Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Proposal Vitamin C Content in Fruit
Caricato da
Nurul AshikinTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Proposal Vitamin C Content in Fruit
Caricato da
Nurul AshikinCopyright:
Formati disponibili
PROPOSAL
ANALYSIS OF VITAMIN C (ASCORBIC ACID) IN 10
FRUITS USING UV- SPECTROPHOTOMETER
ROYAL COLLEGE OF MEDICINE PERAK (UNIKL-RCMP)
SSM MODULE YEARR 2 2012
GROUP 33
RESEARCHERS
NURUL ATIQAH MAHMUD
57260211083
NUR HIDAYU FHATIHAH GHADZALI @GHAZALI
57260211062
NUR SYUHAINA ABU BAKAR
57260211126
MAZRIA AMANI MAZLAN
57260211130
SITI RASHIDAH ABU BAKAR
57260211051
SUPERVISOR
Checked by:
Approved by:
1.
INTRODUCTION
Vitamin C or ascorbic acid is a six-carbon lactone, which is synthesized from glucose
by many animals. Vitamin C is synthesized in the liver in some mammals and in the
kidney in birds and reptiles. However, this is not synthesized in a human body, which
makes it an essential nutrient which human obtained through dietary. Due to
deficiency of vitamin C, human suffer from the potentially lethal disease scurvy.
Vitamin C is largely distributed in fruits and plants that make it accessible to be
obtained. It is abundantly found in watermelon, papaya, oranges, apples, strawberries,
pineapple, mango and guava. It is also found in green leafy vegetables, cabbages,
cauliflower, broccoli and tomatoes.
Vitamin C is one of the important water-soluble vitamins. It is essential for
collagen, carnitine as well as neurotransmitter biosynthesis. Hence, vitamin C is
undeniably an important nutrient for metabolic processes in human. The current US
recommended daily allowance (RDA) for ascorbic acid ranges 100-120-mg/per day
for adults. Many health benefits have been attributed such as being a powerful
antioxidant, anti-atherogenic, anti-carcinogenic, immune-modulator and prevent cold
(K.A Naidu et. al, 2003).
Lately there has been mast production of synthetic or nutraceutical ascorbic
acid in the market to keep up with the demand of vitamin C supplement by the
consumers. A study showed that adults taking synthetic vitamin C supplementation
had serious effect. The result shows that synthetic vitamin C supplementation
decreases the exercise or training efficiency. This is because it depreciates the cellular
adaptations of exercise in the mitochondrial level.
Therefore, it is always best to consume natural vitamin C. Due to that, a
research to measure the content of vitamin C in five local fruits in Malaysia is carried
out to compare the ascorbic acid content between these fruits.
2.
LITERATURE REVIEW
A research study had been done regarding iodimetric determination of ascorbic acid
(vitamin C) in citrus fruits in year 2007. The study is conducted to determine the
ascorbic acid content of the juices from four different types of citrus fruits which are
orange, tangerine, grapefruit and lime. The study showed that orange has the highest
value of ascorbic acid (600 g /mL) followed by grape (446 g /mL) and then
tangerine (415 g /mL). Lime had the least value (306 g /mL). It is concluded that
orange would supply more ascorbic acid per milliliter for body need. From this
research, it is also concluded that the juices of oranges and tangerine are hereby
recommended, preferably, to be taken daily during meal at quantities sufficient to
meet daily vitamin C need (Izuagie et al. , 2007).
Besides, another research is carried out to quantify the total phenolics,
flavonoids and carotenoides, vitamin C contents, reported as antioxidants, in the
extracts of four locally available varieties of durian. In the research, the fruits are
extracted using dichloromethane: pentane (1:1 v/v) and subjected to study the various
quality parameters. The result showed the total of vitamin C contents.
This research also proved that caffeic acid and quercetin are the dominant
antioxidant substances found in durian. From this research, we know that the
bioactivity of ripe durian is high and the total polyphenols are the main contributors to
the overall antioxidant capacity. It is thus recommended to consume durian fruit for
uptake of antioxidant phytochemicals and vitamin C (Ashraf et al. , 2011).
3.
