Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Today
Caricato da
saadiisCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Today
Caricato da
saadiisCopyright:
Formati disponibili
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY - Vol. V - Production of Organic Acids - F. Sanchez-Riera
Encyclopedia of Life Support Systems (EOLSS)
PRODUCTION OF ORGANIC ACIDS
F. Sanchez-Riera
Bio-Technical Resources, Wisconsin, USA
Keywords: Acetic acid, Citric acid, Gluconic acid, Itaconic acid, Lactic acid, Propionic
acid, Pyruvic acid, Succinic acid
Contents
1. Introduction
2. Citric Acid
2.1 Historical Development
2.2 Fermentation Basics
2.3 Fermentation Processes
2.3.1 Production with A. niger
2.3.2 Production with Yeasts
2.4 Product Recovery
3. Lactic Acid
3.1 Historical Development
3.2 Fermentation Basics
3.3 Fermentation Processes
3.3.1 Raw Materials
3.3.2 Process Parameters
3.3.3 Process Configurations
3.4 Product Recovery
4. Acetic Acid
4.1 Overview
4.2 Aerobic Production of Vinegar
4.3 Process Configurations
4.4 Anaerobic Production of Acetic Acid
5. Gluconic Acid
5.1 Overview
5.2 Production Process
6. Itaconic Acid
6.1 Overview
6.2 Production Process
7. Other Acids
7.1 Propionic Acid
7.2 Succinic Acid
7.3 Pyruvic Acid
8. Conclusions
Acknowledgements
Glossary
Bibliography
Biographical Sketch
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY - Vol. V - Production of Organic Acids - F. Sanchez-Riera
Encyclopedia of Life Support Systems (EOLSS)
Summary
The microbial production of several organic acids of industrial importance is discussed.
Organic acids constitute a significant portion of the fermentation market in the world,
and microbiological production is an important economic alternative to chemical
synthesis for many of them.
Acids are treated individually. The most remarkable aspects of the biochemistry of the
producing organisms, most common processes and key process parameters are
discussed in each case. Information about the most recent advances presented in patents
and published studies are also included.
Citric, lactic, gluconic, and itaconic acid are currently produced by fermentation.
Microbial production of acetic acid is important for food applications, in the form of
vinegar. Propionic, succinic, and pyruvic acid are not currently being produced
commercially by fermentation, but have significant potential for future manufacture by
this route.
1. Introduction
Organic acids are broadly distributed in nature, and humans have used them in their
natural sources since early ages. Fermentation processes that involve the production of
acetic and lactic acid have been used in food preparation for centuries. Industrial
production with the microbiological route was started when the acids were identified as
the main product in known fermentation processes. During the first half of the twentieth
century, advances in chemical synthesis offered new manufacturing procedures that
became economically competitive and replaced many fermentation processes. The
situation changed in the 1990s, when further developments in the biotechnology field,
environmental pressures and the vertical integration of the fermentation and corn
processing industries resulted in much improved economics for the biological route.
The production of organic acids discussed here includes those which are currently
manufactured by fermentation in large quantities or which offer potential for future
developments.
2. Citric Acid
2.1 Historical Development
Citric acid (2-hydroxy-1,2,3 propanetricarboxylic acid, C
6
H
8
O
7
) is widely distributed in
plant and animal tissues and fluids. It occurs in rather high concentrations in citrus
fruits, but is ubiquitous in nature as an intermediate in the Krebs cycle, whereby
carbohydrates are oxidized to carbon dioxide.
It was first isolated from lemon juice in 1784, and its commercial production started in
England in 1826 from Italian made calcium citrate. At the beginning of the twentieth
century, Italy had a virtual monopoly with a production of about 10 000 tons. In 1893,
Wehmer observed that citric acid was produced by some species of Penicillium when
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY - Vol. V - Production of Organic Acids - F. Sanchez-Riera
Encyclopedia of Life Support Systems (EOLSS)
grown on sugar solutions, but his attempts to establish a manufacturing process by
fermentation were unsuccessful. The 1917 discovery of citric acid producer strains of
Aspergillus niger, which could grow at pH values around 2.5 to 3.5, led to the first
commercial production by fermentation.
