Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Model Studies of Mixing Phenomena in Stirred Melts
Caricato da
akshukCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Model Studies of Mixing Phenomena in Stirred Melts
Caricato da
akshukCopyright:
Formati disponibili
Canadian Metallurgical Quarterly, Vol. 28, No.1, pp.
19-27, 1989
Printed in Great Britain
0008-4433/89 $3.00+ .00
Canadian Institute of Mining and Metallurgy
Pergamon Press pIc
MODEL STUDIES OF MIXING PHENOMENA IN
STIRRED MELTS
JURGEN MIETZ and FRANZ DETERS
BerlinTechnical University, Str. des 17Juni 135, 1000Berlin 12, Federal Republic of Germany
(Received 15October 1987; in revised form 7July 1988)
Abstract-Mixings were performed in a water model systemof a gas-stirred ladle with both an optical
decolorization method and conductivity measurements at different positions within the vessel. The experi-
mental results show that the rate by which the concentration is homogenized depends on the location of
the measuring probe as well as on the used stirring conditions, e.g. gas flowrate and position of the gas
injection nozzle. Furthermore, anewtheoretical mixingmodel that combines theconcepts of thecirculating
concentration cloud and the two-tank model ispresented. The results of numerical calculations using the
model arecompared with experimental data of mixing inthewater model and in a40-tonne steel ladle.
1. INTRODUCTION
Because of the stochastic nature of mixing, a large number of
measurements were required. The task of acquiring data was
greatly facilitated by the development and implementation of
acomputer controlled experimental deviceand procedure. The
transfer of the experimental water model results to the full
scalesystemsuch as the stirred steel ladle then necessitated the
development of atheoretical model. In the second part of this
paper a new mixing model that combines the concept of the
circulating concentration cloudwiththetypical two-tank model
ispresented.
Mixing of steel in ladles has become important with thedevel-
opment of such processes as continuous casting and secondary
metallurgy. Steel melts are stirred with agas which generates a
flow field within the containment vessel. Mixing proceeds by
the simultaneous action of the directional circulating and the
random turbulent movement of this flowfield [1]. Numerous
experimental studies, mainly with water models, have been
carried out in the last fewyears to study the characteristics of
the flowfields and to assess the overall mixing process in gas
stirred vessels[2-42].
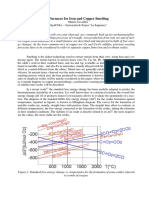
The intensity of mixing isnormally quantified by themixing
timewhichisdefinedasthetimerequired for theconcentration
of a giveninput of tracer to reach a level that iswithin 5% of
the steady state concentration. In several studies relevant to
metallurgical processing, mixingtimeswerefound to berelated Figure 1shows a schematic of the experimental apparatus
to the flow rate of the stirring gas or to the supplied mixing used in the present study. The experiments werecarried out in
power per unit of liquid mass. Furthermore, the effectsof sev- a vessel of acrylic glass having an inner diameter of 630 mm
eral geometrical parameters on mixing time were also inves-' and a height of 1000 mm. The vessel was filledwith distilled
tigated. Someof theseincluded thelocation and diameter of the water to aheight of 580 mm. Thus, thediameter to depth ratio
nozzle, immersion depth of alance, bath depth, and diameter to was 1.1, a value that is representative of a typical steel ladle.
depth ratio [33-38, 40]. In some studies, mixing experiments Nozzles enabling theintroduction of various stirring gaseswere
with various tracer input locations and positions of the meas- embedded in the bottom at a number of locations. The tracer
uring probe werealso carried out [30, 35, 36]. used in this study was an aqueous sodiumhydroxide solution.
