Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
7 Limited Pressure Cycle
Caricato da
captainhassDescrizione originale:
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
7 Limited Pressure Cycle
Caricato da
captainhassCopyright:
Formati disponibili
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M .
Mallikarjuna
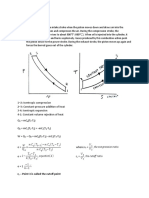
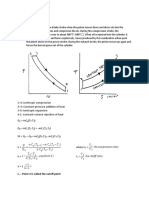
4.6 Limited Pressure Cycle (or Dual Cycle):
This cycle is also called as the dual cycle, which is shown in Fig.4.6. Here the heat
addition occurs partly at constant volume and partly at constant pressure. This cycle is a
closer approximation to the behavior of the actual Otto and Diesel engines because in
the actual engines, the combustion process does not occur exactly at constant volume
or at constant pressure but rather as in the dual cycle.
Process 1-2: Reversible adiabatic compression.
Process 2-3: Constant volume heat addition.
Process 3-4: Constant pressure heat addition.
Process 4-5: Reversible adiabatic expansion.
Process 5-1: Constant volume heat rejection.
3 4
Volume
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Constant Volume
Constant Pressure
4
2
5
Entropy
Fig.4.6. Dual cycle on p-v and T-s diagrams
Air Standard Efficiency:
Heat sup plied = m C v ( T3 - T2 ) + m Cp ( T4 - T3 )
Heat rejected = m C v ( T5 - T1 )
Net work done = m C v ( T3 - T2 ) + m Cp ( T4 - T3 ) - m CV ( T5 - T1 )
m C v ( T3 - T2 ) + m Cp ( T4 - T3 ) - m C v ( T5 - T1 )
ηth =
m C v ( T3 - T2 ) + m Cp ( T4 - T3 )
T5 - T1
ηth = 1 -
( T3 - T2 ) + γ ( T4 - T3 )
P3 v v
Let, = rp ; 4 = rc ; 1 = r
P2 v3 v2
T2 = T1 r γ - 1
T3 = T2 rp = T1 r γ - 1 rp
T4 = T3 rc = T1 r γ - 1 rp rc
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
γ -1 γ -1 γ -1
T5 ⎛v ⎞ ⎛v v ⎞ ⎛r ⎞
=⎜ 4⎟ = ⎜ 4. 2⎟ =⎜ c⎟
T4 ⎝ v5 ⎠ ⎝ v 2 v5 ⎠ ⎝r⎠
γ -1
⎛r ⎞
T5 = T4 ⎜ c ⎟ = T1 rp rcγ
⎝r⎠
T1 rp rcγ - T1
ηth = 1 -
{( T r
1
γ -1
) (
rp - T1 r γ - 1 + γ T1 r γ - 1 rp rc - T1 r γ - 1 rp )}
= 1-
( rp rcγ - 1)
{( rp r γ - 1 - r γ - 1 ) + γ ( rp rc r γ - 1 - rp r γ - 1 )}
1 ⎧⎪ rp rcγ - 1 ⎫⎪
ηth 1 - γ -1 ⎨ ⎬
r ( )
⎪⎩ rp - 1 + γrp ( rc - 1) ⎭⎪
From the above equation, it is observed that, a value of rp > 1 results in an increased
efficiency for a given value of rc and γ. Thus the efficiency of the dual cycle lies between
that of the Otto cycle and the Diesel cycle having the same compression ratio.