Plants were opened in Belgium in 1919 and the US in 1923. Several plants using the
surface process were started in other countries, and in about 10 years around 80% of the
citric acid was produced by fermentation.
Intense research led to the development of the submerged process, which was applied
industrially after the Second World War. More efficient strains, cheaper medium and
better control conditions have resulted in great process improvements, but research in
the field continued through the years.
Several yeast strains have been identified for the production of citric acid from both
carbohydrates and hydrocarbons. However, production from the latter has not been
economically viable.
By 1997 the global capacity was estimated at 840 000 tons per year; demand has been
increasing at a 5% rate from 1988 to 1997 and an average 3.5% growth is expected until
2002.
In the last few years the citric acid business has been mostly consolidated in large
producers back-integrated to raw materials. While production has also increased in
China and India, their capacities are still much lower than that of international
companies.
The main application of citric acid (70%) is in the food and beverage industry, as the
most versatile and widely used acidulant. Its pleasant taste, high water solubility, and
property of enhancing flavors have affirmed its application in this market, which has
been largely responsible for the increases in citric acid demand.
A buffering capacity and the ability to form complexes with heavy metals have driven
the application of citric acid and several of its salts in the pharmaceutical and chemical
processing industry as sequestering agents. The remaining demand is distributed as
follows: 18% in detergents and cleaners, 6% in pharmaceuticals and cosmetics and 6%
in industrial and chemical processing uses.
2.2 Fermentation Basics
The excretion of citric acid is not a normal phenomenon for microorganisms, but it can
accumulate in very large quantities when certain bacteria, yeast, and fungi are grown
under particular constraints.
Appreciable accumulation is the result of severe irregularities in the metabolism caused
by genetic deficiencies or by metabolic imbalances. The definition of the appropriate
conditions to maximize citric acid yield and concentration varies widely depending on
the strain in use and the main carbon source.
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY - Vol. V - Production of Organic Acids - F. Sanchez-Riera
Encyclopedia of Life Support Systems (EOLSS)
Figure 1. Metabolic relationships of citrate metabolism from carbohydrates or n-
alkanes.
The basic metabolic sequences related to citrate metabolism are well established, as
shown in Figure 1. However, the regulation of the pathway and the main points that
control citrate accumulation are still subject to debate, because different conclusions
have been reached in numerous studies. Among the reasons for this is the fact that many
studies have used different strains and conditions, and that there is evidence that
indicates that more than one set of conditions can lead to citric acid accumulation.
A.niger forms citric acid from glucose viaglycolytic catabolism to two moles of
pyruvate and their subsequent conversion to oxaloacetate. Citrate synthase then
condenses oxaloacetate with acetyl CoA to form citrate. The presence of the enzymes of
the tricarboxylic acid cycle has been demonstrated in this organism under various
conditions. For citric acid to accumulate, an imbalance between the metabolic flux in
the glycolytic pathway and the tricarboxylic cycle must occur. This imbalance has been
detected as an increase in the flux through glycolysis with high activity of the glycolytic
enzymes due to culture conditions. A key step in the process is the synthesis of
oxaloacetate by an anaplerotic carbon dioxide fixation catalyzed by pyruvate
carboxylase, which is induced in the presence of high carbohydrate concentrations. This
enzyme and citrate synthase produce an accumulation in the cellular concentration of
citric acid. Some authors suggest that in the stages of production of citric acid, the
tricarboxylic cycle is restricted at isocitrate dehydrogenase. But the nature of the
regulation of overproduction and accumulation of citric acid is still unclear, because
several different mechanisms have been proposed and the existing evidence is not
enough to confirm a single one. When yeasts produce citric acid from alkanes, these
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY - Vol. V - Production of Organic Acids - F. Sanchez-Riera
Encyclopedia of Life Support Systems (EOLSS)
compounds are converted by -oxidation to acetyl CoA, which combines with
oxaloacetate to produce citrate. The anaplerotic glyoxylate cycle replenishes
oxaloacetate (see Cell Thermodynamics and Metabolism).