STUDY RATIONAL
Diets rich in fresh fruits and vegetables can protect us against chronic and
degenerative diseases (Joshipura et al. , 1999). The significant of this study is to study
the content of vitamin C in fresh fruits highly available in the market. Due to the side
effects of the synthetic supplementation of vitamin C, this study is carried out to find
the content of naturally available vitamin C in fresh fruits. This is significant to
educate the public that vitamin C supplementation can be taken naturally from fruits.
It is without a doubt that synthetic, nutraceutical vitamin C capsule available in the
market is modified chemically to prolong their time of consume. With this study, we
can help educate the public about the significant of eating fresh fruits that is readily
available in the market and therefore create awareness to take natural
supplementation, which is cheaper and healthier.
4.
OBJECTIVES
4.1.
GENERAL OBJECTIVES
4.1.1.
To determine the content of vitamin C in nine local fruits and an import fruit
in comparison with supplement vitamin C.
4.2.
SPECIFIC OBJECTIVES
4.2.1. To study the concentration of vitamin C using UV spectrophotometer method
with sodium oxalate acts as stabilizer.
4.2.2. To compare the ascorbic acid content between Orange and nine local fruits in
Malaysia which are:
4.2.2.1.
4.2.2.2.
4.2.2.3.
4.2.2.4.
4.2.2.5.
4.2.2.6.
4.2.2.7.
4.2.2.8.
4.2.2.9.
Guava
Mango
Durian
Pamelo
Banana
Pineapple
Star fruit
Papaya
Water apple/ bell fruit
4.2.3. To compare all the 10 fruits with the supplement vitamin C available in the
market.
4.2.4. To determine which fruits have the highest concentration of vitamin C.
4.2.5. To determine the pH value in each fruits used in the experiment.
4.2.6. To educate the public about the importance of vitamin C in daily diet.
4.2.7. To provide alternative for the public who want cheaper and healthier supply of
vitamin C.
5.0.
METHODOLOGY
There are many analytical methods have been reported to determine vitamin C
concentration such as titrimetry (Gunjan, 2012), electrochemical (Kim, 1989) and
potentiometric method (Leslie et al. , 1941). Some methods are available for
determination of ascorbic acid but only few are found to be used to determine both
forms of vitamin C (L-ascorbic acid and dehydroascorbic acid). It is very difficult to
choose a suitable method to determine the vitamin C contents in fruit juice. All the
methods have their own limitation for different purpose. (Mohammad et al. , 2007).
In this study, the method chosen is direct spectrophotometric method. It is
simple and highly sensitive method for determination of L-ascorbic acid in pure form.
This method used sodium oxalate (0.0056 mol/dm 3) as a stabilizer and molar
absorptivity (which does not require an extraction procedure) was 1.42x104 dm3mol1
cm-1 at 266nm. Beers law is obeyed in concentration range of 0.857-12.0 g ascorbic
acid/cm3 (Amra et al. , 2011).
5.1.
INSTRUMENTS
UV-Visible spectrophotometer using 1cm path length is used to measure the content
of vitamin C in each sample solutions. Digital pH meter is used to determine the pH
value in each sample solutions.
5.2.
REAGENTS
All reagents used are of analytical-reagent grade. Buffer solution (pH = 5.4), Sodium
oxalate solution (0.0056 mol/dm3) and L-ascorbic acid solution (1.13x10-3 mol/dm3).
5.3.
REAGENT PREPARATION
Buffer solution (pH = 5.4)
A mixture of potassium dihydrogenphosphate (0.03 mol/dm 3) and disodium
hydrogenphosphate (8.99x10-4 mol/dm3) is prepared by dissolving 4.08 g of KH 2PO4
(Fluka) and 0.16 g of Na2HPO4.2H2O (Merck) in 100 cm3 of distilled water.
Sodium oxalate solution (0.0056 mol/dm3)
This solution is prepared by dissolving 0.75 g of sodium oxalate (Sigma) in 1000 cm 3
of the buffer solution.
L-Ascorbic acid solution (1.13x10-3 mol/dm3)
A 0.05 g amount of L-ascorbic acid (Riedel-de Han) was dissolved in 250 cm 3 of the
sodium oxalate solution.
5.4.