Mean Effective Pressure:
Workdone
mep =
Displacement volume
m C v ( T3 - T2 ) + m Cp ( T4 - T3 ) - m C v ( T5 - T1 )
=
v1 - v 2
m C v ( γ - 1) T1 ⎛ r - 1 ⎞
v1 - v 2 = ⎜ ⎟
p1 ⎝ r ⎠
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
p1 r ⎧⎪ T3 - T2 γ ( T4 - T3 ) T5 - T1 ⎫⎪
mep = ⎨ + - ⎬
( r -1)( γ - 1) ⎪⎩ T1 T1 T1 ⎭⎪
=
p1 r
( )( )
r - 1 γ - 1
{ ( ) (
r γ - 1 rp - 1 + γ r γ - 1 rp ( rc - 1) - rp rcγ - 1 )}
=
p1 r
( )( )
r - 1 γ - 1
{ {( ) } (
r γ - 1 rp - 1 + γ rp ( rc - 1) - rp rcγ - 1 )}
Indian Institute of Technology Madras
Potrebbero piacerti anche
- Fundamental Frequency TheoryDocumento9 pagineFundamental Frequency TheorytotalnjaNessuna valutazione finora
- Calculation of Time Required To Fill Tanks With Compressed GasDocumento4 pagineCalculation of Time Required To Fill Tanks With Compressed Gas1940LaSalleNessuna valutazione finora
- Minimising Crack Control Reinforcement - Design BookletDocumento21 pagineMinimising Crack Control Reinforcement - Design BookletNitish Ramdawor100% (1)
- C4-1 Lecture 5 - Dynamic Response of BuildingsDocumento75 pagineC4-1 Lecture 5 - Dynamic Response of Buildingselidstone@hotmail.comNessuna valutazione finora
- Many-Body Quantum Theory in Condensed Matter Physics Henrik Bruus and Karsten FlensbergDocumento352 pagineMany-Body Quantum Theory in Condensed Matter Physics Henrik Bruus and Karsten FlensbergSashaGorkorNessuna valutazione finora
- Solution Manual for an Introduction to Equilibrium ThermodynamicsDa EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNessuna valutazione finora
- Gas Turbines Unit 3: Gas Turbines and Jet Propulsion: Classification of Gas Turbines, Analysis of Open Cycle GasDocumento39 pagineGas Turbines Unit 3: Gas Turbines and Jet Propulsion: Classification of Gas Turbines, Analysis of Open Cycle GasJoseph MwansaNessuna valutazione finora
- 2.57 Nano-To-Macro Transport Processes Fall 2004: Ikn A IkxDocumento7 pagine2.57 Nano-To-Macro Transport Processes Fall 2004: Ikn A IkxcaptainhassNessuna valutazione finora
- Wide Beam DesignDocumento6 pagineWide Beam DesignNaveen RevannaNessuna valutazione finora
- Module 8 - FrictionDocumento17 pagineModule 8 - FrictionMadelar, Arc Francis C.Nessuna valutazione finora
- 9 Atkinson CycleDocumento3 pagine9 Atkinson CyclecaptainhassNessuna valutazione finora
- 4.10 Brayton Cycle (Simple Gas Turbine Cycle) :: Fig.4.10. Brayton Cycle On P-V and T-S DiagramDocumento3 pagine4.10 Brayton Cycle (Simple Gas Turbine Cycle) :: Fig.4.10. Brayton Cycle On P-V and T-S DiagrammominjeelaniNessuna valutazione finora
- 10 Lenoir CycleDocumento2 pagine10 Lenoir Cyclecaptainhass100% (1)
- Limited Pressure CycleDocumento4 pagineLimited Pressure Cyclecasu19Nessuna valutazione finora
- 11 - Dr. M. Atia - Gas Power Cycles-Dual CycleDocumento20 pagine11 - Dr. M. Atia - Gas Power Cycles-Dual CycleBahaa RaghebNessuna valutazione finora
- Unit-Ii Diesel, Gas Turbine and Combined Cycle Power PlantsDocumento71 pagineUnit-Ii Diesel, Gas Turbine and Combined Cycle Power Plantsrsankarganesh MECH-HICETNessuna valutazione finora
- Applied Thermodynamics ME250: Submitted ToDocumento12 pagineApplied Thermodynamics ME250: Submitted Tomad eye m00dyNessuna valutazione finora
- B59TC Gas Power Plant NotesDocumento23 pagineB59TC Gas Power Plant Noteshansdavid.aquino2004Nessuna valutazione finora
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocumento5 pagineDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNessuna valutazione finora
- Ideal CyclesDocumento2 pagineIdeal CyclesSofia OrjuelaNessuna valutazione finora
- JEE Advanced Heat and Thermodynamics Important QuestionsDocumento26 pagineJEE Advanced Heat and Thermodynamics Important QuestionsMayank VermaNessuna valutazione finora
- CLS JEEAD-19-20 XI Phy Target-5 Level-2 Chapter-11 PDFDocumento28 pagineCLS JEEAD-19-20 XI Phy Target-5 Level-2 Chapter-11 PDFVISHVESH GOYAL100% (1)
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocumento7 pagineDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNessuna valutazione finora
- Principle of TurbomachineryDocumento159 paginePrinciple of TurbomachineryWalid MohammedNessuna valutazione finora
