 9 Trends and Issues in Nursing PharmacologyDocumento51 paginePHARMA 9 Trends and Issues in Nursing PharmacologyddsadNessuna valutazione finora
- Nciph ERIC6Documento4 pagineNciph ERIC6bejarhasanNessuna valutazione finora
- FDA Clinical Trial Requirements For Medical DevicesDocumento38 pagineFDA Clinical Trial Requirements For Medical DevicesUri HofferNessuna valutazione finora
- Clinical Oncology - Previous Examination Papers 2011Documento12 pagineClinical Oncology - Previous Examination Papers 2011wipi112Nessuna valutazione finora
- STD 12 Science Group B Ready Recknor VDocumento36 pagineSTD 12 Science Group B Ready Recknor Vfree accag100% (2)
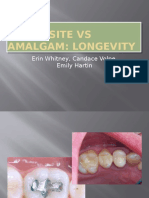
- Composite Vs Amalgam PowerpointDocumento17 pagineComposite Vs Amalgam Powerpointapi-322463366100% (1)
- Orthodontics Treatment For TMD JournalDocumento19 pagineOrthodontics Treatment For TMD JournalEndang SetiowatiNessuna valutazione finora
- Drug Registration and Essential DrugsDocumento42 pagineDrug Registration and Essential DrugsJeyanthakumar RasarathinamNessuna valutazione finora
- DOH HFSRB QOP 01 Form 2 3212019 postedDOHDocumento2 pagineDOH HFSRB QOP 01 Form 2 3212019 postedDOHMarcy100% (1)
- Hempel Vs Cydan Et Al-ComplaintDocumento34 pagineHempel Vs Cydan Et Al-Complaintforbesadmin100% (1)
- Ankleshwar ListDocumento11 pagineAnkleshwar ListMeghayu AdhvaryuNessuna valutazione finora
- Attaining A Working Archwire - Which Sequence?Documento1 paginaAttaining A Working Archwire - Which Sequence?vijayprabugNessuna valutazione finora
- Chapter 1: Structure and Composition: Independent Ethics CommitteeDocumento23 pagineChapter 1: Structure and Composition: Independent Ethics CommitteeBlissful DaysNessuna valutazione finora
- Critical Appraisal Therapy Study (Randomized Controlled Trial)Documento7 pagineCritical Appraisal Therapy Study (Randomized Controlled Trial)Putri Dwi KartiniNessuna valutazione finora
- Multiple Myeloma: Diagnosis & Management: Rakesh Popat UCL Cancer Institute & University College London HospitalsDocumento32 pagineMultiple Myeloma: Diagnosis & Management: Rakesh Popat UCL Cancer Institute & University College London HospitalsjderbyshNessuna valutazione finora
- Consensus of Prostho On Implants-2017Documento148 pagineConsensus of Prostho On Implants-2017Faheemuddin MuhammadNessuna valutazione finora
- Director Clinical Affairs Devices in Minneapolis ST Paul MN Resume Teree OlsonDocumento2 pagineDirector Clinical Affairs Devices in Minneapolis ST Paul MN Resume Teree OlsonTeree OlsonNessuna valutazione finora
- Kei Atân Erawh Zawng Pathian Hnaih Hi A Ha A NiDocumento4 pagineKei Atân Erawh Zawng Pathian Hnaih Hi A Ha A NiMatua AremesNessuna valutazione finora
- Sri Ramachandra University: Regn. No.: ......................... (To Be Filled by Office)Documento8 pagineSri Ramachandra University: Regn. No.: ......................... (To Be Filled by Office)kar_ind4u5636Nessuna valutazione finora
- Guidelines VertigoDocumento16 pagineGuidelines VertigoririnNessuna valutazione finora
- What To Do About Extracranial and Intracranial Stenosis: Home About Issues Categories Back Issues Case Reports ContactDocumento13 pagineWhat To Do About Extracranial and Intracranial Stenosis: Home About Issues Categories Back Issues Case Reports ContactpramitaListyNessuna valutazione finora