Even though numerous investigators have published results It was added into the water by an injection lancethat could be
pertaining to mixing times, only afewhave actually described positioned wheredesired. Usually, 3ml of 0.85 M NaOH solu-
the mixing process itself. Published mixing curves expressing tion wereused.
the transient nature of mixing, obtained for water models as The mixing of the tracer was measured by two methods.
well as from full scale measurements in a steel ladle show One involved the simultaneous measurement of the electrical
damped oscillations caused by arecirculating flowinthevessel conductivity of thewater at three different locations within the
as well as a gradual increase in concentration during injection vessel. Each measurement thus gavethelocalizedconcentration
[3, 4, 25-28, 32-36]. as a function of time. The o.ther method involved the col-
Inaneffort tobetter understand theeffectof thelargenumber orization and decolorization of the indicator phenolphthalein
of parameters onmixing, it was necessary to quantify thetran- that was contained within the water. The volume of the tracer
sient evolution of mixing. As acontribution to this aim, mixing solution was chosen so that locally the water was colored by
experiments wereperformed on awater model of agas stirred phenolphthalein, however, after complete mixing the water
ladle. Moreover, it was necessary to carry out measurements was decolored. The time required for complete decolorization
with various methods and at different positions in the vessel. wastaken toindicatetheendof themixingprocess. Thismethod
19
2. EXPERIMENTAL APPARATUS AND
PROCEDURE
20
J. MIETZ and F. OETERS: MIXING OF STEEL MELTS
flow
meter
air
foto
resistance
array
light
--source
injection
lance
\
\
\
\
\
\
\
,~,e:,.,
\ ,e:,..- = .,
\ ,e:,..- = .I
~~~
',e:,.~1
, .c=. I
I ~I
bl
\ -= I
r=,
~I
t-=-
PI
\I
nozzle /
Fig. 1. Schematic representation of theexperimental setup.
has the advantage that the mixing phenomenon becomes
directly visibleand its progress with timecan beobserved over
an extended local range of the vessel by noting the behavior
and movement of the colored cloud. On the other hand, con-
ductivity measurements givetheexact development of thecon-
centration with timeat localized points only. As a result, both
methods wereused.
The transient colorization of the bath during the mixing
process was monitored with an opto-electronic system con-
sisting of a matrix of single photoresistances of rectangular
shapearrayed onaplate and anormal plate camera into which
thematrix wasplaced. Thephotographic object, i.e. thesection
of the vessel where the coloring appears, was illuminated by a
light source from behind the vessel as shown in Fig. 1. The
object was focused by the camera onto thephotoelectric plate.
Thesignalsfromtheindividual photoresistances wereprocessed
by amultiprogrammer and then relayed to acomputer. Upon
injection of thetracer, theopto-electronic systemthus measured
light absorption and its evolution with time as caused by the
colorization of thebath.
The multiprogrammer performs a number of functions
including thehandling of thegasblowing, theinjection of tracer
with its accompanying handling, and the illumination. It also
has thetask of receivingthesignalsfromtheconductivity cells,
the pH meter and the opto-electronic system. Moreover, it
digitizesand stores theresults and inturn transfers themto the
host computer. These procedures were controlled by com-
puter programs designed for this purpose. Other programs
were written to evaluate and process the original measure-
ments, especially the conversion of the conductivity data into
dimensionless concentrations. Signals from the photoresist-
ances were converted into "grey-intervals" and represented
graphically by hachures of varying intensities.
Shown in Fig. 2arc a sct of results of the optical system. In
theexperiments which yielded theseresults, thetracer solution
was added in the dead zone at the bottom of the vessel. The
flowrate of the stirring gas injected through thecentral nozzle
was 800 ncm3/S. The quick extension' of the colored region
under theedgeof thegas bubble plume zone isclearly evident.
It isto benoted that theweak grey areas at the upper left hand
sideof theschematics arenot colored but rather are caused by
the gas bubbling zone. In addition, an average value for the
entire contour of the colored region was computed and its
development with time recorded. Figure 3 shows the relative
absorption, thus determined, for three different flow rates of
stirring gas. These values were used to compute mixing time
which was taken as the timewhen the relative absorption falls
below 5%.
As stated, conductivity measurements were converted into
concentrations by acalibration formula. Calibration tests were
carried out prior to the main investigations. Dimensionless
concentrations were computed according to equation (1) and
plotted against time.
c(t)
c=-
Coo
(1)
where
c(t) = instantaneous concentration
Coo = concentration after complete mixing.
