Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Physrevlett 22 185
Caricato da
secateDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Physrevlett 22 185
Caricato da
secateCopyright:
Formati disponibili
VOLUME 22' NUMBER 5 PHYSICAL REVIEW LETTERS 3 FEBRUARY 1969
broad, at low temperatures is attributed by us
to the coexistence of regions of pure Ta and of
tantalum-impurity atom precipitates. Although
ordering effects of interstitial atoms in tantalum-
interstitial atom alloys previously have been ob-
served,
' '
in the present case the concentrations
of interstitial atoms were significantly lower (es-
timated to be: N, &40; 0, (100; H,
&20;
C, (40,
in ppm).
The large, sharp maxima seen at 142, 185,
205, and 230 K in Fig. 2 may be qualitatively
un-
derstood as due to ordering transitions of sever-
al impurity species. The differing lattice param-
eters of Ta and of Ta-interstitial-atom precipi-
tate produce large strains at the boundaries of
precipitate "clusters.
"
Near a transition tem-
perature clusters are rapidly fluctuating in size,
and large numbers of Ta nuclei
"see"
the cluster
boundaries. It is known that the linewidth of the
NAR line in Ta is markedly dependent on strains
present in the lattice.
"~"
This strain sensitivity
of the linewidth, combined with the density fluctu-
ations near a transition temperature, produces
a maximum in the NAR linewidth.
Ne acknowledge helpful comments by J. G.
Miller and J. Mishory.
*Research sponsored in part by
the National Science
Foundation under Grant No. GP 7931, and Air Force Of-
fice of Scientific Research, U. S. Air Force, under
Grant No. 65-0841.
H. Suzuki and S. Miyahara, J. Phys. Soc. Japan 21,
2735 (1966), and references therein.
D. G. Westlake, Phil. Mag. 16, 905 (1967), and ref-
erences therein.
D. Q. Westlake, Trans. Met. Soc. AIME 239, 1341
(1967).
D. I. Bolef, J. Appl. Phys. 32, 100 (1961). The V(I)
of the present paper is the same as V(I) of this refer-
ence.
5
R. W. Thompson and O. N. Carlson, J. Less-Com-
mon Metals 9, 354 (1965).
A. J. Malland, J. Phys. /hem. 68, 2197 (1964).
YB. Pederson, T. Krogdahl, and O. Stakkeland, J.
Chem. Phys. 42, 72 (1965).
R. E. Villagrana and G. Thomas, Phys. Status Solidi
9, 499 (1965).
~K. K. Kelly, J. Chem. Phys. 8, 316 (1940).
E. H. Gregory, dissertation, University of Califor-
nia at Los Angeles, 1965 (unpublished).
~~E.
H. Gregory and H. E. Bommel, Phys. Rev. Let-
ters 15, 404 (1965).
PHOTOLUMINESCENCE OF METALS
A. Mooradian
Lincoln Laboratory,
*
Massachusetts Institute of Technology, Lexington, Massachusetts
(Received 31 October 1968)
Radiative recombination in gold, copper, and gold-copper alloys has been observed
arising from transitions between electrons in conduction-band states below the Fermi
level and holes in the d bands generated by optical excitation.
The first observation of optically excited radia-
tive recombination of electrons and holes in met-
als is reported here. This emission involves
states near the Fermi energy, in contrast to the
soft-x-ray emission in which the excited holes
are in the deep-lying bands. The noble metals
gold and copper were studied as well as gold-
copper alloys, but similar results should be ob-
served in other metals.
The luminescence was excited both by an argon-
ion laser which produced in excess of 2 W cw in
either the 4880- or the 5145-A line and by a high-
pressure Hg arc lamp from which the 3000- to
4000-A emission bands were used. Samples were
in the form of ingots, single-crystal slices, or
evaporated films. The spectra did not seem to
be dependent on the type
of sample used. Mea-
surements were made at temperatures from 5 to
300'K. Standard Raman spectroscopy techniques
were used to detect the emitted light and are de-
scribed elsewhere.
