Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Palliative Management of Malignant Ascites Guidance
Caricato da
Silvia MuttiDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Palliative Management of Malignant Ascites Guidance
Caricato da
Silvia MuttiCopyright:
Formati disponibili
This is an official Northern Trust policy and should not be edited
in any way
Palliative Management of Malignant
Ascites Guidance
Reference Number:
NHSCT/11/396
Target audience:
This Trust-wide policy is directed to medical and nursing staff working with adult
patients in the acute hospital setting.
Sources of advice in relation to this document:
Dr Jayne McAuley, Consultant Palliative Medicine
Dr Olivia Dornan, Consultant A & E
Replaces (if appropriate):
Previous Guidance for the Palliative Management of Malignant Ascites (2007)
Type of Document:
Trust Wide
Approved by:
Policy, Standards and Guidelines Committee
Date Approved:
15 December 2010
Date Issued by Policy Unit:
12 April 2011
NHSCT Mission Statement
To provide for all, the quality of service we expect for our families, and ourselves.
Palliative Management of
Malignant Ascites
Guidance
December 2010
Contents
Introduction
1
Purpose of the Guidance
1
Target Audience
1
Responsibilities
1
Equality, Human Rights and DDA
1
Alternative formats
2
Source of advice in relation to this document
2
Policy Statement
2
Management Plan
2
Checklist for Abdominal Paracentesis Consent
5
References
6
1
Palliative Management of Malignant Ascites Guidance
Introduction
Ascites is the accumulation of protein rich fluid in the peritoneal cavity and can be
classed as an exudate or transudate depending on the serum ascites albumin
gradient. It can occur in conditions such as cirrhosis of the liver, heart failure,
tuberculosis but over 90% is secondary to malignancy. The commonest cancers
to cause ascites are ovarian, breast and gastrointestinal. Peritoneal involvement
by the cancer causes an exudate to form with a high albumin content and low
gradient (<11g/L), while less commonly a transudate can form secondary to
cirrhosis or large liver metastases, with a low albumin content and high gradient
(>11g/L).
Purpose of the Guidance
Malignant ascites can give rise to distressing symptoms such as nausea,
vomiting, abdominal distension, pain and breathlessness and carries a poor
prognosis. Treatment should therefore be minimally invasive with minimisation of
the risk of complications and aimed at the relief of symptoms. This guidance has
been developed to support current best practice in the palliation of malignant
ascites at the end of life.
Target Audience
This Trust-wide policy is directed to medical and nursing staff working with adult
patients in the acute hospital setting.
Responsibilities
Directors
Responsibility is delegated to individual Directors who have responsibility to
ensure that this guidance has been disseminated to the clinical staff working
within their area of responsibility.
Clinical staff working in hospital and community environment
It is the responsibility of clinical staff working with adult patients in the acute
hospital setting to be aware of the guidance and to implement it when
appropriate.
Equality, Human Rights and DDA
The guidance is purely clinical in nature and will have no bearing in terms of its
likely impact on equality of opportunity or good relations for people within the
equality and good relations categories.
2
Alternative formats
This document can be made available on request on disc, larger font, Braille,
audio-cassette and in other minority languages to meet the needs of those who
are not fluent in English.
Sources of advice in relation to this document
The guidance author, responsible Assistant Director or Director as detailed on
policy title page should be contacted with regard to any queries on the content of
this policy.
Policy Statement
The management plan should be in line with current best practice but also be
minimally invasive, aim to relieve distressing symptoms and reduce the risk of
additional burden. The palliative management of malignant ascites in the NHSCT
should also be individualised, after an holistic assessment of the patient which
should include their disease stage, current symptom burden, prognosis and
informed goals of treatment.
Management Plan
Assess patient suitability for intervention and record findings in the
patients records.
If patient is too frail for either diuretics or invasive procedures, symptom
management with analgesia and antiemetics should be maximised, with
the Hospital Specialist Palliative Care Teams involvement.
