Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
FTIR Reflection Techniques
Caricato da
Anish BhosaleCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
FTIR Reflection Techniques
Caricato da
Anish BhosaleCopyright:
Formati disponibili
FT-IR Reflection Techniques
Overview Main Principles of Reflection Techniques
Internal Reflection
External Reflection
Summary
Vladimr Setnika
Transmission:
Excellent for solids, liquids and gases
The reference method for quantitative analysis
Sample preparation can be difficult
Reflection:
Collect light reflected from an interface
air/sample, solid/sample, liquid/sample
Analyze liquids, solids, gels or coatings
Minimal sample preparation
Convenient for qualitative analysis, frequently
used for quantitative analysis
Differences Between Transmission and
Reflection FT-IR Techniques
Internal Reflection Spectroscopy:
Attenuated Total Reflection (ATR)
External Reflection Spectroscopy:
Specular Reflection (smooth surfaces)
Combination of Internal and External Reflection:
Diffuse Reflection (DRIFTs) (rough surfaces)
FT-IR Reflection Techniques
FT-IR Reflection Techniques
Infrared beam reflects from a
interface via total internal reflectance
Sample must be in optical contact
with the crystal
Collected information is from the
surface
Solids and powders, diluted in a IR
transparent matrix if needed
Information provided is from the
bulk matrix
Sample must be reflective or on a
reflective surface
Information provided is from the
thin layers
Attenuated Total Reflection (ATR)
- introduced in the 1960s, now widely used
- light introduced into a suitable prism at an angle
exceeding the critical angle for internal reflection
an evanescent wave at the reflecting surface
IRE internal reflection element = ATR crystal
Single Bounce ATR
sample in close contact
with IRE
from the interaction of
the evanescent wave
(exponential decay) with
the sample, a spectrum
can be recorded with little
or no sample preparation
Total internal reflection
critical angle - when the angle of
refraction (r) becomes equal to 90
degrees and Snell's law reduces to:
sin(u) = n(1)/n(2) n(1) n(2)
where (u) is termed the critical angle c
When the critical angle is exceeded for a particular light wave,
it exhibits total internal reflection back into the medium.
Attenuated Total Reflection (ATR)
The larger the angle to the normal,
the smaller is the fraction of light
transmitted, until the angle when
total internal reflection occurs.
Factors influencing ATR analysis
Wavelength of IR radiation
Refractive indexes of sample and IRE n
smp
, n
IRE
Angle of incidence of IR radiation u
Depth of penetration (pathlength) d
P
Sample and IRE contact efficiency
When an incident ray is totally internally reflected at the
interface between two materials of different refractive index,
the intensity of the evanescent field extending into the medium
of lower index decays exponentially with distance from the
boundary:
I
ev
= I
o
exp [-z/d
p
]
z is the distance normal to the optical interface,
I
o
is the intensity at z = 0,
d
p
is the penetration depth
Attenuated Total Reflection (ATR)
Depth of penetration (pathlength) of the infrared beam into
the sample depends on , n
smp
, n
IRE
, u
Attenuated Total Reflection (ATR)
2
IRE smp
2
IRE
) / ( - sin 2
=
n n n
dp
u t
The effective pathlength of the spectrum collected varies with
the wavelength of the radiation:
- longer greater d
P
: d
P
lower at higher wavenumbers
- ATR intensities decreased at higher wavenumbers if
compared to transmission spectra
d
P
typically < 10 m
ATR correction accounts for this variation in effective
pathlength by scaling the ATR spectrum accordingly. Most
FTIR software packages incorporate an ATR correction
algorithm.