- TDHT - 03 - Thermodynamic Cycles (Gas Steam) 1Documento19 pagineTDHT - 03 - Thermodynamic Cycles (Gas Steam) 1djukwe keuzetienNessuna valutazione finora
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocumento8 pagineDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNessuna valutazione finora
- A Cooling Unit To Increase The Efficiency of Gas Turbine PlantDocumento5 pagineA Cooling Unit To Increase The Efficiency of Gas Turbine PlantseventhsensegroupNessuna valutazione finora
- Kmme Te101-3Documento6 pagineKmme Te101-3ayla sözenNessuna valutazione finora
- Final Exam MockDocumento11 pagineFinal Exam Mockb8vfdrjff6Nessuna valutazione finora
- Cycle CalculationsDocumento6 pagineCycle CalculationsmudassarhussainNessuna valutazione finora
- Air Cycle RefrigerationDocumento10 pagineAir Cycle RefrigerationashisNessuna valutazione finora
- Gas TurbineDocumento7 pagineGas TurbineVishal BediNessuna valutazione finora
- Marine Diesel (WEEK - 3) Thermodynamics: Jurusan Teknik Sistem Perkapalan ITS SurabayaDocumento38 pagineMarine Diesel (WEEK - 3) Thermodynamics: Jurusan Teknik Sistem Perkapalan ITS SurabayafaridNessuna valutazione finora
- Gasturbine 1 160120155226Documento52 pagineGasturbine 1 160120155226Muhammad Afif NaufalNessuna valutazione finora
- 3-TurboEngine Components CharacteristicsDocumento10 pagine3-TurboEngine Components CharacteristicsAbdou JirariNessuna valutazione finora
- Otto CycleDocumento5 pagineOtto CycleSaraju NandiNessuna valutazione finora
- Mechanical Upto Ic Engine SpecifocationnDocumento22 pagineMechanical Upto Ic Engine SpecifocationnlechuznimaNessuna valutazione finora
- Otto Cycle, Fuels, Combustion PDFDocumento39 pagineOtto Cycle, Fuels, Combustion PDFAchmad Rizal FirmansyahNessuna valutazione finora
- Prelim Ito Na PreDocumento8 paginePrelim Ito Na PreJethro Briza GaneloNessuna valutazione finora
- Gas Power CyclesDocumento140 pagineGas Power CyclesMohammed AlsirajNessuna valutazione finora
- Tegar Ari Widianto 16306141051 Penyelesaian SoalDocumento10 pagineTegar Ari Widianto 16306141051 Penyelesaian SoalTegar Ari WidiantoNessuna valutazione finora
- 04 - Thermodynamic - Cycles - (Joule - B) PDFDocumento8 pagine04 - Thermodynamic - Cycles - (Joule - B) PDFAntonio Di FioreNessuna valutazione finora
- Chap 23Documento15 pagineChap 23민규Nessuna valutazione finora
- Ideal Engine CycleDocumento20 pagineIdeal Engine CycleMulugeta WoldeNessuna valutazione finora
- Applied Thermodynamics and Heat Transfer - Unit IDocumento37 pagineApplied Thermodynamics and Heat Transfer - Unit IArun ShankarNessuna valutazione finora
- ICE - Lecture From MapuaDocumento48 pagineICE - Lecture From MapuaMarcial Jr. MilitanteNessuna valutazione finora
- Power Cycles 1 - 1 PDFDocumento6 paginePower Cycles 1 - 1 PDFclarkmaxNessuna valutazione finora
- crs2 1Documento2 paginecrs2 1Chayut NuntadusitNessuna valutazione finora
- Thermo Cycles PDFDocumento29 pagineThermo Cycles PDFWaseem Abbas AttariNessuna valutazione finora
- 4 EngineCyclesAnalysisDocumento9 pagine4 EngineCyclesAnalysisNatthaphon NaosookNessuna valutazione finora
- Compres Turbine Heating (Combustion) 2: g-1 G g-1 G g-1 GDocumento7 pagineCompres Turbine Heating (Combustion) 2: g-1 G g-1 G g-1 GMrityunjay KrNessuna valutazione finora
- Lecture 5 - Gas Turbine EnginesDocumento11 pagineLecture 5 - Gas Turbine EnginesShailani HossainNessuna valutazione finora
- Derive An Expression For Efficiency of Diesel CycleDocumento3 pagineDerive An Expression For Efficiency of Diesel CycleME-Pratham JainNessuna valutazione finora
- CHE3161 Semester1 2010 Solutions PDFDocumento14 pagineCHE3161 Semester1 2010 Solutions PDFkumiristineNessuna valutazione finora
- Internal Combustion EnginesDocumento13 pagineInternal Combustion EnginesEunice Joy VillegasNessuna valutazione finora
- Lecture Notes OnDocumento200 pagineLecture Notes Onananth k r100% (3)
- AERO213: Aeroengines: AERO213 School of Engineering DR David JC Dennis 44831Documento9 pagineAERO213: Aeroengines: AERO213 School of Engineering DR David JC Dennis 44831Ahmed ElgamalNessuna valutazione finora
- Thermodynamics ConsidertionsDocumento20 pagineThermodynamics ConsidertionsCoordinador LaboratoriosNessuna valutazione finora