Even though a large number of microorganisms can produce citric acid, the fungus
Aspergillus niger has remained as the organism of choice for commercial production. In
the last few years, production with the yeast Yarrowia lipolytica (asexual form
Saccharomycopsis, formerly Candida) has increased significantly. Some other
organisms include A. wentii, A. carbonarius, A. aculeatus, A. awamori, Candida
tropicalis, C. oleophila, C. guilliermondii, and C. citroformans. A significant effort has
been devoted to obtain strains with higher yields and better production efficiency, but
the strains actually used in manufacture and the selection methods for strain
improvements have been kept secret, and information about them is not generally
available. Cell improvements for citric acid production through gene cloning and
expression are not straightforward because, in general in these long pathways, the
contribution of a single enzyme has very limited success in increasing overall
productivity. Limitations and improvements are rarely based on a single step, and the
regulatory enzymes may be less important to the overall productivity of the organism
than the processes of uptake of substrate or excretion of the acid.
2.3 Fermentation Processes
Different fermentation processes have been developed for the different strains and
carbon sources. A. niger and Y. lipolytica are used in commercial processes. Surface and
submerged methods have been used with A. niger and either carbohydrates or n-alkanes
can be the carbon source in processes using yeasts.
2.3.1 Production with A. niger
In order to achieve abundant excretion of citric acid, the growth of the strain must be
restricted. High sugar concentrations favor high glycolytic flux, but limitations in either
nitrogen or phosphorous are required as well as very low levels of certain heavy metals,
including iron and manganese. Various media have been described in the literature, and
the general requirements for production with A. niger can be summarized as follows:
high level of a carbon source (60-240g l
-1
), low level of a nitrogen source (2-3g l
-1
), low
level of a phosphate source (1.5-3.0g l
-1
), deficiency of manganese (<0.000001g l
-1
),
low level of iron (<0.0013g l
-1
), low level of zinc (0.00025g l
-1
), high aeration and low
pH (2 or 3).
Beet and sugar cane molasses and glucose syrups are the main carbon sources utilized
industrially. The composition of the molasses is variable and testing and pre-treatments
are required. The main problem is related to the levels of zinc, iron, magnesium, and
manganese. The manganese concentration is of critical importance in the process as it
has been demonstrated that this ion regulates several enzymes, including those related to
citric acid export. Control of the level of trace metals is achieved by addition of sodium
or potassium ferrocyanide. The optimum addition level must be above that needed to
chelate the metals; these compounds may also contribute to a certain degree of growth
inhibition. Trace metal levels can also be reduced by ion exchange, but this is only
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY - Vol. V - Production of Organic Acids - F. Sanchez-Riera
Encyclopedia of Life Support Systems (EOLSS)
applicable to substrates with low salt concentrations, such as corn syrups. Optimum
mineral combinations and strains resistant to metal level regulation have been pursued.
Either nitrogen or phosphate limitations favor citric acid production. Nitrogen sources
such as ammonium sulfate and ammonium nitrate are commonly used in concentrations
between 1 to 3g l
-1
. The type and concentration of the nitrogen source affect cell growth
and citric acid formation. Phosphate content is more important in fermentations where
the trace metal concentration is not tightly controlled, but low phosphate levels are
usually recommended. Magnesium is required both for growth and citric acid
production. Other nutrients such as lower alcohols (methanol and ethanol) and lipid
materials in vegetable oils have been shown to increase citric acid production. The
effect of alcohols is related to trace metal concentrations, and they can be inhibitory
when used with purified substrates.