Attenuated Total Reflection (ATR)
Critical Angle depends on n
IRE
and n
smp
- increasing n
IRE
decreasing u and d
P
|
|
.
|
\
|
=
IRE
smp
n
n
1
sin
c
u
high values of n
IRE
needed
Materials of ATR crystals (IRE elements)
Attenuated Total Reflection (ATR)
Attenuated Total Reflection (ATR)
Materials of ATR crystals (IRE elements)
Zinc Selenide ZnSe
- preferred for all routine applications, limited use with
strong acids and alkalies, surface etched during prolonged
exposure to extremes of pH, complexing agents (ammonia
and EDTA) will also erode its surface because of the
formation of complexes with the zinc
AMTIR
- as a glass from selenium, germanium and arsenic,
insolubility in water, similar refractive index to zinc
selenide, can be used in measurements that involve strong
acids
Attenuated Total Reflection (ATR)
Materials of ATR crystals (IRE elements)
Germanium Ge
- high refractive index, used when analyzing samples have a
high refractive index
Silicon Si
- hard and brittle, chemically inert, affected only by strong
oxidizers, well suited for applications requiring temperature
changes as it withstands thermal shocks better then other
ATR materials, hardest crystal material offered except for
Diamond, which makes it well suited for abrasive samples
that might otherwise scratch softer crystal materials, below
1500 cm
-1
usefulness limited
Attenuated Total Reflection (ATR)
Materials of ATR crystals (IRE elements)
Diamond
- for analysis of a wide range of samples, including acids,
bases, and oxidizing agents, scratch and abrasion resistant,
expensive, intrinsic absorption from approximately 2300 to
1800 cm
-1
limits its usefulness in this region (5% transmission)
Attenuated Total Reflection (ATR)
For thin films, the ATR spectra are the same as
transmission spectra.
For thick films, the absorption bands are more intense at
longer wavelengths.
As the angle of incidence approaches the critical angle, the
bands tend to broaden on the long wavelength side and the
minima are displaced to longer wavelengths (lower
wavenumbers). Dispersion type spectra are observed very
close to and below critical angle.
Experimental Setup horizontal arrangement (HATR)
Small sampling area
- use for strong absorbers
- solid samples, liquids
Attenuated Total Reflection (ATR)
Single Bounce ATR Multi-Bounce ATR
Broad sampling area provides
- greater contact with the
sample
- use for weak absorbers or
dilute solutions
Experimental Setup
Attenuated Total Reflection (ATR)
Attenuated Total Reflection (ATR)
Attenuated Total Reflection (ATR)
Multi-Bounce ATR
SAMPLE
ATR element
Circle ATR
Incoming
Light
Exiting
light
Attenuated Total Reflection (ATR)
Scheme of the Circle ATR Cell
Attenuated Total Reflection (ATR)
Scheme of the Circle ATR Cell
Attenuated Total Reflection (ATR)
ATR Spectra
Attenuated Total Reflection (ATR)
ATR Spectra
Attenuated Total Reflection (ATR)
IR Spectra of Methanol
Metanol ATR , nek or igovno
2
4
6
A
b
s
o
r
b
a
n
c
e
Metanol ATR , kor ek ce tlous tk y
2
4
6
A
b
s
o
r
b
a
n
c
e
Metanol kyv eta AgCl, 0,082 mm
0,2
0,4
0,6
A
b
s
o
r
b
a
n
c
e
1000 2000 3000 4000
Wav enumber s ( cm- 1)
ATR (ZnSe 45
o
), ATR uncorrected
ATR (ZnSe 45
o
), corrected
Transmittion in the cell
Attenuated Total Reflection (ATR)
ATR Spectra of Rubber Diamond vs Germanium IRE
Refractive indexes: Ge=4
Diamond=2.4
Summary:
- Versatile and non-destructive technique for
variety of materials soft solid materials, liquids,
powders, gels, pastes, surface layers, polymer films,
samples after evaporation of a solvent ..
- Requires minimal or no sample preparation
- Useful for surface characterization, opaque samples
Attenuated Total Reflection (ATR)
Limitation: sensitivity is typically 3-4 orders of
magnitude less than transmission
Specular vs Diffuse Reflection
Specular reflection is defined as light reflected from a
smooth surface (such as a mirror, any irregularities in
the surface are small compared to ) at a definite angle,
whereas diffuse reflection is produced by rough
surfaces that tend to reflect light in all directions. There
are far more occurrences of diffuse reflection than
specular reflection in our everyday environment.