SAMPLE
10 raw material of fresh fruits used in this study are purchased from a local
supermarket on the day of analysis. Guava (Psidium guajava), mango (Mangifera
indica), durian (Durio zibethinus), pamelo (Citrus grandis), banana (Musa spp),
pineapple (Ananas comosus), star fruit (Averrhoa carambola), papaya (Carica
papaya), water apple (Syzygium samarangense). The fruits chosen are in ripest state
(according to assumption).
5.5.
SAMPLE PREPARATION
All the 10 sample of fruits are washed thoroughly with water and the juices are
extracted manually using juice squeezer. All samples are filtered to remove pulp and
seed. After that they are stored in already labelled plastic containers. A small amount
of fruit juice is sufficient for the determination of ascorbic acid in these samples.
5.6.
GENERAL PROCEDURE
Transfer a portion of the sample solution containing 60 300 g of L-ascorbic acid to
a 25 cm3 standard flask. Dilute to the mark with the (0.0056 mol/dm 3) sodium oxalate
solution and measure the absorbance at 266 nm against the sodium oxalate solution as
a blank.
5.7.
DETERMINATION OF L-ASCORBIC ACID IN FRUIT SAMPLES
Transfer an accurately weighed amount of powder obtained from several tablets into a
100 cm3 volumetric flask, dissolve and make up to the mark with the (0.0056
mol/dm3) sodium oxalate solution. Filter and dilute a suitable aliquot of the filtrate to
50 cm3 with the stabilizer solution. Take an aliquot of the final solution and determine
the ascorbic acid content as described under general procedure.
5.8.
DETERMINATION pH IN FRUIT SAMPLES
Set the digital pH meter to set mode and insert the electrode into the solution samples.
Wait the reading to stabilize before record the value appeared on the display. Always
rinse the electrode before use it to test other sample solutions.
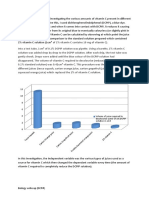
6.0.
RESULTS
Plan for data analysis and interpretation
Table 1: Biochemical characteristics of different types of fruits in Malaysia
Types of fruits
Vitamin C Contents(mg/100g)
pH
Orange
Bell fruit
Guava
Banana
Mango
Papaya
Starfruit
Pineapple
Durian
Pomelo
Figure 1: Experimental and literature values of vitamin C content for fresh
tropical fruits
7.0.
REFERENCES
K.A Naidu (2003) Vitamin C in human health and disease is still a mystery? An
overview, Nutrition Journal, 2(7), 1.
Joshipura, Alberto. A, JoAnn E.M, Meir J. S, Eric B.R, Frank E. S, Charles H.H,
Donna S, Walter C.W (1999) Fruits and vegetables Intake in Relation to Risk
of Ischemic Stroke, The Journal of American Medical Association (JAMA),
282(13), 1237-1239.
Ashraf, M. A., Maah, M. J., Yusoff, I., Mahmood, K., & Wajid, A. (2011). Study of
Antioxidant Potential of Tropical Fruit. International Journal of Bioscience,
Biochemistry and Bioinformatics, 1(1), 53-57.
Izuagie, A. A., & Izuagie, F. O. (2007). Iodimetric Determination of Ascorbic Acid
(Vitamin C) in Citrus Fruits. Research Journal of Agriculture and Biological
Sciences, 3(5), 367-369).
Gunjan Kashyap & Mangla Dave Gautam (2012). Analysis of Vitamin C in
Commercial and Naturals substances by Iodometric Titration found in Nimar
and Malwa region. Journal of Scientific Research in Pharmacy, 2277-9469.
Kim HJ. (1989). Determination of total vitamin C by ion exclusion chromatography
with electrochemical detection. US National Library of Medicine National
Institutes of Health Journal, Jul-Aug; 72(4): 681-6.
Leslie J. Harris, L. W. Mapson & Y. L. Wang (1941). A Simple Potentiometric
Method for Determining Ascorbic Acid, Suitable for Use with Coloured
Extracts. Nutritional Laboratory, University of Cambridge and Medical
Research Council.
Mohammad Mizanur Rahman Khan & Mohammad Mazedul Hosain (2007). Analysis
of Vitamin C (ascorbic acid) Contents in Various Fruits and Vegetables by
UV-spectrophotometry. Bangladesh J. Sci. Ind. Res. 42(4), 417-424, 2007.
