Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Insulin Oma
Caricato da
stbmi123Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Insulin Oma
Caricato da
stbmi123Copyright:
Formati disponibili
Clinical Case
Insulinoma
JS, 42/M
c/o-
Recurrent episodes of
unconsciousness x 6
months
Prolonged sleep
Fasting
From last 2 months patient
used to eat frequently to avoid
these symptoms
Subsequently patient gained
weight due to overeating
Fasting
-- H/o sweating and
palpitation during these
episodes
-- No other neurologic
symptoms
Patient used to recover
after taking glucose water
weight due to overeating
History..
No history of-
Bone pains
Abdominal pain
Urinary calculi
Headache Headache
Visual disturbances
Broadening of facies
Diabetes mellitus
Hypertension
Diabetic in family
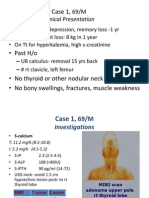
Examination
No cutaneous lesions
No bony deformities
No marfanoid habitus
No acromegaly No acromegaly
Pulse Rate- 86/min
BP- 120/70 mm Hg
Systemic examination:
unremarkable
Investigations
Hormone Value Normal range
S. Insulin 116.0 mIU/L Undetectable
C-peptide 14.22 U/L Below detectable
72-hr supervised fasting hypoglycemia testing- hypoglycemia in 6 hours
C-peptide 14.22 U/L Below detectable
limits
Blood sugar 32 mg/dl 70-110
Prolactin 263 mIU/L 0-400
Calcium 8.4mg/dl 8.4-10.5
GH <0.17 Normal
Clinical diagnosis
Hyperinsulinemic hypogylcemia (?Insulinoma)
Imaging- CECT abdomen
Lesion in distal body of
pancreas
Well defined
21 mm
Intense arterial
enhancement
Surgical plan
Laparotomy with Enucleation-
Intraoperative USG
Bidigital palpation
Intra-op USG
Intra-op USG
Intra-op USG
Pancreatic Exploration
Intra-operative USG
showed single
lesions-
At distal body of
pancreas
MPD not seen MPD not seen
Bi-digital palpitation:
confirms findings
Enucleation done
Post operative course
Developed Diabetes Ketoacidosis on POD 1-
managed with IV fluids and Insulin infusion
Started orally on POD 3
Gradual increase in abdominal drain on POD 5
USG-
No evidence of peripancreatic collection
Drain fluid amylase/serum amylase > 3times
Post op
Diagnosis: Pancreatic fistula
Managed with keeping patient Nil per oral
Antibiotic coverage
Nutritional support
Control fistula with abdominal drain
Surgical Management of
Insulinoma
Introduction
Incidence Insulinoma commonest
neuroendocrine tumor of the
pancreas
Four in every 1 million persons
Age at presentation 45-50 years Age at presentation 45-50 years
Male: Female 2:3
Location Throughout pancreas
Sporadic 90% less than 2cm, Single, and
Benign
Etio-pathogenesis
Sporadic Familial
90%
10%
Associated with MEN 1
90%
Lesions are
Solitary
Benign
Encapsulated
Associated with MEN 1
Lesions are
Early onset
Multifocal
Increased risk of malignancy
Localization Techniques
Preoperative
Non invasive-
Transabdominal USG
CECT/ CT Angiography
MRI
Intraoperative-
Palpation
USG-
Open
Laparoscopic
MRI
PET
Invasive-
Angiography
ASVS
PVS
Endoscopic ultrasound
Laparoscopic
Localization studies- Noninvasive
Author Year Institute n USG CT MRI PET
Varma 2011 Birmingham 40 62 82
Christ 2009 Switzerland 6 25 17 100 (GLP-1R)
Kimberly 2009 Mayo clinic 237 61 55 42
Espana-Gomez 2009 Mexico 34 45 91
Guettier 2009 NIH 45 14 32 25
Nikfarjam 2008 Massachusetts 61 14 62 63
Paul 2008 Vellore 18 63 83
Tseng 2007 Taipei 17 6 23 18
Indications
1. Failed Non- Invasive Localization
2. Re-operations
Modalities
Pre-operative localization
Invasive
Modalities
1. Endoscopic USG
2. Angiography