What kind of reflections
account for the column
of light reflected off
the water?
What would we see on
the water if it were
perfectly flat, unmoving?
Specular vs Diffuse Reflection
- introduced in the 1960s, much wider use in the 1970s
- light is reflected from a smooth (mirror-like) sample
at a definite angle to record its spectrum
- spectroscopic technique for films deposited on, or
pressed against reflective surfaces
Specular Reflection
= External Reflection Spectroscopy
Angle of incidence = Angle of reflection
- if surface absorbs a
wavelength of light
its relative intensity
is decreased
~20-60
o
I
d
medium 0
sample 1
medium 2
R
01
T
01
F
T
01
FT
12
T
01
F
2
R
12
T
01
F
2
R
12
T
10
T
01
F
3
R
12
R
10
T
01
F
4
R
12
R
10
2
etc
etc
F = exp(-4pk
1
d/l)
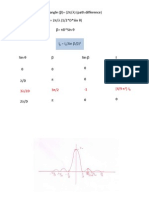
Light Rays Through Thin Films
If the surface is smooth like a mirror:
reflection and the incidence angles are equal
reflected beam retains the polarization
characteristics of the incidence beam
Thin layers: 0.5-20 m angle ~20-60
o
spectra
similar to transmission ones
Monomolecular layers: angle ~60-85
o
spectra
predominatly a function of the refractive index
derivative shape of the bands arising from
superposition of extinction coefficient and
dispersion of refractive index
Specular Reflection
K. Yamamoto and H. Ishida, Vibrational Spectrosc., 8, 1 (1994)
Refractive
index
Absorbance
index
Specular Reflection
Specular Reflection
Experimental Setup
- selection of incident angle
Incident angle influences
- effective pathlength
- polarized IR response
Specular Reflection
Experimental Setup
K. Yamamoto and H. Ishida, Vibrational Spectrosc., 8, 1 (1994)
Refractive
index
Absorbance
index
Specular Reflection
Specular Reflection Spectra
Specular Reflection
Correction of Restrahlen bands
IR spektrum bez k or ekc e
IR spektrum po korekci podle Kr amer se a Kroniga
0,0
0,1
0,2
0,3
0,4
0,5
0,6
0,7
0,8
0,9
1,0
A
b
s
o
r
b
a
n
c
e
500 1000 1500
Wav enumber s ( cm- 1)
Specular Reflection
Specular Reflection Spectra
Specular Reflection
Summary:
- non-destructive measurement of thin layers or
monolayers
- coatings on polished metals e.g.varnishes
- surface characterization
Limitation: Spectra depend on refractive index
- used for testing of lubricated surfaces of hard
disks, to degradation studies of a protective
coating on the surface; analysis of polymers on the
surface of food containers and many others
Diffuse Reflection
Diffuse Reflectance Infrared Fourier Transform Spectroscopy
(DRIFTs)
Spectra of powders and rough
surfaces can be recorded by
illuminating these surfaces and
collecting sufficient scattered
radiation with ellipsoids and
paraboloids.
- fast measurement of powdered samples
- low repeatability of spectral data
- complicated physical description of the effect
shape of particles, compactness of samples
refractive index of particles
reflectivity and absorption characteristics of particles
Diffuse Reflection
Mechanisms generating infrared spectrum of a powder
Diffuse Reflection
Experimental Setup
Diffuse Reflection
Experimental Setup Different Geometry
Diffuse Reflection
DRIFTs Sample Preparation
- powder simply placed into the sample cup and
analyzed (no sample preparation)
- if the sample is too absorbent, it must be diluted in
a nonabsorbent matrix (KBr, KCl,)
- no pellet pressing required
- particle size smaller than 10 m (i.e. not exceeding
the wavelength of the incident radiation) preferred
- or paper disc coated on a surface by SiC as an
abrasive material grinding of sample
- ideal for pharmaceutical and forensic applications
Diffuse Reflection
DRIFTs Spectra y axe units
From the theoretical standpoint, there is no linear relation
between band intensity and concentration (as valid in
transmission), and quantitative analyses by the DRIFTS
method are therefore rather complicated.