3. EXPERIMENTAL RESULTS
3.1. Centric gas injection
3.1.1. Tracer addition in the dead zone. In the first series of
experiments, the tracer was injected into the dead zone at the
bottom of thevessel. Thisposition waschosen asit isimportant
under practical considerations. Figure 4 shows for a gas flow
rate of 1000ncm
3
/S the dimensionless concentration vs time
curves obtained from the three conductivity probes located at
positions denoted on the schematics. It is to be noted that
traces shown inFig. 4aswell asinsubsequent figuresrepresent
averages of up to 30individual experiments.
With reference to Fig. 4, it is seen that at position I,i.e.
J. MIETZ and F. OETERS: MIXING OF STEEL MELTS 21
gas flow rate'
800 [Ncm"3/s]
t = 1(s]
ti
t. __ --measuring area
It>
I
\ .----inj ect ion
t =2 [5] t = 2.5 [5]
Fig. 2. Results of the decolorization measurements. Measured absorption at different times.
t = 3 [5]
100 V
g
: 400 [ Nc m" ' 3/ s ]
10
V
G
: : [Ncm"'3/s]
'I
1000
E
80
,'-- B
pos i t i on 1
t..)
6
c
60
c:
~
0
u
...,
40
(J) 4
c.
'-
(J)
0
~
en
20 .c
s
----------
co
I~
10
2030..A-
40
. 50
60
50
t i me (8)
W
'I
, 1
\ I
\I
, "
1 !
V
s
= 1000 (Ncm"3/s1 I
-~ f
100 V
g
: 800 [ Nc m
A
3/ s ]
I
E
80
~
c
60
c:
_ 6t
2-illj
0
.~
U \ 1
. 4
pos i t i on 2
\ I
...,
40
til \1
C.
til
'-
~
,
0
. 2
I
(I)
20 .c
.~ co
00
"C
10 20 30 40 50
-'60
50
t i me [s]
Fig. 3. Measured total absorption vs time at different gas flow rates
(centric nozzleposition, tracer addition inthe dead zone).
Fig. 4. Dimensionless concentration vstimeat different positions inthe
vessel (centric nozzle position, tracer addition in the dead zone). 1=
position of tracer injection. 1, 2, 3resp. =positions of concentration
measurement. V
g
=volumeflowrate of stirring gas.
100 V
g
: 1200 [ Nc m
A
3/ s ]
I
E
80
. 8
~
~Ezj
c
60
c . 6 V
G
= 1000 [ Nc m
A
3/ s ]
\ 1
0
\ 1
.~
u
pos i t i on 3
\1
...,
40
(J) . 4
\ c..
en
Co.
~
I 0
. 2
(J)
20 .c
~ rc
0
0
"C
10 20 30 40 50 60
50
t i me [5]
adjacent to the tracer input point, the concentration increases
very rapidly and subsequently falls back gradually to the final
concentration level. At positions 2 and 3, i.e. near the wall
immediately below the water surface and somewhat lower and
more distant from the wall respectively, the concentration
increases gradually and only after adelay time. Thetimelagof
several seconds for these two positions represents the flowing
time between the tracer input position and the measuring
points.
J. MIETZ and F. OETERS: MIXING OF STEEL MELTS 22
50
40
~
30
OJ
E
.r-!
~
0)
.S 20
X
.r-!
E
10
0
0
5
V
G
= 500
I
[ Nc m" 3! s ]
4
I
~
pos i t i on 1
I
. _ - - - .
c: 3
0
.0\ ~ "
---
-.- -.-- -~
u
~
C/) 2 I' \ /
\
C/) \/
\\ ~
v
\,
t
mi x .~
\ \
00 \ \
"C
10 20 30 40 50 60
,"
t i me [5]
\0,
\ "
+"~
+',
,
5
' -+-
< >
V
G
= 500
I
[ Nc : m" 3! s ]
4
I
---
~
pos i t i on 2
I
2,
pos i t i on 1
+
c: 3
\J,'
0\/
0
3' + pos i t i on 2
u
\ /
0 pos i t i on 3
C/) 2 \/
l' \I
C/) v
~ I
.~
/
2
500 1000 1500
"C
00
10 20 30 40 50 60
g as f l ov l r at e [ Nc m" 3/ s ]
t i me [5]
Fig. 5. Mixing timevsgas flowrate at different measuring positions in
thevessel (centric nozzleposition, tracer addition inthe dead zone).