3. Trans-hepatic Portal Venous Sampling (THPVS)
4. Selective arteriography with Calcium stimulation
test (SACST)
Mathur A et al. Insulinoma. Surg Clin N Am 89 (2009): 1105-1121
Arterial Stimulated Venous Sampling
First described by Doppman in 1991
Technique
Selective cannulation of Splenic A, SMA and Gastroduodenal arteries via
femoral artery
Femoral venous catheter directed toward right hepatic vein for sample
collection
Calcium gluconate (5ml bolus of 0.01- 0.025mEq / Kg) in saline is injected
Samples taken at 0, 30,60, 90, 120 and 180 sec
A two fold increase in insulin in 30s or 60s sample or both regionalize the
tumour
Guettier JM et al.The NIH Experience. J CEM 2009; 94: 1074-1080
Not used in most centers as it is
Invasive
Technically difficult
Expensive
It may be appropriate when an Insulinoma is strongly
suspected but all non-invasive imaging tests are
negative
Re-operative setting
Ritzel RA et al. Rev Diabetic Stud 2004
Intra-operative Localization
1. Digital Palpation
2. USG (Open/
Adjuncts
1. Intra-op glucose
monitoring
Laparoscopic)
monitoring
2. Intra-op insulin assay
3. Frozen section biopsy
Intraoperative palpation
Technique-
Enter lesser sac
Expose pancreas
Mobilize pancreas Mobilize pancreas
Palpate regions of
pancreas systemically
Insulinoma feels like a firm
nodule within soft
parenchyma
Intra operative palpation
Palpation 90%
sensitive in
experienced hands
Limitations- Limitations-
1. Tumors deep within the
pancreatic parenchyma
and uncinate process
2. Patients with previous
attack of pancreatitis
3. Fatty pancreas
Intraoperative USG
UCSF protocol-
Enter lesser sac
Expose pancreas
Mobilize pancreas
Palpate pancreas
USG done by 7.5- 10 MHz
transducer
Scanned longitudinally
Considered positive if
sonolucent mass detected in
both transverse and longitudinal
imaging planes
Intra-operative localization
Ravi etal. Ann R Coll Surg Engl 2007; 89: 212 - 217
Treatment
Sporadic
Familial
Sporadic
Familial
Malignant
Sporadic insulinoma
Majority are benign, solitary lesion
Enucleation is the procedure of choice
Important to remove the tumor with capsule to prevent
future recurrence future recurrence
Resections may be required in certain situations
Risk of recurrence- 5% at 10 years and 7% at 20 years
Service FJ et al. Mayo Clin proc 1991; 66 (7): 711-9
Sporadic
Surgery
Resection
Enucleation
Small benign tumors
2-3 mm from the MPD
Away from major vessels
Tumor abutting MPD or major vessels
Suspicion of malignancy
Hard Infiltrating tumor
Lymph node involvement
Distal dilatation of pancreatic duct
Multiple Insulinoma in body & tail
*Ramage JK. etal. Gut 2005;54(suppl 4):i1 - 16
Pylorus preserving Whipples Distal pancreatectomy splenectomy
Resections
Pylorus preserving Whipples Distal pancreatectomy splenectomy
Mid-body Pancreatectomy
Subtotal Pancreatectomy
Laparoscopic surgery
Favorable features-
More than 90% of Insulinomas are benign ,solitary and
intrapancreatic intrapancreatic
Tumors in the tail and body can be approached easily
Laparoscopic ultrasound is 90-95% sensitive in detecting
Insulinomas
Post op morbidity
Pancreatic fistula (most common)- 15- 43%
Pancreatic pseudo-cyst- 4-6%
Respiratory infection- 10-25%
Splenic infarction- 4-7%
Abdominal abscess- 4-7% Abdominal abscess- 4-7%
Failure of surgical procedure (persistent hypoglycemia)- 5-
15%
Recurrence- 5- 21%
Iatrogenic diabetes- 5-10%
Mathur A et al. Surg Clin N Am 2009; 89 : 1105-1121
Lap Vs Open
Variable Open Lap p value
Blood loss 212ml 185ml 0.24