The first industrial process was the surface one, which is still employed in some cases as
it is simpler to operate, has lower energy costs than submerged processes and is less
sensitive to the trace metals composition. However, it is much more labor intensive. The
mycelium is grown as a surface mat in a large number of shallow trays (see Microbial
Cell Culture). The carbohydrate source is diluted to 15%, pretreated, and the pH is
adjusted to between 5 and 7 to allow the spores to germinate. The medium is then
sterilized and pumped into the trays. The culture is inoculated with spore suspensions.
Air is blown through the fermentation chamber, where temperature is maintained at
30C and humidity between 40 and 60%. Aeration supplies the oxygen requirements
and also removes the heat generated. As the fermentation progresses, the pH decreases
to about 2.0. The fermentation is completed in between 7 and 15 days with yields
around 70%. The fermented liquor is separated from the mycelium for further
processing.
The submerged process is the most commonly used either in stirred tank reactors or
tower fermentors. The process is run in batch. Fermentors are equipped with aeration
systems capable of maintaining high dissolved oxygen levels, which is critical for high
citric acid production. Medium preparation involves the dilution of the carbon source to
between 15 and 27% and also the pre-treatment procedures to eliminate manganese and
reduce the level of the other critical trace metals. Different combinations of metal
concentration and pre-treatments have been described and patented, and there is not a
unique set of acceptable conditions. Initial pH values are between 2.5 to 3.0;
fermentation temperature is between 28 and 35C; inoculation is performed with spores
or with preculture mycelia in a 1:10 ratio. The characteristics of the inoculum and of the
medium influence the culture morphology, which impacts citric acid yield. The
formation of small pellets with a smooth and hard surface results in a broth with lower
energy requirements and better mass transfer characteristics. The fermentation runs
from 5 to 10 days depending on the process.
Fed-batch and even continuous processes have been proposed (see Microbial Cell
Culture). As accumulation of citric acid is only partially growth associated, the
continuous process is less efficient and fed-batch processes do not support significant
enough gains to become standard. Because of the dissociation between growth and
production, the use of immobilized mycelia has been attempted in several laboratories.
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY - Vol. V - Production of Organic Acids - F. Sanchez-Riera
Encyclopedia of Life Support Systems (EOLSS)
Mycelium has been immobilized in alginate beads, collagen, and polyurethane foam.
Productivity rates are still too low and the process is too cumbersome to be of industrial
interest. The increase in the available knowledge about fermentation conditions, and the
development of more accurate models that support better controlled fermentations, have
resulted in more significant improvements than these process configurations.
The simplest process for the production of citric acid is the solid state one known as
Koji process (see Food Fermentation and Processing). It was initially developed for the
use with solid raw materials (sweet potato fibrous residues, rice, and wheat bran).
Several new studies have applied the process to different waste materials such as fruit
pomace and sugar cane residues. The carbohydrate source is soaked with water to about
70% moisture, sterilized, placed in trays, and inoculated with conidia of A. niger. The
starch is hydrolyzed by amylase produced by the fungus and converted to citric acid in
from 4 to 5 days. This process is primarily considered to satisfy small demands for citric
acid in decentralized economic systems.
2.3.2 Production with Yeasts
Production of citric acid with yeasts is also applied commercially. Yeast cells can
tolerate higher initial concentration of sugar, have a higher fermentation rate and are
insensitive to metals, which reduces the pre-treatments required. Several processes
using different strains have been patented. Production with yeast is always performed in
a submerged culture. The original process, patented in 1968, controlled the pH of the
fermentation above 5.5 with addition of calcium carbonate. In some later processes, the
pH was allowed to fall to values between 3 and 4 after cell growth. Several yeast genera
were proposed for citric acid production, with most emphasis in various species of
Candida. The main disadvantage of the use of yeasts is the formation of isocitric acid as
a by-product, but various additives have been tested to reduce its production. The use of
halogen-substituted alkanoic mono or di-substituted acids, lead acetate and other
substances has been patented, and many mutant strains with reduced isocitrate
production have been selected. An osmophilic strain that can convert sugar
concentrations as high as 28% without pre-treatment of the molasses has also been
patented.