'
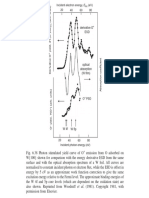
Figure 1 shows the luminescence spectra of
gold at 300 and
10'K
and copper at
300'K
with the
excitation source being the 4880-A argon laser
line. The emission spectra were totally unpolar-
ized and did not vary with polarization of the in-
cident laser beam which is consistent with lumi-
nescence from cubic cyrstals. The spectrum on
the long-wavelength side is cut off by the strong
absorption near the plasma edge while the short-
wavelength side is limited by the energy
avail-
a.ble from the pumping source. The emission
tail on the high-energy side of the laser occurs
from thermal smearing of the electron and hole
distributions and in the case of gold is seen to
disappear at low temperature. In order to make
185
VGLvME 22, NUMBER $ PHYSICAL RKVIKW LKTYKP S 3
I'zsRm. Rv 1969
15
I-
(A
10
GOLD, 500'K
GOLD, 10'K
COPPER, 500'K
4880 A EXCITATION
ENERGY
)k
FERMI
CONDUCTION
BAND
LIJ
I-
CL
'~
d bands
0
4000 5000 6000
,
7000 8000
WAVELENGTH (A)
FIG. 1. Photoluminescence spectra of gold and cop-
per. Exciting light was incident on the sample surface
at an angle of about 10 deg from the surface while the
emitted light was collected normal to the surface. The
spectra are uncorrected for system response.
PXXX/EXYEEX///XXX/XWPXY/EXEi
kF
FIG. 2. Schematic band structure of a noble metal
showing the excitation and recombination transitions.
certain that the observed emission was not due
to some other source such as Raman scattering
from electrons,
'~'
different excitation wavelengths
of the argon laser ranging from 4579 to 5145 A
were used, as well as the 3000- to 4000-A emis-
sion lines from a Hg arc lamp. In all cases the
luminescence peaked at the same energy and the
high-energy tail increased slightly with higher
photon-energy pumping. There was no signifi-
cant change in the emission spectrum when the
angle of observation varied from forward to back-
ward with the laser near grazing incidence. The
present results are not to be confused with the
optically excited plasma radiation reported in
silver and potassium which shoms no difference
in frequency between the incident and emitted ra-
diation. This plasma radiation was interpreted
in terms of light scattering which involved sur-
face roughness and which showed zero intensity
for emission normal to the surface. The present
results are quite unambiguous as the laser exci-
tation provides not only a discrete exciting ener-
gy
but a well-defined propagation direction and
polarization. No correction was taken into ac-
count for reabsorption in the present work.
Emission was also observed in copper -goM al-
loys which peaked at wavelengths between that
for pure copper and gold. The dependence of the
peak emission wavelength on alloy composition
was consistent with the absorption thresholds
which have been reported recently for Cu-Au al-
loys.
4
The details of the excitation and recombination
mechanisms are shown in Fig. 2 where the band
structure for a typical noble metal is represent-
ed by a simple model which includes an s-P con-
duction band and two sets of & bands. The 4
bands, indicated by the cross -hatched regions,
in fact are made up of a number of closely lying
bands in k space. The interaction between the
conduction ba, nd and the d bands at their cross-
over is also neglected for simplicity. Excitation
occurs from states in the upper
& bands to levels
at and above the Fermi energy. Because of the
small photon momenta the interband transitions
are assumed to be direct. Indirect recombi:sa-
tion cannot be ruled out but woold have to involve
the participation of a phonon or an impurity to
provide the necessa, ry momentum. If the photo-
excited holes in the d band thermalize before re-
combining, they would collect at the zone bounda-
ry since the 4 bands slope upward slightly with
increasing &. The radiative transitions mould
then have to be indirect. The onset of emission
in this case would be significantly shifted to low-
er energy from the laser exciting line. No such
shift is observed. It seems more likely that the
emission arises from direct recombination of
conduction-band electrons below the Fermi ener-
gy
with holes in the & band that have been scat-
tered to momentum states less than the Fermi
momentum, kF. Band calculations made on the
noble metals such as copper' and gold indicate
that the 4 bands can be relatively flat at some re-
gions in
4'
space less than 4F. This might allow
photoexcited holes to be scattered into a suffi-
cient range of states in 4 space to account for the
observed broad linewidths. Gold showed an ap-
preciable sharpening and shift of its spectrum at
low temperature mhich is attributed in part to a
VOLUME 22, NUMBER 5 PHVSreAJ. REVIEW XEYTERS $ FEBRUARY 1969
change of the plasma absorption edge as mell as
narrowing of the distribution of electrons and
holes. The copper spectrum also narrowed
slightly and shifted from 5930 to about 5800 A in
going from 300 to 10'K.