- expressed in linear units Kubelka-Munk (roughly correspond
to absorbance in transmission KBr pellet technique)
Diffuse Reflection
DRIFTs Spectra y axe units
- DRIFTs bands stronger than expected absorption from weak
IR bands compensation by Kubelka-Munk conversion
- f(R) is called Kubelka-Munk function
R
absolute reflectance of the sampled layer
k molar absorption coefficient a absorptivity
- proportional to the fraction of transmitted light
s diffusion (scattering) coefficient
- proportional to the fraction of diffused light
creates a linear relationship for spectral intensity relative
to sample concentration
k = 2.303 a c
f(R) = 2.303 a c/s
s ~ const. f(R) = 2.303 a c
( )
( )
s
k
s
c
a
R
R
R f = =
. . 303 . 2
2
1
2
Diffuse Reflection
DRIFTs Spectra y axe units
linear relationship assumed when:
- infinite sample dilution in a non-absorbing matrix, i.e. KCl,
KBr, (such that k = 0 and R = 1)
- a constant scattering coefficient
- infinitely thick sample layer
These conditions achieved for:
- highly diluted samples
- small particle samples (the scattering coefficient is a function
of sample size and packing)
- sample layer of at least 1.5 mm
With proper sample preparation DRIFTs can provide
ppm sensitivity and high quality results.
T Armaroli et al. Oil & Gas Science and Technology Rev. IFP, 59 (2004), 215.
Diffuse Reflection
DRIFTs Spectra
Diffuse Reflection
IR Spectra of CaCO
3
C
a
C
O
3
,
K
B
r
t
a
b
l
e
t
a
C
a
C
O
3
v
K
B
r
,
D
R
I
F
T
S
0,1
0,2
0,3
0,4
0,5
0,6
0,7
0,8
0,9
1,0
A
b
s
o
r
b
a
n
c
e
1000 2000 3000 4000
Wav enumber s ( cm- 1)
DRIFTs
KBr pellet
Diffuse Reflection
IR Spectra of 1,2-Bis(diphenyl phosphino)ethane
DRIFTs
KBr pellet
T Armaroli et al. Oil & Gas Science and Technology Rev. IFP, 59 (2004), 215.
Diffuse Reflection vs Transmission
Zeolite Y14 (Si/Al of
13.6)
a) transmission spectrum,
selfsupported pure powder
pellet, after overnight
activation at 723 K under
vacuum
b) diffuse reflection
spectrum after 10 h
activation under
nitrogen.
spectra recorded at 298 K
Diffuse Reflection
Pt/Al
2
O
3
DRIFTs spectra, variation in species at the
surface during 1 h of nitration at 423 K (background
first spectrum, activated surface).