The production of citric acid from n-alkanes was developed as an alternative when
hydrocarbons were a cheap carbon source. A process using Yarrowia lipolytica was able
to achieve more than 200g l
-1
citric acid using C
14
-C
16
n-paraffins. A final pH between 2
and 3 allowed for the recovery of the free acid without the need to produce a salt. The
fermentation was completed in about 6 days. The system is not being used
commercially because it cannot compete economically with the utilization of
carbohydrates.
2.4 Product Recovery
Citric acid cannot be recovered directly from the fermented liquor by crystallization
because of excess of impurities. In the classic protocol, citric acid is precipitated as
calcium citrate by the addition of lime. The washed precipitate is treated in aqueous
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY - Vol. V - Production of Organic Acids - F. Sanchez-Riera
Encyclopedia of Life Support Systems (EOLSS)
suspension with H
2
SO
4,
yielding gypsum as a by-product. The citric acid solution is
concentrated by vacuum evaporation and crystallized at low temperatures.
Solvent extraction can also be applied with the advantage of avoiding the formation of
gypsum. Different solvents can be used; the extraction step is usually carried out at low
temperature and the solvent is stripped with hot water. A mixture of n-octyl alcohol and
tridodecylamine has been recommended for citric acid used in food and drug
applications.
Some other recovery techniques have been studied and patented. They include the
combination of membrane filtration with adsorption resins, the use of different anionic
exchange resins and the application of electrodialysis. The main objective of these
studies has been the development of economic recovery processes that eliminate the
formation of gypsum and reduce the environmental impact of citric acid manufacture.
-
-
-
TO ACCESS ALL THE 26 PAGES OF THIS CHAPTER,
Visit: http://www.eolss.net/Eolss-sampleAllChapter.aspx
Bibliography
Demain A. and Solomon N., eds. (1985). Biology of Industrial Microorganisms, pp. 143184, 359397.
Menlo Park, CA: The Benjamin/Cummings Publishing Co. [General biology of Lactic, Acetic, Popionic
bacteria and Aspergillus.]
Grewal H. S. and Kalra K. L. (1995). Fungal production of citric acid. Biotechnology Advances 13 (2),
209234.
Kharas, G, Sanchez-Riera, F., and Severson, D. (1994). Polymers of lactic acid. In Plastics from
Microbes (ed. D. Mobley). Cincinnati, OH: Hanser Publishers. pp. 93103. [Detailed description of lactic
acid fermentation, existing processes and production of polylactates.]
Mattey M. (1992). The production of organic acids. Critical Reviews in Biotechnology 12(1/2), 87132.
Moo-Young, M., ed. (1985). Comprehensive Biotechnology, Vol. 3. Elmsford, NY: Pergamon Press. pp.
665778. [Detailed information on production of citric, lactic, acetic,gluconic, itaconic and propionic
acids.]
Nagodawithana T. W. and Reed G., eds (1998). Nutritional Requirements of Commercially Important
Microorganisms. Milwaukee, WI: Esteekay Associates. pp. 236297. [Up to date review of nutritional
information for microorganisms that produce citric and lactic acids.]
Norton S. and Vuillemard J .-C. (1994). Food bioconversions and metabolite production using
immobilized cell technology. Critical Reviews in Biotechnology 14(2), pp. 193224. [Application of
immobilization techniques in the production of organic acids.]
Rehm H.-J . and Reed G., eds (1983). Biotechnology, Vol. 3. Basel, Switzerland: Verlag Chemie. pp. 387
478. [Review of biochemistry and process information in the production of several organic acids.]