Consider diuretics if abdomen not tense and symptoms mild or post
paracentesis to reduce re-accumulation of ascitic fluid. There is some
evidence that diuretics work best where there is evidence of portal
hypertension (1).
o check U&E & BP (baseline)
o if no contra-indications commence Spironolactone 100-200mg
mane
dose increase 100mg every 3-7 days
usual maintenance dose 300mg/day
o If no response after 2 weeks, but renal function satisfactory and
patient still suitable, consider addition of Furosemide 40mg mane
(response may take
10-28
/
7
to be evident).
3
o monitor BP (hypotension), U&E (hyperkalemia, renal function),
patients general condition, response (symptoms, abdominal girth,
weight loss) and discontinue medication if no evidence of benefit
after 1 month or if significant side effects develop.
Consider paracentesis if abdomen tense or symptoms moderate to
severe.
Paracentesis
Should only be undertaken by doctor competent in this procedure, or
under trainer supervision if part of practical skills training.
Give appropriate explanation and obtain written consent.
Ultrasound evaluation +/- marking of drainage site may be required if
concern over diagnosis, suspicion of bowel obstruction or loculation of
fluid.
IV fluids are not routinely required but patients with portal hypertension
secondary to massive liver metastases or heptocellular carcinoma +/-
cirrhosis may be at risk of hypovolaemia.
IV albumin has no proven role in paracentesis for malignant related
ascites (2) but if evidence of concomitant portal hypertension +/-
cirrhosis, especially in the setting of large volume paracentesis (more
than 5 litres/24 hours), an IV albumin infusion should be considered (6-
8g/L of ascitic fluid drained).
Drain should be in for the shortest time possible to reduce
complications especially infection and burden to the patient.
Procedure
Record baseline pulse, BP and check U&E. Platelet count (plt > 40) and
coagulation screen (INR < 1.4) should be checked in at risk patients
(previous chemotherapy / advanced liver disease) and corrective steps
taken if appropriate. Stop routine anticoagulation 48 hours before the
procedure.
If hypotensive, dehydrated or severe renal impairment, consider IV
hydration with 0.9% sodium chloride (150mls per litre of ascitic fluid
removed) and drain more slowly, ( 1L/hr and 3L overall).
Procedure should be performed by a doctor and nurse together to ensure
aseptic technique.
Have patient empty bladder and lie on bed at 45-90.
Choose drain site avoid scars, tumour masses, distended bowel or
bladder, liver, inferior epigastric artery. Preferred sites lower quadrants
lateral to the rectus muscle.
Put on sterile gloves.
Sterilize site with iodine.
Place sterile draping towels.
4
Anaesthetise chosen site by injecting 1 or 2% Lidocaine, including onto
peritoneum (max. 10mls).
A small nick is performed using a scalpel.
Introduce the catheter assembly of a Bonanno suprapubic catheter
through the abdominal wall by using short thrusts at first obliquely up to
the peritoneum and then perpendicularly to penetrate the peritoneum,
when resistance disappears. If position correct fluid should flow freely
when vent plug removed. If peritoneal fluid is very proteinaceous manual
aspiration with a 3 way valve and syringe may be necessary.
Disengage the needle from the catheter hub. Holding the needle to use it
as a guide, advance catheter until the suture disc is flat against the skin.
Connect the adapter clamp as directed and secure it with tape to the
abdominal wall.
Alternatively the trochar in peritoneal aspiration pack could be used if
clinician more familiar with it, time limited or no Bonanno catheter
available.
Connect the drainage bag to the catheter, emptying it as necessary.
Observations are recorded hourly until 2 hours post-procedure.
If patient dizzy during drainage clamp drain and record pulse and BP, if
hypotensive slow drainage as above or stop if appropriate.
Drain until rate slows or stops (usually 5L and 6 hrs, maximum 24 hrs).
Remove drain.
If minimal leakage from puncture site apply dressing pad.
If significant leakage persists make a purse string suture to control.
Monitor for complications especially pain, hypotension, haemorrhage and
infection and treat accordingly.
Aim to drain as quickly as possible so drain normally out within 24 hours.
The patient can be discharged a few hours after the drain is removed if
observations stable.
Other treatments
Insertion of a permanent indwelling paracentesis catheter (pleurx drain)
into the abdomen can be considered in palliative care patients who are
experiencing distressing symptoms from the rapid reaccumulation of the
fluid despite paracentesis on a weekly or fortnightly basis.