Amra Selimovi, Mirsad Salki & Amel Selimovi (2011). Direct Spectrophotometric
Determination of L-ascorbic acid in Pharmaceutical Preparations Using
Sodium oxalate as a Stabilizer. International Journal of Basic & Applied
Sciences IJBAS-IJENS Vol: 11 No: 02.
Potrebbero piacerti anche
- Jaggery ProcessDocumento4 pagineJaggery ProcessAsh1ScribdNessuna valutazione finora
- Atomic Adsorption SpectrosDocumento14 pagineAtomic Adsorption SpectrosRzi Danil Ishutin0% (1)
- Electrogravimetry: Electrogravimetric Analysis V Semester Inorganic ChemistryDocumento2 pagineElectrogravimetry: Electrogravimetric Analysis V Semester Inorganic Chemistryvandv printsNessuna valutazione finora
- Gravimetric Sulfate DeterminationDocumento3 pagineGravimetric Sulfate DeterminationkatlegoNessuna valutazione finora
- Research ProposalDocumento6 pagineResearch ProposalrubyshreeNessuna valutazione finora
- Green Chemistry and Its Role For Sustainability: PresenterDocumento16 pagineGreen Chemistry and Its Role For Sustainability: PresenterMandeep BatraNessuna valutazione finora
- Bio ReportDocumento8 pagineBio ReportTharshini_Indr_6713Nessuna valutazione finora
- ConclusionDocumento1 paginaConclusionNitin KashyapNessuna valutazione finora
- Biology November ReportDocumento3 pagineBiology November ReportIndrani GoswamiNessuna valutazione finora
- Gobena's Thesis Commented 1Documento69 pagineGobena's Thesis Commented 1Gobena AdebaNessuna valutazione finora
- S Determination of Caffeine in BeveragesDocumento5 pagineS Determination of Caffeine in BeveragesVioleta Grigoras100% (1)
- Purification of Surface Water From Orange Peel WastesDocumento4 paginePurification of Surface Water From Orange Peel WastesJohn Bryan Aldovino100% (1)
- Simultaneous Determination of Salicylic Acid and Acetylsalicylic AciDocumento5 pagineSimultaneous Determination of Salicylic Acid and Acetylsalicylic Aciiabureid7460Nessuna valutazione finora
- Yeast LabDocumento4 pagineYeast LabAtikah OlivaNessuna valutazione finora
- LONG CAFFEINE UVLong Caffeine UvDocumento8 pagineLONG CAFFEINE UVLong Caffeine UvzaNessuna valutazione finora
- Computer Investigatory Project CBSE Class 12Documento10 pagineComputer Investigatory Project CBSE Class 12sidharth1196100% (3)
- Biology ProjectDocumento9 pagineBiology ProjectAbdul Sami0% (1)
- 6 - Toxicants Formed During ProcessingDocumento45 pagine6 - Toxicants Formed During Processingismailmannur100% (1)
- Ascorbic Acid ExperimentDocumento10 pagineAscorbic Acid ExperimentJoa YupNessuna valutazione finora
- Chemistry Investigatory Project OnDocumento15 pagineChemistry Investigatory Project OnjibinNessuna valutazione finora
- Dokumen - Tips - Toxicants Formed During Food Processing 2 PDFDocumento45 pagineDokumen - Tips - Toxicants Formed During Food Processing 2 PDFHồ Ngọc Thanh HiềnNessuna valutazione finora
- I EVALUATION OF THE TOXICITY OF THE METHANOLIC EXTRACT OF MORINGA OLEIFERA LEAVES ON MICEDocumento53 pagineI EVALUATION OF THE TOXICITY OF THE METHANOLIC EXTRACT OF MORINGA OLEIFERA LEAVES ON MICEAdetolaNessuna valutazione finora
- Chromatography - The Most Versatile Method of Chemical AnalysisDocumento438 pagineChromatography - The Most Versatile Method of Chemical AnalysisdnabrasilNessuna valutazione finora
- Vitamin C Write-UpDocumento5 pagineVitamin C Write-UpannafiiNessuna valutazione finora