Diffuse Reflection
DRIFTs spectra of Ca oxalate + KBr (5/95 w/w)
Summary:
Attenuated Total Reflection (ATR)
- structural information from the surface thick layers
Specular Reflection (smooth surfaces)
- measurement of thin layers or monolayers
(coatings on metals, surface characterization)
Diffuse Reflection (DRIFTs) (rough surfaces)
- structural information is from the bulk matrix
FT-IR Reflection Techniques
Potrebbero piacerti anche
- Francis Francis x1 Espresso Machine Circuit Board Schematic and Wiring Diagram v2Documento2 pagineFrancis Francis x1 Espresso Machine Circuit Board Schematic and Wiring Diagram v2frox123100% (2)
- Topcom Outdoor 2010 Manuel MultiDocumento152 pagineTopcom Outdoor 2010 Manuel Multifrox123Nessuna valutazione finora
- Reflect Ance SpectrosDocumento3 pagineReflect Ance SpectrosRoss JonesNessuna valutazione finora
- ATR PrinciplesDocumento21 pagineATR PrinciplesKassimNessuna valutazione finora
- ATRAN611Documento3 pagineATRAN611Sunita TipmontaNessuna valutazione finora
- Glossary of TermsDocumento11 pagineGlossary of TermsalayuNessuna valutazione finora
- ATR Theory and ApplicationDocumento3 pagineATR Theory and ApplicationjorgchanNessuna valutazione finora
- FTIR InstrumentationDocumento35 pagineFTIR InstrumentationNgurah Mahasvira100% (1)
- Attenuated Total Reflectance FTIRDocumento4 pagineAttenuated Total Reflectance FTIRFabrício RamosNessuna valutazione finora
- Components of Optical Instruments Chapter 7Documento70 pagineComponents of Optical Instruments Chapter 7Noranisza Mahmud100% (1)
- Speed of Light Air Speed of Light MediumDocumento11 pagineSpeed of Light Air Speed of Light MediumkishoreputhaNessuna valutazione finora
- Construction of Optical FiltersDocumento34 pagineConstruction of Optical FiltersAvnish KumarNessuna valutazione finora
- Kuliah Infra MerahDocumento110 pagineKuliah Infra Merahmuhlisun azimNessuna valutazione finora
- Presentation 1Documento38 paginePresentation 1Kiruthick DonNessuna valutazione finora
- 17 Infrared LTDDocumento59 pagine17 Infrared LTDMichelle ChicaizaNessuna valutazione finora
- I R SpectrosDocumento20 pagineI R Spectrosbiswajit_baruah6487Nessuna valutazione finora
- Presented by - Sayali P. Patil. Nikita R. Bothare. Dhiraj GuptaDocumento34 paginePresented by - Sayali P. Patil. Nikita R. Bothare. Dhiraj GuptaDhiraj GuptaNessuna valutazione finora
- Topic 3: Reflectarray Antennas Reflectarray Antennas at Terahertz FrequenciesDocumento26 pagineTopic 3: Reflectarray Antennas Reflectarray Antennas at Terahertz FrequenciesFer Martinez de la RivaNessuna valutazione finora
- Nephelometry TurbidimetryDocumento18 pagineNephelometry TurbidimetrySandeep Kumar Thatikonda0% (1)
- Emp580 RS 2 EmrDocumento19 pagineEmp580 RS 2 EmrdeshmukhgeolNessuna valutazione finora
- Chapter Three Light As Wave MotionDocumento11 pagineChapter Three Light As Wave MotionSabrina NevilleLongbottom RitterNessuna valutazione finora
- SpectrosDocumento37 pagineSpectrosKiruthick DonNessuna valutazione finora
- X Ray ReflectivityDocumento14 pagineX Ray Reflectivityjmdq2000Nessuna valutazione finora
- Module 1 Introduction To LightingDocumento26 pagineModule 1 Introduction To LightingchrismaineNessuna valutazione finora
- AAO NNU Refractive Index, Snells Law, Prism, Refraction by Single CurvesDocumento21 pagineAAO NNU Refractive Index, Snells Law, Prism, Refraction by Single Curvesnurfatminsari almaidinNessuna valutazione finora