If required this procedure can be carried out in the radiology department at
Belfast City Hospital at the request of the patients oncologist. These
catheters are tunnelled to reduce the risk of infection.
Early studies suggest that this technique can provide a safe and effective
option for those patients with refractory malignant ascites who have a
prognosis of more than 3 months but for whom oncology treatment is no
longer an option (6,7).
For ongoing management of the catheter refer to NHSCT guidance on
skin-tunnelled indwelling (pleurx) catheters, 2010 (NHSCT/10/356).
5
Checklist for Abdominal Paracentesis Consent
Discuss the purpose of the procedure and the related complications listed below.
Allow opportunity for questions to be asked and appropriately answered, before
completion of the standard Trust consent form.
Common complications
A feeling of weakness and tiredness for 1 to 2 days.
Some leakage of the fluid from the drain site, once drain removed.
Nausea and/or vomiting.
Possible complications
Low blood pressure.
Kidney failure.
Infection.
Bleeding.
Puncture of an organ in the abdomen.
6
References
1. Runyon B, Hoefs J. Ascitic fluid analysis in malignancy-related ascites.
Hepatology 1988; 8: 1104-1109.
2. Stephenson J, Gilbert J. The Development of Clinical Guidelines on
Paracentesis for Ascites Related to Malignancy. Palliative Medicine 2002; 16:
213-218.
3. Regnard C, Hockley J. Ascites in a Clinical Decision Guide to Symptom
Relief in Palliative Care (5
th
edition) Radcliffe Medical Press, 2003.
4. Guidelines for the Management of Malignant Ascites St Francis Hospice
www.palliativedrugs.com newsletter October 03.
5. St. Josephs Mercy Hospice. Guidelines for the Management of Malignant
Ascites, www.palliativedrugs.com newsletter April 04.
6. Keen A, Fitzgerald D, Bryant A, Dickinson HO. Management of drainage for
malignant ascites in gynaecological cancer. The Cochrane Database of
Systematic Reviews 2010, Issue 1, CD0077e94.
7. Mercandante S, Intravaia G, Ferrara P, Villari P, David F. Peritoneal
catheter for continuous drainage of ascites in advanced cancer patients.
Support Care Cancer 2008; 8: 975-8.
8. Fleming ND, Alvarez-Secord A, Von Gruenigen V, Miller MJ, Abernethy AP.
Indwelling catheters for the management of refractory malignant ascites: a
systematic literature overview and retrospective chart review. Journal of Pain
and Symptom Management 2009; 38: 341-9.
Potrebbero piacerti anche
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- Conceps in Clinical Pharmacokinetics PDFDocumento294 pagineConceps in Clinical Pharmacokinetics PDFCareyTran100% (4)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Anti-Inflammatory Handbook - 1st Edition 2023Documento116 pagineThe Anti-Inflammatory Handbook - 1st Edition 2023king_zeusxNessuna valutazione finora
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- BPS Pharmacotherapy Bro - Ef1Documento6 pagineBPS Pharmacotherapy Bro - Ef1Wahab EhsanNessuna valutazione finora
- Lulu's Crafts Giraffe Lovey PatternDocumento5 pagineLulu's Crafts Giraffe Lovey PatternSilvia Mutti100% (1)
- Definition:-The Partograph Is A Simple Chart orDocumento12 pagineDefinition:-The Partograph Is A Simple Chart orBharat ThapaNessuna valutazione finora
- Cats AfghanDocumento4 pagineCats AfghanStregatta100% (3)
- Health Care Delivery System in IndiaDocumento24 pagineHealth Care Delivery System in Indiarizwan89% (9)
- Modern Healthcare ArchitectureDocumento15 pagineModern Healthcare ArchitectureKanak YadavNessuna valutazione finora
- Example Complete Mentoring Action PlanDocumento3 pagineExample Complete Mentoring Action PlanMike100% (1)