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY - Vol. V - Production of Organic Acids - F. Sanchez-Riera
Encyclopedia of Life Support Systems (EOLSS)
U.S. Patent and Trademark Office (various dates). Patent information on processes for the production of
individual acids.
Biographical Sketch
Fernando Sanchez Riera received his chemical engineering degree from the Universidad Nacional de
Tucumn, Argentina, in 1980. He became a fellow of the National Research Council (CONICET)
working at PROIMI (Planta Piloto de Procesos Industriales Microbiolgicos) where he started his
specialization in fermentation processes, while focusing on anaerobic treatment of agroindustrial wastes
for the production of methane. From 1986 to 1988 he was a postdoctoral fellow at the Chemical
Engineering Department at MIT (Massachusetts, USA) where his research efforts focused on
fermentation control. Upon return to PROIMI as an Assistant Researcher, he worked on ethanol
production with Zymomonas and its integration with waste utilization. In 1991, after a short period as a
Visiting Scientist at the University of Wisconsin (Wisconsin, USA), he moved to Bio-Technical
Resources (Manitowoc, Wisconsin, USA). There he has been involved in the development and
optimization of several fermentation processes including the production of lactic acid, a subject on which
he co-authored a book chapter, and biotransformations for the production of chemicals by fermentation
and enzymatic processes.
Potrebbero piacerti anche
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5795)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- Lactic Acid Energy SystemDocumento15 pagineLactic Acid Energy SystemroemriqwewqeNessuna valutazione finora
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- 1 - Phase 1 - Biochemistry Handout For Video Lecture 1 Carlo SaezDocumento20 pagine1 - Phase 1 - Biochemistry Handout For Video Lecture 1 Carlo SaezNikki ValerioNessuna valutazione finora
- Niacin and German New Medicine - Glen Russell Puna Wai Ora Mind Body Cancer Clinic Psycho Oncology - The 6 Phases of Cancer EbookDocumento95 pagineNiacin and German New Medicine - Glen Russell Puna Wai Ora Mind Body Cancer Clinic Psycho Oncology - The 6 Phases of Cancer EbookEbook Pdf100% (3)
- 3200 Types of Waste Heat RecoveryDocumento30 pagine3200 Types of Waste Heat Recoverysaadiis100% (2)
- Biochemistry Answer Key-BLUE PACOPDocumento26 pagineBiochemistry Answer Key-BLUE PACOPLEIGH100% (2)
- Conditioning For The MMA FighterDocumento4 pagineConditioning For The MMA Fighterdavid bourne0% (1)
- Carbohydrate Metabolism-1Documento22 pagineCarbohydrate Metabolism-1Marwah100% (2)
- PALOMADO Midterm Microbiology 2020Documento9 paginePALOMADO Midterm Microbiology 2020MARLON PALOMADONessuna valutazione finora
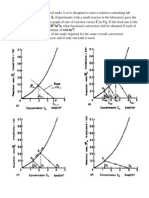
- Kmol/m xl0 M /S, What Fractional Conversion Will Be Obtained If Each of M ?Documento2 pagineKmol/m xl0 M /S, What Fractional Conversion Will Be Obtained If Each of M ?saadiisNessuna valutazione finora
- Citric Acid ProductionDocumento20 pagineCitric Acid ProductionDiana MuñozNessuna valutazione finora
- Furnace FiguresDocumento1 paginaFurnace FiguressaadiisNessuna valutazione finora
- Constants For DaubertDocumento1 paginaConstants For DaubertsaadiisNessuna valutazione finora
- CP302 Example 02 OKDocumento4 pagineCP302 Example 02 OKsaadiis100% (1)