- Intro To ATRDocumento3 pagineIntro To ATRtahanaeimaei13Nessuna valutazione finora
- Week 05 - Reflection and Refraction (Part I)Documento21 pagineWeek 05 - Reflection and Refraction (Part I)inushanth inuNessuna valutazione finora
- FIBRE OPTICS Final 26.02.2021Documento43 pagineFIBRE OPTICS Final 26.02.2021Mahir MittalNessuna valutazione finora
- 11-Infrared SpectrosDocumento38 pagine11-Infrared SpectrosSri DayantiNessuna valutazione finora
- SpectrophotometryDocumento70 pagineSpectrophotometryaugusteNessuna valutazione finora
- AI Unit IDocumento78 pagineAI Unit Isatya narayanaNessuna valutazione finora
- IV. Laser Diode (LD) or Semiconductor LaserDocumento27 pagineIV. Laser Diode (LD) or Semiconductor LaserSagar BhardwajNessuna valutazione finora
- Advanced Spectroscopy: 1. General AspectsDocumento56 pagineAdvanced Spectroscopy: 1. General AspectsMohamed DahmaneNessuna valutazione finora
- An Introduction To Infrared SpectrosDocumento88 pagineAn Introduction To Infrared Spectrosrejie magnayeNessuna valutazione finora
- Optical Wave Guide: by Dong Na (PH.D)Documento18 pagineOptical Wave Guide: by Dong Na (PH.D)Ahsan SaleemNessuna valutazione finora
- FTIRDocumento19 pagineFTIRvsnmurthy1Nessuna valutazione finora
- Lecture 7 Rayleigh PDFDocumento34 pagineLecture 7 Rayleigh PDFWendirad BeshadaNessuna valutazione finora
- Phase Angle (2 /) (Path Difference) 2 / (1/2 D Sin) D SinDocumento59 paginePhase Angle (2 /) (Path Difference) 2 / (1/2 D Sin) D SinanujayanNessuna valutazione finora
- Nephelometry TurbidimetryDocumento28 pagineNephelometry TurbidimetrySandeep Kumar Thatikonda80% (5)
- As H 23b OpticsDocumento48 pagineAs H 23b OpticsHany ElGezawyNessuna valutazione finora
- Analytical Instrumentation ConceptsDocumento20 pagineAnalytical Instrumentation ConceptsshashankNessuna valutazione finora
- Sistec Notes Attenuation in Optical FiberDocumento22 pagineSistec Notes Attenuation in Optical Fibermanishsoni30Nessuna valutazione finora
- Radar BasicsDocumento69 pagineRadar BasicsAdhwareshBharadwajNessuna valutazione finora
- Fourier Transform Infrared (FT-IR) Spectroscopy: Theory and ApplicationsDocumento35 pagineFourier Transform Infrared (FT-IR) Spectroscopy: Theory and ApplicationsLeeyie LimNessuna valutazione finora
- Beam Characteristics - Olympus IMSDocumento3 pagineBeam Characteristics - Olympus IMSSanat MoshaverNessuna valutazione finora
- OpticsDocumento31 pagineOpticsRahul SinghNessuna valutazione finora
- Fabry-Pérot Interferometer: TheoryDocumento5 pagineFabry-Pérot Interferometer: TheorychandreshwarNessuna valutazione finora
- Ultrasonic Testing (Ut) : This Is The Method, Ultrasound (20,000Hz) Is Used To Find The Defect Up ToDocumento30 pagineUltrasonic Testing (Ut) : This Is The Method, Ultrasound (20,000Hz) Is Used To Find The Defect Up ToKrishnamoorthi GovinthanNessuna valutazione finora
- L14 UltrasoundDocumento27 pagineL14 UltrasoundLinh PhanNessuna valutazione finora
- Optical Lithography PDFDocumento16 pagineOptical Lithography PDFprashn80Nessuna valutazione finora
- Reflectance in Thin Films - All 13.10 DatDocumento19 pagineReflectance in Thin Films - All 13.10 DatAnaNessuna valutazione finora
- Introduction To AntennasDocumento47 pagineIntroduction To AntennasShailesh PathakNessuna valutazione finora