- Bobble Stitch HandbagDocumento6 pagineBobble Stitch HandbagRodrigo Pera67% (3)
- Crochet SweaterDocumento1 paginaCrochet SweaterSilvia MuttiNessuna valutazione finora
- Vintage Arm Warmer - Wholelottaknotting PDFDocumento5 pagineVintage Arm Warmer - Wholelottaknotting PDFSilvia MuttiNessuna valutazione finora
- Spice of Life Afghan - US VersionDocumento28 pagineSpice of Life Afghan - US VersionSilvia MuttiNessuna valutazione finora
- Beautiful Shells Blanket Stitch GuideDocumento3 pagineBeautiful Shells Blanket Stitch Guideioana_cozNessuna valutazione finora
- Drugs and Cosmetics Act and Schedule YDocumento60 pagineDrugs and Cosmetics Act and Schedule Yapi-381097682% (11)
- The Emerging Role of Serratiopeptidase in Oral Surgery: Literature UpdateDocumento5 pagineThe Emerging Role of Serratiopeptidase in Oral Surgery: Literature UpdatejklhjNessuna valutazione finora
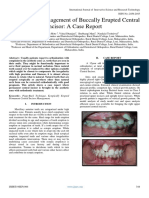
- Orthodontic Management of Buccally Erupted Central Incisor: A Case ReportDocumento3 pagineOrthodontic Management of Buccally Erupted Central Incisor: A Case ReportInternational Journal of Innovative Science and Research Technology100% (1)
- Medical BillDocumento1 paginaMedical BillVansh SaxenaNessuna valutazione finora
- Employer mental health policyDocumento5 pagineEmployer mental health policyMICHA FERRERNessuna valutazione finora
- Turkish Students' Poor Oral Hygiene HabitsDocumento5 pagineTurkish Students' Poor Oral Hygiene HabitsAamirNessuna valutazione finora
- Occupational Health A. Occupational HealthDocumento3 pagineOccupational Health A. Occupational HealthEry ShaffaNessuna valutazione finora
- Top Ten Health Issues/Problems Experienced in The PhilippinesDocumento3 pagineTop Ten Health Issues/Problems Experienced in The Philippinesangelus008Nessuna valutazione finora
- Situation 1Documento18 pagineSituation 1Maler De VeraNessuna valutazione finora
- Competency Appraisal 2: Integrating Health Education, Collaboration and Team WorkDocumento25 pagineCompetency Appraisal 2: Integrating Health Education, Collaboration and Team WorkKissy BesarioNessuna valutazione finora
- Unit Plan Nursing ManagementDocumento4 pagineUnit Plan Nursing ManagementSURAJ2792100% (1)
- Managerial Finance - Business ReportDocumento29 pagineManagerial Finance - Business ReportCythia NgNessuna valutazione finora
- South Sudan CD2030 2020Documento2 pagineSouth Sudan CD2030 2020anjelopaul2017Nessuna valutazione finora
- Bag Technique Lecture Notes PDFDocumento4 pagineBag Technique Lecture Notes PDFtanmai nooluNessuna valutazione finora
- Riverpark Dental of HowellDocumento8 pagineRiverpark Dental of HowellRiverpark Dental HowellNessuna valutazione finora
- Hebert 2016 - Guía Canadiense de ACVDocumento26 pagineHebert 2016 - Guía Canadiense de ACVAilen CastroNessuna valutazione finora
- Competing Thru The New Marketing MixDocumento6 pagineCompeting Thru The New Marketing MixRM ValenciaNessuna valutazione finora
- Daftar Pustaka Sanitasi dan Kesehatan MasyarakatDocumento4 pagineDaftar Pustaka Sanitasi dan Kesehatan MasyarakatDyahSavitriKusumoningtyasNessuna valutazione finora
- CareMore: Innovative Healthcare DeliveryDocumento30 pagineCareMore: Innovative Healthcare DeliveryPartnership to Fight Chronic DiseaseNessuna valutazione finora
- POZ 2020 HIV Drug Chart HighDocumento5 paginePOZ 2020 HIV Drug Chart HighReni BaliNessuna valutazione finora
- WTG IsraelDocumento63 pagineWTG IsraelOlajuwon Okubena100% (1)
- NursingDocumento20 pagineNursingCrazy StrangerNessuna valutazione finora
- King Khalid Hospital Viral Screening ReportDocumento1 paginaKing Khalid Hospital Viral Screening ReportCristal TannerNessuna valutazione finora