- 4A F16 Exp 04 Absorbance and Fluorescence Spectros PDFDocumento27 pagine4A F16 Exp 04 Absorbance and Fluorescence Spectros PDFWanqing HeNessuna valutazione finora
- Food MCB II NotesDocumento73 pagineFood MCB II NotesRichard Simon KisituNessuna valutazione finora
- ThesisDocumento175 pagineThesisselvaraj2482100% (2)
- Chemical Equilibrium in A Liquid PhaseDocumento7 pagineChemical Equilibrium in A Liquid PhasePevie Anne Tenebroso100% (1)
- Enzyme, A Substance That Acts As ADocumento7 pagineEnzyme, A Substance That Acts As AAnanyaNessuna valutazione finora
- Estimation of Sugar by HAGEDORN - JensonDocumento3 pagineEstimation of Sugar by HAGEDORN - JensonTrung Mai VanNessuna valutazione finora
- Determination of The PH Value of Water Using A PH MeterDocumento7 pagineDetermination of The PH Value of Water Using A PH MeterRome Dionisio Komicho100% (1)
- Advantages of Liquid Liquid Extraction SystemDocumento5 pagineAdvantages of Liquid Liquid Extraction Systemkumarsachin3801100% (1)
- Complete AAS N ICPDocumento14 pagineComplete AAS N ICPMaxvicklye Rayner100% (1)
- DCPIP Write UpDocumento2 pagineDCPIP Write UpHashim Chishty0% (1)
- Cutm Examination HandbookDocumento73 pagineCutm Examination HandbookTarun Kumar PradhanNessuna valutazione finora
- Lab Manual For Down Stream Process LabDocumento31 pagineLab Manual For Down Stream Process LabShaasvath AishvaryaNessuna valutazione finora
- Chemistry Project On Presence of Insecticides Pesticides in Fruits VegetablesDocumento9 pagineChemistry Project On Presence of Insecticides Pesticides in Fruits VegetablesIshu SinghNessuna valutazione finora
- Chemistry Investigatory Project ReportDocumento9 pagineChemistry Investigatory Project ReportRaj ChaudhariNessuna valutazione finora
- Atr Ft-Ir Imaging of Acetic AcidDocumento10 pagineAtr Ft-Ir Imaging of Acetic AcidMon RonquilloNessuna valutazione finora
- C FABCON FINALEtotooHD720pDocumento39 pagineC FABCON FINALEtotooHD720pChloegelo MendozaNessuna valutazione finora
- Experiment On Vitamins - CONGSONDocumento3 pagineExperiment On Vitamins - CONGSONShayne Angelique CongsonNessuna valutazione finora
- Biology ProjectDocumento4 pagineBiology ProjectAnthony FalconNessuna valutazione finora
- Vitmin C ReportDocumento14 pagineVitmin C ReportOdongo TonnyNessuna valutazione finora
- Make Biochemistry Manual: Serum Calcium EstimateDocumento3 pagineMake Biochemistry Manual: Serum Calcium Estimatehaseeb ShafaatNessuna valutazione finora
- Chemistry Investigatory ProjectDocumento12 pagineChemistry Investigatory ProjectHuzaifa LokhandwalaNessuna valutazione finora
- Chemistry Investigatory ProjectDocumento16 pagineChemistry Investigatory Project10A 1 ANSH PANARANessuna valutazione finora
- FTIR Spectrometer AnalysisDocumento16 pagineFTIR Spectrometer AnalysisßraiñlĕsšȜĭnšteĭñNessuna valutazione finora
- Harmonized FTPE Curriculum-FinalDocumento222 pagineHarmonized FTPE Curriculum-Finalprashant_cool_4_u100% (1)
- Main Project TopicsDocumento1 paginaMain Project Topics1234cbriNessuna valutazione finora
- Measuring The Vitamin C Content of Foods and Fruit Juices Ss 36Documento1 paginaMeasuring The Vitamin C Content of Foods and Fruit Juices Ss 36Aji CuteMadNessuna valutazione finora
- Tissue CultureDocumento32 pagineTissue CultureYash WardhanNessuna valutazione finora