Figure 5 shows mixing times for the three probes as deter-
mined with the 5% criterion. As may be expected, mixing time
is reduced as gas flow rate is increased. At the position of the
tracer addition, mixing time is longest which is a result of the
low flow velocities at this location. The curves drawn in Fig. 5
were obtained by a regression analysis of the measured values
according to the following function:
t
mix
=aV~
where
a,b =constants obtained from regression analysis and
V
g
=volume flow rate of stirring gas.
The exponent, b, for position 2 was found to be -0.54 while
that for position 3 was - 0.38. These values agree with .pub-
lished data [25, 27, 30-33, '38, 39]. The exponent for position 1
was determined to be only - 0.06. This indicates that mixing
in the dead zone is much less influenced by flow conditions than
it is in the upper part of the vessel.
3.1.2. Tracer addition in the plume zone. In the second series
of experiments, the tracer was added in the gas plume zone
about 25 cm below the surface. Figure 6 presents the dimen-
sionless concentration traces for three locations as noted on the
accompanying schematics. Also shown is the position of the
tracer injection lance. In these experiments the probes were
positioned at nearly the same locations as in the first exper-
imental series with the exception that the two probes near the
top surface were placed on opposite sides, whereas in the first
series they were located on the same side. The gas flow rate for
the results shown in Fig. 6 was 500 ncm
3
js.
The first trace shown in Fig. 6 was obtained for position I
which corresponds to the center of the toroidal loop in the
upper part of the vessel. Initially, the concentration exceeds the
final value by a considerable margin. From the maximum the
concentration then gradually falls to its final value. Such acurve
indicates that during the first circulation of the tracer enriched
5
V
G
= 500
I
.~
[ Nc m" 3! s ]
4
~
pos i t i on 3
c: 3
0
u
(J) 2
(f ]
\j- ~
.~
"C
00
10 20 30 40 50 60
t i me [5]
(2)
Fig. 6. Dimensionless concentration vs time at different positions in
the vessel (centric nozzle position, tracer addition in the plume). 1=
position of tracer injection. 1, 2, 3 resp. =positions of concentration
measurements. V
g
=volume flowrate of stirring gas.
liquid, a large part of this volume comes into the region where
the probe is positioned. As this region has only a low flow
velocity, it behaves like a storage device from which the tracer
is gradually transferred into the surrounding liquid by random
movement. Thus, the decrease in concentrat.ion is slow. The
middle figure shows the concentration trace for the dead zone.
Contrary to the upper figure, the tracer is not itnitially enriched,
however, after a certain latent time, gradually rises to the final
value. The bottom figure shows the concentration trace for the
position of strong flow. It reveals a damped oscillation of the
concentration. This oscillation typifies the circulating character
of the flow. The enriched region moves likl~ a cloud in the
direction of the flow and simultaneously extends by random
turbulent diffusion, thus gradually decreasing in concentration.
After each loop the enriched region passes the probe and pro-
duces a peak on the trace. Thus, the time between two maxima
represents the circulation time. At least three circulations are
afforded before the concentration is equalized. This number is
a measure of the interaction between directional and random
turbulent flows. This means that for the upper figure directional
flow is relatively unimportant compared to mixing by random
diffusion as only one peak in the concentration trace is
observed.
23
J. MIETZ and F. OETERS: MIXING OF STEEL MELTS
50
+
- t y pe a)
l=
10
l'
pas .
'it
~~3
1- .0\/
* pas .
- t y pe b)
t=
' I
pos i t i on 3
\!
40
8
*
*
*
*
,
*
*
.~
\.
\
~ *
-..
,.
30
Q)
6
\.
I
ID
.~
\.