- The Spectral Theory of Toeplitz Operators. (AM-99), Volume 99Da EverandThe Spectral Theory of Toeplitz Operators. (AM-99), Volume 99Nessuna valutazione finora
- C*-Algebra Extensions and K-Homology. (AM-95), Volume 95Da EverandC*-Algebra Extensions and K-Homology. (AM-95), Volume 95Nessuna valutazione finora
- Recommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsDa EverandRecommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsE. F. G. HeringtonNessuna valutazione finora
- Group 15. Mechanics of Fluids: Vocabulary of Mechanics in Five Languages: English/German/French/Polish/Russian, Vol. 2Da EverandGroup 15. Mechanics of Fluids: Vocabulary of Mechanics in Five Languages: English/German/French/Polish/Russian, Vol. 2Valutazione: 1.5 su 5 stelle1.5/5 (3)
- An Introduction to Equilibrium Thermodynamics: Pergamon Unified Engineering SeriesDa EverandAn Introduction to Equilibrium Thermodynamics: Pergamon Unified Engineering SeriesNessuna valutazione finora
- Q FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004Documento5 pagineQ FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004 Guest Lecture by Prof. Mildred S. DresselhausDocumento10 pagine2.57 Nano-to-Macro Transport Processes Fall 2004 Guest Lecture by Prof. Mildred S. DresselhauscaptainhassNessuna valutazione finora
- Q FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004Documento7 pagineQ FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNessuna valutazione finora
- Lecture 18Documento6 pagineLecture 18captainhassNessuna valutazione finora
- d dT J Lq L dx dx: q=J (Π - Π) q=J (Π - Π)Documento6 pagined dT J Lq L dx dx: q=J (Π - Π) q=J (Π - Π)captainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004 - Lecture 15 Guest Lecture by Prof. DresselhausDocumento10 pagine2.57 Nano-to-Macro Transport Processes Fall 2004 - Lecture 15 Guest Lecture by Prof. DresselhauscaptainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004:) N N N N (NDocumento8 pagine2.57 Nano-to-Macro Transport Processes Fall 2004:) N N N N (NcaptainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004Documento9 pagine2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNessuna valutazione finora
- Lecture 14Documento8 pagineLecture 14captainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004: F M F F FDocumento5 pagine2.57 Nano-to-Macro Transport Processes Fall 2004: F M F F FcaptainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004: I T KXDocumento8 pagine2.57 Nano-to-Macro Transport Processes Fall 2004: I T KXcaptainhassNessuna valutazione finora
- N F T e U F T F TD D: 2.57 Nano-to-Macro Transport Processes Fall 2004Documento6 pagineN F T e U F T F TD D: 2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004: DT Q K T Q DX Q (W/M K (W/M-K) Is Thermal ConductivityDocumento5 pagine2.57 Nano-to-Macro Transport Processes Fall 2004: DT Q K T Q DX Q (W/M K (W/M-K) Is Thermal ConductivitycaptainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004: V L KK VKK N VDocumento7 pagine2.57 Nano-to-Macro Transport Processes Fall 2004: V L KK VKK N VcaptainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004: Ae Be CeDocumento7 pagine2.57 Nano-to-Macro Transport Processes Fall 2004: Ae Be CecaptainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004: Nlms 2Documento7 pagine2.57 Nano-to-Macro Transport Processes Fall 2004: Nlms 2captainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004: Ja (j-1) A (j+1) ADocumento9 pagine2.57 Nano-to-Macro Transport Processes Fall 2004: Ja (j-1) A (j+1) AcaptainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004: A Wigner-Seitz Primitive Unit CellDocumento6 pagine2.57 Nano-to-Macro Transport Processes Fall 2004: A Wigner-Seitz Primitive Unit CellcaptainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004: E HN MDocumento10 pagine2.57 Nano-to-Macro Transport Processes Fall 2004: E HN McaptainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004Documento7 pagine2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004Documento7 pagine2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004Documento6 pagine2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNessuna valutazione finora