- Difference Between AC and DC MotorDocumento2 pagineDifference Between AC and DC MotorYrty KdfgNessuna valutazione finora
- (18530) Sheet 1 Electrochemistry B PDFDocumento99 pagine(18530) Sheet 1 Electrochemistry B PDFAnuragPandey100% (1)
- Prac 4 ReportDocumento15 paginePrac 4 ReportAvanthi Moodley100% (1)
- Chemistry Investigatory ProjectDocumento11 pagineChemistry Investigatory ProjectAmalendu Sundar MandalNessuna valutazione finora
- CHE555 Assignment 1 Mac 2015Documento2 pagineCHE555 Assignment 1 Mac 2015Jaja TeukieNessuna valutazione finora
- Ref 3Documento12 pagineRef 3ANDRES FELIPE MOSQUERA MARTINEZNessuna valutazione finora
- 265 2416 3 PB PDFDocumento5 pagine265 2416 3 PB PDFjessica sidhartaNessuna valutazione finora
- Yarkwanand OketundeDocumento8 pagineYarkwanand Oketundejihansalmaazhaarfirdaus00Nessuna valutazione finora
- Acido Ascorbico en Jugos de Fruta y Su Rata de Perdida Por AlamacenamientoDocumento4 pagineAcido Ascorbico en Jugos de Fruta y Su Rata de Perdida Por AlamacenamientoYuliana PerezNessuna valutazione finora
- HFMD N MeningitisDocumento19 pagineHFMD N MeningitisNurul AshikinNessuna valutazione finora
- Fracture Classification and Eponym Fractures: Supervisor: DR SyarifahDocumento56 pagineFracture Classification and Eponym Fractures: Supervisor: DR SyarifahNurul AshikinNessuna valutazione finora
- Obsteric EmergenciesDocumento48 pagineObsteric EmergenciesNurul AshikinNessuna valutazione finora
- Alzheimer FolioDocumento15 pagineAlzheimer FolioNurul AshikinNessuna valutazione finora
- PBL 1 ReportDocumento17 paginePBL 1 ReportDian Ratri CNessuna valutazione finora
- (Rath & Pauling) - Heart Disease, Ascorbate, Lysine and Linus Pauling by Jeffrey Dach MDDocumento21 pagine(Rath & Pauling) - Heart Disease, Ascorbate, Lysine and Linus Pauling by Jeffrey Dach MDpedpixNessuna valutazione finora
- Formulasi Sediaan ParenteralDocumento47 pagineFormulasi Sediaan ParenteralThania RaniNessuna valutazione finora
- A. K. Tripathi and Mukesh Gautam PDFDocumento6 pagineA. K. Tripathi and Mukesh Gautam PDFLingga NimpunaNessuna valutazione finora
- Fdocuments - in Kangen Water IndiaDocumento62 pagineFdocuments - in Kangen Water IndiaNitin MhatreNessuna valutazione finora
- The Pharmacology of Equisetum Arvense-A Review: Prof DR Ali Esmail Al-SnafiDocumento12 pagineThe Pharmacology of Equisetum Arvense-A Review: Prof DR Ali Esmail Al-SnafiBekaNessuna valutazione finora
- Varun II SeminarDocumento15 pagineVarun II Seminarchidananda sNessuna valutazione finora
- Canadian Journal of Diabetes: Malika Hamdiken MSC, Samira Bouhalit MSC, Zine Kechrid PHDDocumento9 pagineCanadian Journal of Diabetes: Malika Hamdiken MSC, Samira Bouhalit MSC, Zine Kechrid PHDbelenNessuna valutazione finora
- Diabet Zaharat-Adriana Ciobanu 0753590863Documento8 pagineDiabet Zaharat-Adriana Ciobanu 0753590863Adriana CiobanuNessuna valutazione finora
- Microbicidal Effect of Embilica Officianalis On Black Pigmented Bacteria in Patients With Periimplant MucositisDocumento10 pagineMicrobicidal Effect of Embilica Officianalis On Black Pigmented Bacteria in Patients With Periimplant MucositisARAVINDAN GEETHA KRRISHNANNessuna valutazione finora