/
E
4J /
.~
,
./
-I-J C
,
"-
./
CJ
' w. . . . . _ _ -
./
OJ
\
.~
c
20
\
4J
4
.~
rt:
X
,
t
r-t
.~
t
'- ....
::J
=
U
...
...
L
..... t_ ri
- -+
- t
u
10
---
---
2
t
0
0
a 500 1000 1500
0 500 1000 1500
g as f l ow r at e [Ncm"'3/s]
g as f l ow r at e [Ncm"'3/s]
Fig. 7. Mixing timevsgasflowrate for different types of concentration
vstimecurves (centric nozzleposition, tracer addition in theplume).
The results presented thus far were averaged from a large
number of single experiments. When each experiment was
viewedasaseparate entity, it was remarkable to note the large
differences which were more than just statistical in nature. In
fact, especially at position 1(Fig. 6), completely different con-
centration traces were generated by what should have been
ide~tical experiments. In explaining this, the decolorization
method described above was found to be more useful. Tests
showed that the colored cloud generated by the addition of
the tracer was not always equally distributed in each radial
direction-sometimes it moved in a preferred direction. If the
position of the conductivity probe did not liein this direction,
the first circulation transferred little enriched volume to the
probe. Hence, alatent period followed by agradual increasein
the concentration was observed. If however the probe was
located inthe preferential direction of the cloud, enriched vol-
ume was transferred to the probe by the first circulation. As a
result, amaximumwasnoted with asubsequent decreaseto the
final concentration. Thus, thedecolorization method confirmed
theobservations madewiththeconductivity probes. Thereason
for this phenomenon lies in the eventual positioning of the
gas bubbling column. In many cases the distribution of tracer
enriched liquid was not axisymmetric because of slight incli-
nations of the bubbling column. This was judged to be the
major cause of the differences in the individual concentration
traces.
Associated withthevarious concentration traces areremark-
able differences in the mixing times. Figure 7presents mixing
timesfor twotypes of concentration curvesplotted asafunction
of gas blowing rate. For the range of flow rates investigated,
the mixing times for curves of type (b) where the probe was
located inthepreferential direction of the cloud aremore than
twiceas long as those for type (a). Moreover, themixingtimes
for type (a) become shorter with increasing gas blowing rate
whereas those for type (b) remain nearly constant. Type (a)
Fig. 8. Circulation timevsgas flowrate (centric nozzleposition, tracer
addition in theplume).
mixing times can be expressed by a power function similar to
that givenby equation (2). The exponent (b) was found to be
-0.37, a value which is in good agreement with published
results. Mixingtimesfor type(b) curves showedno suchdepen-
dence.
Figure 8 shows the circulation times for different gas flow
rates obtained fromconcentration traces at position 3(seeFig.
6). The shape of the curve is somewhat surprising, however it
canberationalized by considering theflowpatterns at different
gas flowrates. As gas flowrate is increased, the flowvelocity
of the liquid increases and hencethe recirculating volume flow
rate increases. On the other hand, with increasing volume flow
theenriched concentration cloud reaches deeper regions of the
vessel and thus the circulation loop is enlarged. This implies
that therearetwoeffectsresponsible for thecharacteristic shape
of the curve plotted in Fig. 8. One is the increase of volume
flowof liquid with higher gas flowrates whilethe other is the
enlargement of thecirculating loop implying agreater distance
of travel for one circulation. Since neither effect is mutually
exclusive, the result isacurveof the type shown in Fig. 8.
3.2. Eccentric gas injection
To study the effect of the radial position of the gas injection
nozzle on the mixing process, additional experiments were
carried out with aneccentric location of thenozzleat rlR = 2/3
(where r = radial displacement and R = radius of the vessel).