Hospital stay 9 days 9days 0.66
Op Time 201min 193min 0.32
Complication 15% 27% <0.05
Melanie L. Richards. WJS 2009
MEN 1 Syndrome asso
Insulinoma
Multiple tumors- (80-85%)
More chances of malignancy and recurrence
Distal pancreatectomy + pancreatic head enucleation-
recommended*
Risk of recurrence is 21% at 20yrs follow up**
*Imamura M.World J Gastroenterol. 2010
*Machado MC. Clinics (Sao Paulo). 2012
** Tucker etal. Br J Surg 2006 93:264-275
Occult insulinoma
5-15% in various series
Most common site of occult Insulinoma- head of pancreas
Terminate the surgical procedure
Re-confirm the biochemical diagnosis
More extensive localization before re-operation (SACST)
During re-exploration no tumor identified , then do a
pancreatic resection as suggested by calcium angiogram
(Enlightened resection rather than blind resection)
Abboud etal. World J Gastroenterol ;2008
Malignant Insulinomas
Incidence: 5-12% of all Insulinomas*
The only definite criteria for diagnosis is presence of metastasis
They tend to be larger mean 4.7cm(4-9 cm)
Aggressive treatment recommended in the presence of metastatic
disease with concurrent resection of primary tumor along with
synchronous metastasis
Palliative resection indicated when >90% tumor can be removed
Is also indicated for symptom relief both from hormonal and
mechanical effects
*Hirshberg etal. Cancer 2005 104(2)264-272
Tucker etal. Br J Surg 2006 93:264-275
Non Insulinoma Persistent Hyperinsulinism
Syndrome
(NIPHS)
Persistent hyperinsulinemic hypoglycemia (PHH)
Difficult to diagnose from Insulinoma
4% of all PHH in adults 4% of all PHH in adults
Characterized by islet cell hyperplasia
Anlauf M et al. Am J Surg Pathol 2005; 29:524-533
Features
Hyperinsulinemic neuroglycopenia (usually
postprandial)
Negative 72- hr fast
Negative perioperative imaging studies for Negative perioperative imaging studies for
insulinoma
Positive selective arterial calcium stimulation test
Service FJ etal. Surgery 2005;128:937-45
Outcomes of Surgery for NIPHS
Witteles RM etal. Arch Surg 2001;136:656-663
Take home message
Biochemical confirmation of diagnosis is must for further evaluation
Role of Imaging is to localize and not to diagnose Insulinoma
CECT/MRI depending upon institutional protocol
ASVS in cases of non localized tumors
Enucleation/ Enlightened resection is the mainstay and is the
only curative treatment option available
THANK YOU THANK YOU
Potrebbero piacerti anche
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5795)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- LPL - Lpl-Rohini (National Reference Lab) Sector - 18, Block - E Rohini DELHI 110085Documento8 pagineLPL - Lpl-Rohini (National Reference Lab) Sector - 18, Block - E Rohini DELHI 110085le sage0% (1)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Adhesive Capsulitis Presentation May 2009Documento29 pagineAdhesive Capsulitis Presentation May 2009cm4100% (1)
- Hypervitaminosis D After ParathyroidectomyDocumento8 pagineHypervitaminosis D After Parathyroidectomystbmi123Nessuna valutazione finora
- Case 4Documento24 pagineCase 4stbmi123Nessuna valutazione finora
- Case 1Documento18 pagineCase 1stbmi123Nessuna valutazione finora
- Case 3Documento15 pagineCase 3stbmi123Nessuna valutazione finora
- ER Case 2Documento13 pagineER Case 2stbmi123Nessuna valutazione finora
- Respiratory MCQs LJDocumento7 pagineRespiratory MCQs LJfjghNessuna valutazione finora
- Head InjuryDocumento55 pagineHead InjuryDakaNessuna valutazione finora