- Chemical Composition and Antioxidant Capacity of Lettuce: Comparative Study of Regular-Sized (Romaine) and Baby-Sized (Little Gem and Mini Romaine) TypesDocumento11 pagineChemical Composition and Antioxidant Capacity of Lettuce: Comparative Study of Regular-Sized (Romaine) and Baby-Sized (Little Gem and Mini Romaine) TypesRahmania OktaNessuna valutazione finora
- SocoDocumento4 pagineSocoJerwilyn LicuasenNessuna valutazione finora
- 1 Hadzovic DzuvoDocumento7 pagine1 Hadzovic DzuvoRoy WilsonNessuna valutazione finora
- Antioksidan Tamarindus IndicaDocumento5 pagineAntioksidan Tamarindus IndicaFadli PutrantoNessuna valutazione finora
- HormonesBalance Superfoods Powerherbs Guides FINALDocumento28 pagineHormonesBalance Superfoods Powerherbs Guides FINALAndrada Faur100% (2)
- Alpha-Lipoic Acid As A Dietary SupplementDocumento12 pagineAlpha-Lipoic Acid As A Dietary SupplementKeyvanNessuna valutazione finora
- Definition of Empowered: Having The Knowledge, Confidence, Means, or Ability To Do Things or Make Decisions For OneselfDocumento10 pagineDefinition of Empowered: Having The Knowledge, Confidence, Means, or Ability To Do Things or Make Decisions For OneselfJaydee PacionNessuna valutazione finora
- Acceptability of Gumamela Hibiscus Rosa SinensisDocumento28 pagineAcceptability of Gumamela Hibiscus Rosa SinensisEdwin Balbon Jr.Nessuna valutazione finora
- 23 1Documento5 pagine23 1Cang Haedar100% (1)
- Chemistry F5 (Chp.5)Documento12 pagineChemistry F5 (Chp.5)Nurul DiyanaNessuna valutazione finora
- Development of Herbal Tea Using Moringa Olifera, Elsholtzia Communis and Alpinia Galanga: A Sensory Acceptance StudyDocumento8 pagineDevelopment of Herbal Tea Using Moringa Olifera, Elsholtzia Communis and Alpinia Galanga: A Sensory Acceptance StudyIJAR JOURNALNessuna valutazione finora
- 810822-Menara MGRN Krim (1116) 1x15kg Carton-B240 PDFDocumento4 pagine810822-Menara MGRN Krim (1116) 1x15kg Carton-B240 PDFQA RCPINessuna valutazione finora
- Moisture Rich Shower Cream LAVENDER & ORCHID& Olive Oil & Honey & Obliphicha Oil780mlDocumento20 pagineMoisture Rich Shower Cream LAVENDER & ORCHID& Olive Oil & Honey & Obliphicha Oil780mlSaayesha RamNessuna valutazione finora
- Super FoodsDocumento72 pagineSuper Foodsfarrah Z100% (1)
- Effect of Newer Antioxidants On The Bond Strength of Composite On Bleached EnamelDocumento6 pagineEffect of Newer Antioxidants On The Bond Strength of Composite On Bleached EnamelFilipe QueirozNessuna valutazione finora
- Comment protéger les acides gras polyinsaturés à longues chaînes oméga 3 (AGPI ‐‐ LC ω3) vis‐à‐vis de l'oxydationDocumento22 pagineComment protéger les acides gras polyinsaturés à longues chaînes oméga 3 (AGPI ‐‐ LC ω3) vis‐à‐vis de l'oxydationjb100% (1)
- Ebook Concise Pathology For Exam Preparation PDF Full Chapter PDFDocumento77 pagineEbook Concise Pathology For Exam Preparation PDF Full Chapter PDFdarrel.hoffman255100% (28)
- DR Brownstein A Novel Approach To Treating Cornavirus Using Nutritional and Oxidative TherapiesDocumento19 pagineDR Brownstein A Novel Approach To Treating Cornavirus Using Nutritional and Oxidative Therapiessame shitNessuna valutazione finora
- RegistrationDocumento9 pagineRegistrationhkalertNessuna valutazione finora
- Evaluation and Standardization of Process For Development of Carrot CandyDocumento134 pagineEvaluation and Standardization of Process For Development of Carrot CandyAnand SinghNessuna valutazione finora