Figure 9 presents dimensionless concentration traces for the
three positions noted on the schematics. The flowrate was set
at 1000ncm
3
js. The three conductivity probes werepositioned
on the vertical plane which crosses both the nozzle and the
center of the vessel. All three locations showed damped oscil-
lation behavior. As a consequence, these locations were
assumed to bewithin themain circulating flowof liquid. How-
ever, the results for acentric nozzlewerenot comparable. For
example, position 2 in Fig. 6, i.e. with centric gas injection,
24
10
I
8
~
6 t::
0
U
en
4
en
~
2
.~
00
"C
10
I
8
r..i
6 t::
0
u
en 4
tr.I
~
2
.~
'C
J. MIETZ and F. OETERS: MIXING OF STEEL MELTS
V G = 500 [ Nc : m" ' 3/ s ]
pos i t i on 1
7
,
'1,
I' 0
'"
" I
10 20 30 40 50 60
t i me [5]
V G = 500 [ Nc : m" 3/ s ]
pos i t i on 2
I
,
I
'11
, I
"II
""2
20 30 40 50 60
t i me Is]
v G = 500 [ Nc m" 3/ s ]
pos i t i on 3
:t
,3
:
,
,
III'
II
"
"
10 20 30 40 50 60
t i me [5]
10
I
8
r..i
6 t::
0
U
tr.I 4
ttl
~
2
.~
'C
Fig. 9. Dimensionless concentration vs time at different positions in
the vessel (eccentric nozzle position, tracer addition in the plume).
I = position of tracer injection. 1, 2, 3 resp. = positions of con-
centration measurement. V
g
=volume flowrate of stirring gas.
behaved like a dead zone with its characteristic long incubation
period and the gradual concentration increase up to the final
value. However, at the same position with eccentric gas stirring
(as shown in the second curve of Fig. 9), a recirculating flow
was apparent. This different mixing behavior for various nozzle
positions was caused by the formation of different flow patterns.
Flow measurements during stirring indicated that the dead zone
at the bottom of a vessel can be largely eliminated by using an
eccentric nozzle position instead of a centric one [13,41].
Figure 10presents circulation times obtained at various pos-
itions plotted as a function of gas flow rate. Circulation times
were obtained from concentration traces as described in an
earlier section. These results show that the circulation time
steadily decreases with increasing gas flow rate. This was not
the case for centric gas injection as shown in Fig. 8. The fact
that the measured circulation times for a constant gas flow rate
are independent of position over a wide range of locations
indicates that the eccentric gas injection conditions used in this
study eliminated dead zones. Only a main recirculating flow
existed. The curve in Fig. 10 can be regressed by the following
power function
t
c
=aV~
where t
c
is the circulation time.
+
+ pos i Ulon 1
* positilon 2
'# position 3
posit:lon 4
o posit:lon 5
pos i t:LOn 6
o pos i t:lon 7
< > posit:lon B
(:, posit:lon 9
20
~
-5'6
I... ..
10, -2,3
s-- (l'i''''
, I
7 B 1.4
15
\ ~
\
~
\
\
\
t i Q>
+#1?' .... < >
.... ef.-. ~ A
4f -:r- -J -> - _~
r-----~----,
10B V -
0
.
36
g
[l)
E
..-1
~
c:: 10
o
.~
~
1'0
.....
:::::J
U
.~
u 5
o ...L.J....-J
o 500 1000 1500
gas f lOH rate [Ncm" 3/s)
Fig. 10. Effect of gas flowrate and probe position on circulation time.
For eccentric gas injection, the length of the circulation loop
does not depend on the gas flow rate, so that, the volume
contacted by the circulating liquid isessentially constant. There-
fore, the main parameter determining the circulation time
should be the circulation volume flow rate of liquid. From the
theory of the gas bubbling column, the voluITleflow rate in the
plume zone VI,pcan be calculated from the following [6, 10, 16,
31,42]
v = KTi'(1/3)
I,p g
(4)
where K= constant (dependent on geometrical and physical
factors).
Moreover, it can be assumed that the recirculating flow rate,
V
c
, is mainly controlled by the volume flow rate of liquid in the
bubble plume zone. Thus,
(5)
The circulation time, t
e
, is defined by the ratio of the liquid
volume, V, to the circulating volume flow rate, T /
c
'
(6)
The exponent b in equation (3), as determined by regression
analysis, yielded a value of -0.36 which is in good agreement
with the result produced by the theoretical approach embodied
by equations 4-6.