- Chapter 9 Mass TransferDocumento64 pagineChapter 9 Mass TransfersaadiisNessuna valutazione finora
- HAZOP Case Studies For Workshop SessionDocumento4 pagineHAZOP Case Studies For Workshop Sessionsaadiis100% (2)
- 413 Topic v-3 (Run-Around Coil Systems, Regenerative Heat Ex Changers and Pinch Technology)Documento41 pagine413 Topic v-3 (Run-Around Coil Systems, Regenerative Heat Ex Changers and Pinch Technology)Preethish PrakashNessuna valutazione finora
- Matlab and Gams DifferenceDocumento20 pagineMatlab and Gams DifferencesaadiisNessuna valutazione finora
- Physical Education (1ST Sem - Module 1 Dance)Documento18 paginePhysical Education (1ST Sem - Module 1 Dance)mary roseNessuna valutazione finora
- Energy and Respiration MSDocumento52 pagineEnergy and Respiration MSWaheed SherazNessuna valutazione finora
- Wardlaws Perspectives in Nutrition A Functional Approach 1st Edition Byrd Bredbenner Test BankDocumento38 pagineWardlaws Perspectives in Nutrition A Functional Approach 1st Edition Byrd Bredbenner Test Bankedricduyenuc1uw100% (25)
- Prokaryotic Cells Are Also CalledDocumento25 pagineProkaryotic Cells Are Also CalledArdo RamdhaniNessuna valutazione finora
- Content List For MdcatDocumento5 pagineContent List For MdcatMuhammad AbdullahNessuna valutazione finora
- Metabolisme EritrositDocumento50 pagineMetabolisme EritrositRamdhana ZaqifahNessuna valutazione finora
- Aakash Rank Booster Test Series For NEET-2020Documento14 pagineAakash Rank Booster Test Series For NEET-2020Anish TakshakNessuna valutazione finora
- 9 Dissacharides Metabolism Compatibility ModeDocumento16 pagine9 Dissacharides Metabolism Compatibility ModeAubrey Nativity Ostulano YangzonNessuna valutazione finora
- Pathophysiology 5th Edition Copstead Test BankDocumento35 paginePathophysiology 5th Edition Copstead Test Bankturnmiddayu0ppsx100% (12)
- Fermentation in CyanobacteriaDocumento33 pagineFermentation in CyanobacteriaDiego Andres Ortiz ValleNessuna valutazione finora
- MuscularDocumento61 pagineMuscularFranz DiazNessuna valutazione finora
- Articulo Pichia PastorisDocumento10 pagineArticulo Pichia PastorisSergio A Mtz BhaNessuna valutazione finora
- Course Review KeyDocumento24 pagineCourse Review KeyHermann Dejero LozanoNessuna valutazione finora
- Sample Lab ReportDocumento7 pagineSample Lab ReportPutri Syalieyana0% (1)
- By: Herdianty Kusuma H, SST., FTR., M.KesDocumento3 pagineBy: Herdianty Kusuma H, SST., FTR., M.KesTaufik Dwi KurniawanNessuna valutazione finora
- 8 GlycolysisDocumento48 pagine8 GlycolysisnsjunnarkarNessuna valutazione finora
- RespirationDocumento100 pagineRespirationColin RiddellNessuna valutazione finora
- Microbial MetabolismDocumento31 pagineMicrobial MetabolismHolin DolanNessuna valutazione finora
- Intro To Metabolism Glycolysis Krebs Cycle ETCDocumento104 pagineIntro To Metabolism Glycolysis Krebs Cycle ETCAnna Sofia ReyesNessuna valutazione finora
- Aerobic Respiration Power PointDocumento35 pagineAerobic Respiration Power PointJeremy WongNessuna valutazione finora
- Genbio Quatter 2Documento5 pagineGenbio Quatter 2Shakira UyNessuna valutazione finora
- at The End of This Lecture, Student Will Be Able ToDocumento30 pagineat The End of This Lecture, Student Will Be Able ToAntoNessuna valutazione finora
- Integration of MetabolismDocumento40 pagineIntegration of Metabolismseada JemalNessuna valutazione finora