The intensity of recombination radiation for di-
rect transitions between electrons and holes is
given
bye
t(hu)
&f
IM(k)~*f
(k)f(k)
. (1)
Here &()t&) is the intensity per unit range of pho-
ton energy,
~ is the interband matrix element,
fz(k)
and
fI,
(k) are, respectively, the electron
and hole distribution functions which may not be
uniform in space, and ~ is the surface corre-
sponding to the
energy &e(k)-&Ii(k)
=h&
in wave-
vector (k) space. Because the line shape involves
the energy-momentum relation of the various
bands, the spectra might help to elucidate the
band structure of these metals. The role of ex-
citons in the recombination has been neglected
since the high electron densities should effective-
ly screen out any exciton bound state. However,
there has been some speculation' about the exis-
tence of excitons in metals and perhaps the lumi-
nescence studies would help to demonstrate this
effect.
In both metals the peak emission was consis-
tent with the optically observed energy between
the upper
d band and the Fermi energy: 2.0 eV
for copper and 2.2 eV for gold. Similar transi-
tions between the conduction band and the lower
d band should also be observed for sufficiently
high pump energies. The transitions should oc-
cur around 4 eV. Further experiments are in
progress to observe this transition. The maxi-
mum integrated efficiency of recombination ne-
glecting corrections for reabsorption was esti-
mated to be on the order of 1 part in
10'
.
The observation of interband radiative recom-
bination in metals may very well provide another
technique to help investigate some properties of
the band structure of metals as well as to deter-
mine the nature of scattering mechanisms of ex-
cited electrons and holes. Photoluminescence
might also be observed for transitions between
higher lying bands in semiconductors and semi-
metals and could be accessible to study by laser
excitation techniques.
The author would like to thank Dr. G. F. Dres-
selhaus for many informative discussions and
Mr. D. J. Wells for assistance with the measure-
ments.
*Operated with support from the U. S. Air Force.
~A. Mooradian, Phys. Rev. Letters 20, 1102 {1968).
2A. Mooradian, in Proceedings of the International
Conference on Light Scattering from Solids, New York,
1968 (to be published).
For a review article on this effect which contains
pertinent references, see W. Steinmann, Phys. Status
Solidi 28, 487 (1968).
4P.
O. Nilsson, A. Persson, and S. Hagstrom, Solid
State Common. 6, 297 {1968).
5G. A. Burdick, Phys. Rev. 129, 138 (1963); B. Se-
gall, Phys. Rev. 125, 109 (1962); E. C, Snow and J. T.
Waber, Phys. Rev. 157, 570 (1967); H. L. Davis, J. S.
Faulkner, and H. %. Joy, Phys. Rev. 167, 601 (1968);
E. C. Snow, Phys. Rev. 171, 785 (1968).
A. Mooradian and H. Y. Fan, Phys. Rev. 148, 873
(1966).
7M.
H. Cohen, in Optical Properties and Electronic
Structure of Metals and Alloys, edited by F. Abeles
(North-Holland Publishing Company, Amsterdam, The
Netherlands, 1966), p. 66.
BD.
Beaglehole, Proc. Phys. Soc. (London) 85, 1007
(1965), and 87, 461 (1966).