4. MIXING THEORY
(3)
4.1. Fundamentals
In order to describe the mixing process, two types of models
were developed. These are referred to as turbulent and tank
models. Turbulent models enable one to simulate mixing from
first principles, however they require much computation. On
the other hand, tank models use empirical parameters and as
J. MIETZand F. OETERS: MIXING OF STEELMELTS 25
v
--- V
N
Fig. 11. Combinedrecirculationandtwo-tankmodel.
sucharemuch more simple. Moreover, they areflexibleintheir
application to various vessel shapes.
Results published in theliterature [3,4, 15, 18,24-28, 31-36,
38] as well as the results of the present work suggest that the
following parameters are important in the modelling of the
mixingprocess. Theseare: the recirculating flow, theturbulent
diffusion of the enriched cloud, and the exchange between the
dead zone and bulk volume. A model that includes these par-
ameters was developed. The model draws upon the concepts
of the recirculating cloud and the two-tank model. Figure 11
presents a schematic of the model. The simultaneous effect of
directional and random flow was modelled by a number of
tanks in series through which a flow is circulating [43]. this
model ischaracterized by three parameters:
-the total volume, V
tOb
-the recirculation volume flow rate through the tanks, V,
and
-the number of tanks, N.
Thetank-in-series model wasthen combined with thetwo-tank
model. Thetwo-tank model has two characteristic parameters:
-the exchange volume flowrate, V
d
and
-the ratio of the dead volume V
d
to the remaining volume,
Vtot- Vd
Inthecombined model theremaining volume isidentical to the
volume of the tanks in series. Thus, the combined model has,
intotal, the following fiveparameters:
-the recirculating volume flowrate, V;
-the total volume, V
tot
;
-the ratio of the exchange volume flow rate to the recir-
culation volume flowrate, Vdl V;
-the ratio of the dead volume to thetotal volume, Vdl V
tot
;
-the number of tanks in series, N.
The combined model thus contains five characteristic par-
ameters and as such should be able to describe the mixing
process insufficientdetail to enable it to answer practical ques-
tions. A mathematical description of the model is published
elsewhere [22].
4.2. Application of the combined model to experimental results
Figure 12illustrates the degree of agreement between com-
puted and measured concentration traces for a typical experi-
ment. Experimental curves are presented on the left hand side
inFig. 12awhileFig. 12bshowsthecorresponding results from
the mathematical model. The sizeof the dead volume, V
d
, was
measured from visual observations of decolorization experi-
ments. Thebasis for determining the recirculating volume flow
rate, V, was the volume flowat the upper part of the gas
bubbling column as computed from theory [6, 10, 16, 31, 42].
Comparison of Figs 12aand b shows good agreement between
calculated and measured results. Thus, the model is able to
describe the mixing behavior of a gas-stirred water model sys-
temof the typeused here.
Confirmation of its validity for the stirring of steel ladles
must be established. Measurements of the concentration of a
tracer, added to asteel ladle, as afunction of timewerecarried
out in Swedenby T. Lehner et at. [3,4, 28].
Experiments werecarried out ina6-tonne ladleusing copper
as the tracer and in 40- and 50-tonne melts using radioactive
gold asthe tracer. Sincethe Froude number and the height-to-
diameter ratio for both the water model and steel ladle experi-
ments were equal, geometrical and physical similarity criteria
werefulfilled. Thus, it should bepossibletocalculatethemixing
behavior in the steel melt by using the same dimensionless
parameters as for the water model. Only the ratio of the dead
volume to the total volume was increased from 0.17 for the
water model to 0.25 for the steel ladle. The use of a higher
fraction for the dead zone was viewed as reasonable because
for the ladle the gas was blown into the melt through a lance,
whereas inthewater model experiments anozzleinthebottom
wasused. A comparison of thecomputed and measured results
for the 40-tonne steel ladle is shown in Fig. 13. It is apparent
that mixing in the steel ladle can bedescribed by theproposed
model.