- 1.optical FibersDocumento23 pagine1.optical FibersYashNessuna valutazione finora
- EN ASFA AU Koplik - Infrared - Spectros PDFDocumento25 pagineEN ASFA AU Koplik - Infrared - Spectros PDFalfitra mahendraNessuna valutazione finora
- Chap 4: RadiationDocumento40 pagineChap 4: RadiationOng Teck ChongNessuna valutazione finora
- E 349 - 72 R99 Rtm0os1sruqDocumento7 pagineE 349 - 72 R99 Rtm0os1sruqJuanNessuna valutazione finora
- Chapter 3-Fall-2022-C411Documento35 pagineChapter 3-Fall-2022-C411hesham khaledNessuna valutazione finora
- Terrestrial PropagationDocumento30 pagineTerrestrial PropagationKrishna DuttNessuna valutazione finora
- First Module Part 1Documento53 pagineFirst Module Part 1hrithikNessuna valutazione finora
- DSP-W215 A1 Manual v1.00 (DI)Documento31 pagineDSP-W215 A1 Manual v1.00 (DI)frox123Nessuna valutazione finora
- Installation and User ManualDocumento19 pagineInstallation and User Manualfrox123Nessuna valutazione finora
- Vogel - Boric AcidDocumento5 pagineVogel - Boric Acidfrox123Nessuna valutazione finora
- EUBP Guidelines Seedling LogoDocumento2 pagineEUBP Guidelines Seedling Logofrox123Nessuna valutazione finora
- Installation and User ManualDocumento19 pagineInstallation and User Manualfrox123Nessuna valutazione finora
- OrganoCatalisys Angew 2004 PDFDocumento38 pagineOrganoCatalisys Angew 2004 PDFfrox123Nessuna valutazione finora
- The Williamson AmplifierDocumento44 pagineThe Williamson Amplifierfrox123Nessuna valutazione finora
- Atac Nuline Cone Plate Viscometer Data Sheet Rev 02Documento2 pagineAtac Nuline Cone Plate Viscometer Data Sheet Rev 02frox123Nessuna valutazione finora
- MomentiveDocumento4 pagineMomentivefrox123Nessuna valutazione finora
- Programmateur Nez de Robinet E-Drip2: 3/4'' 1'' 0,5 - 8 BarsDocumento6 pagineProgrammateur Nez de Robinet E-Drip2: 3/4'' 1'' 0,5 - 8 Barsfrox123Nessuna valutazione finora
- Brand AQAGloss-Brochure - Product Overview Polymer Dispersions Architectural Coatings-EnglishDocumento6 pagineBrand AQAGloss-Brochure - Product Overview Polymer Dispersions Architectural Coatings-Englishfrox123Nessuna valutazione finora
- Raspberry Pi OpenVPN Server TutorialDocumento9 pagineRaspberry Pi OpenVPN Server Tutorialfrox123Nessuna valutazione finora
- Brochure Matting Agents EDocumento60 pagineBrochure Matting Agents Efrox123Nessuna valutazione finora
- IP Camera CGI - English VersionDocumento28 pagineIP Camera CGI - English Versionfrox123Nessuna valutazione finora
- Torlon Processing and PostprocessingDocumento26 pagineTorlon Processing and Postprocessingfrox123Nessuna valutazione finora
- OPS v3.1 Documentation Version 1.2.12 enDocumento161 pagineOPS v3.1 Documentation Version 1.2.12 enfrox123Nessuna valutazione finora
- XplijstDocumento18 pagineXplijstfrox123Nessuna valutazione finora
- Enigma Sim ManualDocumento22 pagineEnigma Sim Manualfrox123Nessuna valutazione finora
- X Pag EngineDocumento54 pagineX Pag Enginefrox123Nessuna valutazione finora
- Brand Luwipal Brochure Amino ResinsDocumento8 pagineBrand Luwipal Brochure Amino Resinsfrox123Nessuna valutazione finora
- Auto Zoom Electronic Flash Unit: Please Read This Operating Manual Carefully First For Proper UseDocumento96 pagineAuto Zoom Electronic Flash Unit: Please Read This Operating Manual Carefully First For Proper Usefrox123Nessuna valutazione finora