Potrebbero piacerti anche
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5795)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Lambda 365 UV/Vis Spectrophotometer With UV Lab Software: Image Not Found Image Not FoundDocumento1 paginaLambda 365 UV/Vis Spectrophotometer With UV Lab Software: Image Not Found Image Not FoundsecateNessuna valutazione finora
- En 4j2 4fDocumento71 pagineEn 4j2 4fRafael HernandezNessuna valutazione finora
- Multiplexers DemultiplexersDocumento17 pagineMultiplexers Demultiplexerslvsaru100% (1)
- PW160-7K S 0411Documento890 paginePW160-7K S 0411ado_22100% (3)
- Je5b00789 Si 001Documento7 pagineJe5b00789 Si 001secateNessuna valutazione finora
- Extraction Titanium Dioxide (Tio,) From Ilmenite and Titaniferous SlagDocumento6 pagineExtraction Titanium Dioxide (Tio,) From Ilmenite and Titaniferous SlagsecateNessuna valutazione finora
- Surfactant-Assisted Synthesis of Batio Nanoparticles by Micro-Emulsion MethodDocumento4 pagineSurfactant-Assisted Synthesis of Batio Nanoparticles by Micro-Emulsion MethodsecateNessuna valutazione finora
- AGENDA ITEM: 650-472 Load Combinations 8 Ballot: P Determined in F.4.1Documento8 pagineAGENDA ITEM: 650-472 Load Combinations 8 Ballot: P Determined in F.4.1secateNessuna valutazione finora
- Improved Temperature Compensation For in Situhumic-Like and Tryptophan-Like Fluorescence Acquisition in Diverse Water TypesDocumento4 pagineImproved Temperature Compensation For in Situhumic-Like and Tryptophan-Like Fluorescence Acquisition in Diverse Water TypessecateNessuna valutazione finora
- E-Books: PublicationsDocumento4 pagineE-Books: PublicationssecateNessuna valutazione finora
- Copper in Drinking Water by Anodic Stripping Voltammetry at The Sctrace Gold Using The 946 Portable Va AnalyzerDocumento2 pagineCopper in Drinking Water by Anodic Stripping Voltammetry at The Sctrace Gold Using The 946 Portable Va AnalyzersecateNessuna valutazione finora
- Accepted Manuscript: RSC - Li/pccpDocumento11 pagineAccepted Manuscript: RSC - Li/pccpsecateNessuna valutazione finora
- Ab-416 3 enDocumento20 pagineAb-416 3 ensecateNessuna valutazione finora
- Surface Interaction Energy Simulation of Ceramic Materials With Epoxy ResinDocumento4 pagineSurface Interaction Energy Simulation of Ceramic Materials With Epoxy ResinsecateNessuna valutazione finora
- Pancreatic JournalDocumento4 paginePancreatic JournalsecateNessuna valutazione finora
- Zinc Oxide Nanostructures For Optoelectronic and Energy DevicesDocumento3 pagineZinc Oxide Nanostructures For Optoelectronic and Energy DevicessecateNessuna valutazione finora
- Quattro ESEM DatasheetDocumento4 pagineQuattro ESEM DatasheetsecateNessuna valutazione finora
- Journal of Colloid and Interface ScienceDocumento8 pagineJournal of Colloid and Interface SciencesecateNessuna valutazione finora
- Prestressed Concrete Developments in Japan: Ben C. Gerwick, JRDocumento11 paginePrestressed Concrete Developments in Japan: Ben C. Gerwick, JRsecateNessuna valutazione finora
- Fig. 6.38Documento1 paginaFig. 6.38secateNessuna valutazione finora
- Meso-Porous Silicon-Coated Carbon Nanotube As An Anode For Lithium-Ion BatteryDocumento8 pagineMeso-Porous Silicon-Coated Carbon Nanotube As An Anode For Lithium-Ion BatterysecateNessuna valutazione finora
- AaaaaaaaaaaaaaaaaaaaaaaaaaaaDocumento1 paginaAaaaaaaaaaaaaaaaaaaaaaaaaaaasecateNessuna valutazione finora
- High Temperature NTC Batio - Based Ceramic Resistors: Ying Luo, Xinyu LiuDocumento4 pagineHigh Temperature NTC Batio - Based Ceramic Resistors: Ying Luo, Xinyu LiusecateNessuna valutazione finora
- Boon Lak Horn 2016Documento5 pagineBoon Lak Horn 2016secateNessuna valutazione finora
- Sonochemical and Microwave-Assisted Synthesis of Linked Single-Crystalline Zno RodsDocumento6 pagineSonochemical and Microwave-Assisted Synthesis of Linked Single-Crystalline Zno RodssecateNessuna valutazione finora
- Ceramics International: Gao-Yu Zhao, Yu Zhang, Lin Jiang, Hong-Mei ZhangDocumento5 pagineCeramics International: Gao-Yu Zhao, Yu Zhang, Lin Jiang, Hong-Mei ZhangsecateNessuna valutazione finora