- AppendectomyDocumento8 pagineAppendectomyDark AghanimNessuna valutazione finora
- World Mental Health DayDocumento2 pagineWorld Mental Health Dayanon_678889745Nessuna valutazione finora
- BSN 3A - A4 Grand Case Study - RevisedDocumento49 pagineBSN 3A - A4 Grand Case Study - RevisedIvan FernandezNessuna valutazione finora
- Viral InfectionsDocumento59 pagineViral InfectionsSajin AlexanderNessuna valutazione finora
- JIA - Information For TeachersDocumento4 pagineJIA - Information For TeachersragingreaderNessuna valutazione finora
- Examination of The Gastrointestinal TractDocumento44 pagineExamination of The Gastrointestinal TractDrNaveen Singh Rajpurohit Kadu100% (1)
- User Guide For The Participant Use Data File: American College of Surgeons National Surgical Quality Improvement ProgramDocumento40 pagineUser Guide For The Participant Use Data File: American College of Surgeons National Surgical Quality Improvement Programbobobobo12341212Nessuna valutazione finora
- Pediatric Assessment TriangleDocumento2 paginePediatric Assessment TriangleVanessa BergollaNessuna valutazione finora
- Pemeriksaan BMDDocumento43 paginePemeriksaan BMDMisael ChristianNessuna valutazione finora
- Cardiac Glycosides 815Documento19 pagineCardiac Glycosides 815SanskritiNessuna valutazione finora
- Assessment Nursing Diagnosis Scientific Explanation Planning Nursing Intervention RationaleDocumento2 pagineAssessment Nursing Diagnosis Scientific Explanation Planning Nursing Intervention Rationalekimglaidyl bontuyanNessuna valutazione finora
- Journal of Clinical Neuroscience: Imran Ahmad, Farooq Azam RathoreDocumento5 pagineJournal of Clinical Neuroscience: Imran Ahmad, Farooq Azam RathorekhairaniNessuna valutazione finora
- EHS Guidelines OrigDocumento111 pagineEHS Guidelines OrigDorin SăcrieruNessuna valutazione finora
- IFM+Toolkit+Table+of+Contents v19Documento13 pagineIFM+Toolkit+Table+of+Contents v19khaled dewanNessuna valutazione finora
- Case StudyDocumento30 pagineCase StudyNur SolehahNessuna valutazione finora
- Daftar Obat DR - Sps DalamDocumento3 pagineDaftar Obat DR - Sps DalamKlinik Sosa Graha MedikaNessuna valutazione finora
- Hematology LaboratoryDocumento3 pagineHematology LaboratoryDee GeeNessuna valutazione finora
- Cardiac GlycosidesDocumento5 pagineCardiac Glycosidesapi-347182709100% (1)
- ABO-Rh Blood Typing With Synthetic Blood: Teacher'S Manual and Student GuideDocumento32 pagineABO-Rh Blood Typing With Synthetic Blood: Teacher'S Manual and Student GuideMylan Gaston100% (1)
- Impact of Illness On The FamilyDocumento30 pagineImpact of Illness On The FamilyguevarrajanelleruthNessuna valutazione finora
- Ascorbic Acid (Vitamin C) - Side Effects, Interactions, Warning, Dosage & UsesDocumento4 pagineAscorbic Acid (Vitamin C) - Side Effects, Interactions, Warning, Dosage & UsesMuhammad ZuhriNessuna valutazione finora
- Triage in Polytauma: Prof. Dr. A. Chandrasekaran M.S., PH.D.Documento73 pagineTriage in Polytauma: Prof. Dr. A. Chandrasekaran M.S., PH.D.ShrutiNessuna valutazione finora
- Cardiovascular FitnessDocumento5 pagineCardiovascular FitnessAlieza JimenezNessuna valutazione finora
- 117 Rle 3MDocumento2 pagine117 Rle 3Malaisahmae02Nessuna valutazione finora
- Questions About KidneysDocumento30 pagineQuestions About KidneysKristine Mae AbrasaldoNessuna valutazione finora
- Coarctation of Aorta (Paed)Documento18 pagineCoarctation of Aorta (Paed)NadiaMichu100% (1)