- L-05 (GDR) (Et) ( (Ee) Nptel)Documento11 pagineL-05 (GDR) (Et) ( (Ee) Nptel)nvnmnitNessuna valutazione finora
- 2.57 Nano-to-Macro Transport Processes Fall 2004Documento8 pagine2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNessuna valutazione finora
- Problem Solving On D C Machines PDFDocumento16 pagineProblem Solving On D C Machines PDFSelvaraj ParamasivanNessuna valutazione finora
- L-44 (GDR) (Et) ( (Ee) Nptel)Documento15 pagineL-44 (GDR) (Et) ( (Ee) Nptel)yashaswiyellapragadaNessuna valutazione finora
- L 07 (GDR) (Et) ( (Ee) Nptel)Documento15 pagineL 07 (GDR) (Et) ( (Ee) Nptel)Aneurys DuranNessuna valutazione finora
- Three-Phase Induction Motor: Version 2 EE IIT, KharagpurDocumento8 pagineThree-Phase Induction Motor: Version 2 EE IIT, KharagpurHarsh PatelNessuna valutazione finora
- Headlamp Vibration PDFDocumento8 pagineHeadlamp Vibration PDFbasavaraj mittalakattechikkappaNessuna valutazione finora
- Susumu Yasuda - Prediction of LiquefactionDocumento64 pagineSusumu Yasuda - Prediction of Liquefactiontedy suristiantoNessuna valutazione finora
- 1 EXP Mechanical Vibration (Corrected)Documento4 pagine1 EXP Mechanical Vibration (Corrected)AbhiNessuna valutazione finora
- Overview of Tuned Liquid Dampers and Possible Ways of Oscillation Damping Properties ImprovementDocumento6 pagineOverview of Tuned Liquid Dampers and Possible Ways of Oscillation Damping Properties Improvementmostafa shahrabiNessuna valutazione finora
- Dy DX: Lny 5 X +LNCDocumento12 pagineDy DX: Lny 5 X +LNCWenaBacxNessuna valutazione finora
- Relationship Between Mortar and Early Age StrengthDocumento24 pagineRelationship Between Mortar and Early Age StrengthMuhammadWazimAkramNessuna valutazione finora
- Geotech 2Documento171 pagineGeotech 2nithinvlNessuna valutazione finora
- Exploring Gas Laws: Learning Goals: Once You Have Completed This Activity, You Should Understand The Concepts ofDocumento5 pagineExploring Gas Laws: Learning Goals: Once You Have Completed This Activity, You Should Understand The Concepts ofAdam CrabbNessuna valutazione finora
- 6CCP3212 Statistical Mechanics Homework 2Documento4 pagine6CCP3212 Statistical Mechanics Homework 2Hameed ul haqNessuna valutazione finora
- 1st Law of ThermodynamicsDocumento4 pagine1st Law of Thermodynamicst8e7w2koNessuna valutazione finora
- Grade XI SCIENCE - Holiday Homework & WorksheetsDocumento19 pagineGrade XI SCIENCE - Holiday Homework & WorksheetsReeja MathewNessuna valutazione finora
- LandauDocumento21 pagineLandauLevema SiraNessuna valutazione finora
- Numerical Evaluation of Dynamic ResponseDocumento22 pagineNumerical Evaluation of Dynamic Responsegabrielitos7891Nessuna valutazione finora
- Find The Stress and Strain Diagram For Concrete and Timber.: Assgnment Reg 162Documento16 pagineFind The Stress and Strain Diagram For Concrete and Timber.: Assgnment Reg 162SeLamah RahmanNessuna valutazione finora
- Unit 10-Short QuestionsDocumento5 pagineUnit 10-Short QuestionssajjaddrNessuna valutazione finora
- SyllabusDocumento2 pagineSyllabusMohamed Ayman MoshtohryNessuna valutazione finora
- 1 Performance Task G8 Science Q1Documento5 pagine1 Performance Task G8 Science Q1jaja genterolaNessuna valutazione finora
- Fluid MechanicsDocumento4 pagineFluid MechanicsAmarjit KeneNessuna valutazione finora
- 1 s2.0 S1674775522001718 MainDocumento12 pagine1 s2.0 S1674775522001718 Mainilham halikNessuna valutazione finora
- SuspensionDocumento12 pagineSuspensionvivek kumarNessuna valutazione finora
- (Ebook) - Engineering - Patran Nastran Student TutorialDocumento72 pagine(Ebook) - Engineering - Patran Nastran Student TutorialnooraniaNessuna valutazione finora
- Algebra and Trigonometry 5th Edition Blitzer Solutions Manual Full Chapter PDFDocumento67 pagineAlgebra and Trigonometry 5th Edition Blitzer Solutions Manual Full Chapter PDFmatthewjordankwnajrxems100% (14)
- DCC40142 - Scheme Assignment 1 Set 1Documento6 pagineDCC40142 - Scheme Assignment 1 Set 1F1026 AzamuddinNessuna valutazione finora
- Modeling of Gravity Concentration Unit Operations: A. K. MajumderDocumento78 pagineModeling of Gravity Concentration Unit Operations: A. K. MajumderSwarnaRakshitNessuna valutazione finora