- 10.1016/j.tsf.2016.12.036: Thin Solid FilmsDocumento34 pagine10.1016/j.tsf.2016.12.036: Thin Solid FilmssecateNessuna valutazione finora
- A) From W. J. Stark, L. Mädler, M. Maciejewski, S. E. Pratsinis, and A. Chem. Comm., 588-589 (2003) - Reproduced by Permission of The RoyalDocumento1 paginaA) From W. J. Stark, L. Mädler, M. Maciejewski, S. E. Pratsinis, and A. Chem. Comm., 588-589 (2003) - Reproduced by Permission of The RoyalsecateNessuna valutazione finora
- A Study On Pushover Analysis Using Capacity Spectrum Method Based On Eurocode 8Documento13 pagineA Study On Pushover Analysis Using Capacity Spectrum Method Based On Eurocode 8ephNessuna valutazione finora
- Have A One Track Mind: Lorna WingDocumento4 pagineHave A One Track Mind: Lorna WingsophilauNessuna valutazione finora
- Vallorbs Guide Cut Vs Rolled ThreadsDocumento3 pagineVallorbs Guide Cut Vs Rolled ThreadsOrlando AriasNessuna valutazione finora
- The Influence of Social Media On Crowd Behavior and The Operational EnvironmentDocumento78 pagineThe Influence of Social Media On Crowd Behavior and The Operational EnvironmentangryTXNessuna valutazione finora
- Lecture On Dictionaries - Jupyter NotebookDocumento13 pagineLecture On Dictionaries - Jupyter NotebookruchikaNessuna valutazione finora
- Conductivity Type of Extrinsic Semiconducting Materials: Standard Test Methods ForDocumento6 pagineConductivity Type of Extrinsic Semiconducting Materials: Standard Test Methods ForRob GridleyNessuna valutazione finora
- K230F Equipment ManualsDocumento166 pagineK230F Equipment ManualsHui ChenNessuna valutazione finora
- Toyota Toyota+sienna+2013+manualDocumento1 paginaToyota Toyota+sienna+2013+manualNur Cholik Widyan Sa0% (1)
- Government Polytechnic, Pune: ET2107 - NODocumento8 pagineGovernment Polytechnic, Pune: ET2107 - NOG012 Bhise AniketNessuna valutazione finora
- AWS Certified Developer - AssociateDocumento2 pagineAWS Certified Developer - AssociateKS ReddyNessuna valutazione finora
- Bulletin: ICC International Court of ArbitrationDocumento30 pagineBulletin: ICC International Court of ArbitrationPriscila Machado MartinsNessuna valutazione finora
- Visual Programming NotesDocumento0 pagineVisual Programming NotesSamuel VictorNessuna valutazione finora
- SPPU Report FormatDocumento50 pagineSPPU Report FormatAmit Devidas AherNessuna valutazione finora
- Edi WowDocumento11 pagineEdi WowfantasighNessuna valutazione finora
- CDI1Documento40 pagineCDI1Leonino Angelica Aiko S.Nessuna valutazione finora
- Tugas Mek I-9 OktDocumento2 pagineTugas Mek I-9 OktAlifani SofiNessuna valutazione finora
- Updated Eva ResumeDocumento1 paginaUpdated Eva Resumeapi-534826411Nessuna valutazione finora
- Additional Instructions For Mailing Your Package: Drop Off LocatorDocumento2 pagineAdditional Instructions For Mailing Your Package: Drop Off LocatorEthanNessuna valutazione finora
- Lesson Plan in Mathematics Grade9Documento6 pagineLesson Plan in Mathematics Grade9Abegail VillanuevaNessuna valutazione finora
- Iso SpinDocumento5 pagineIso SpinjhgsgsNessuna valutazione finora
- Probability DistributionsDocumento167 pagineProbability DistributionslihaiyangNessuna valutazione finora
- Chapter 2 - Analysis of Steam Power Plant CycleDocumento61 pagineChapter 2 - Analysis of Steam Power Plant Cyclerrhoshack100% (1)
- Kinematics of A Novel Nine Degree of Freedom Configurable Gough-Stewart PlatformDocumento19 pagineKinematics of A Novel Nine Degree of Freedom Configurable Gough-Stewart PlatformNeider NadidNessuna valutazione finora
- ZXR10 8900 Series: Hardware Installation ManualDocumento109 pagineZXR10 8900 Series: Hardware Installation ManualErnestoLopezGonzalezNessuna valutazione finora
- E-Rpms Portfolio (Design 3) - Depedclick-1Documento42 pagineE-Rpms Portfolio (Design 3) - Depedclick-1angeliNessuna valutazione finora
- Add Maths F4 Topical Test 3 (E)Documento3 pagineAdd Maths F4 Topical Test 3 (E)HANIFAH50% (2)
- Janice C. Vergel: ObjectiveDocumento3 pagineJanice C. Vergel: ObjectiveRency Reiz JhaniceNessuna valutazione finora