5. CONCLUSIONS
The results of the mixing experiments show that the shapes
of the concentration traces strongly depend on the location of
the tracer input as well as on the position of the measuring
probes and the location of the gasinjection nozzle. Variability
in mixing times can be significant especially when the stirring
gas is injected by a centrally positioned nozzle. In such cases,
regions of very low flow velocities, i.e. dead zones, are found
to occur at thebottom of thevessel. Mixing timeswerelongest
intheseareas.
The adoption of an eccentric gasnozzlelocation can beused
to avoid dead zones. Practical applications for stirring should
makeuseof thisfact. It wasalsoobserved that circulation times
for bulk recirculating flow are independent of probe location.
This indicates that only onemain circulating loop exists under
this condition.
By combining the concepts of the tank-in-series model and
thetwo-tank model, anewrecirculation model was developed.
The measured and calculated values produced by this model
for a 40-tonne steel ladle and for a water model of this ladle
were found to be in good agreement. This leads to the con-
clusion that, provided the dimensionless parameters are the
same for the ladle and water model, water model results can
be applied to full-scale plant situations. This is of practical
importance because it implies that the mixing behavior of ves-
selswithdifferent shapes andflowconditions canbedetermined
with water models coupled with our mathematical model.
Acknowledgements- This researchworkwassupportedfinanciallyby
theGermanFederal Ministryof ResearchandTechnologywhichis
gratefullyacknowledged.
Theauthorswouldalsoliketo thank ProfessorFrank Mucciardi
Department of Miningand MetallurgicalEngineering,McGill Uni~
versity,forhishelpinpreparingtheEnglishversionof ourmanuscript
forpublication.
26
J. MIETZ and F. OETERS: MIXING OF STEEL MELTS
5
V
G
= 500
5
I
[ Nc m' " 3! s ]
I
CiO (t)
4
I
4
t.i pos i t i on 1
I
~
c: 3 I
C 3
0
\ ~ 1
0
U
u
2 I,
.0\ 1
2 CJ)
\ 1 CJ)
CJ)
\1 CJ)
o~
~
u
~
.~
00
.~
"'C
10 20 30 40 50 60
"C
0 10 20 30 40 50 60
t i me [5]
t i me [5]
1
5
V
G
= 500
5
I
[ Nc m" 3! s ]
I
I
c d (t)
4 4
~
pos i t i on 2 I
~
c: 3
\J,'
c 3
0
0
u u
2
\ 1
2 CJ) \1 CJ)
CJ)
U tn
~
1 ~
.~
2
.~
~
"C 0
0 10 20 30 40 50 60
"C 0
0 10 20 30 40 50 60
t i me [ s ]
t i me [5]
5 5
I
A
v
G
= 500 [ Nc m' " 3! s ]
I
c 3 (t)
4
I
4 ~
U
pos i t i on 3 I
U I
c: 3
- 3
c 3
0 \ 1
/
0 0
U
\ 1
U
CJ) 2 \ I
tn 2
en
\I-
\' tn
~
U
~
.~ .~
"'C
00
10 20 30 40 50 60
"C
00
10 20 30 40 50 60
t i me [ s ] t i me [5]
(a) measu red data
(b) calculated data
Fig. 12. Dimensionless concentration vs time at different positions in the vessel (measured and calculated
data). I =position of tracer injection. 1,2,3 resp. =positions of concentration measurements. V
g
=volume
flowrate of stirring gas.
4
I
3
c
.~
./.J
10
t..
./.J
2 c
Q.I
U
C
0
U
tn
tn
~
.~
'C
0
0
3. T. Lehner, Scaninject I, Lulea, Sweden, paper 11(1977).
4. J. Szekely, T. Lehner and C. W. Chang, I ronmaking Steelmaking
6,285 (1979).
5. J. SzekelyN. H. EI-Kaddah and J. H. Grevet, Seaninject II, Lulea,
Sweden, paper 5(1980).
6. T. C. Hsiao, T. Lehner and B. Kjellberg, Scand. J. Metallurgy 9,
105(1980).
7. N. EI-Kaddah and J. Szekely, I ronmaking Steelmaking 6, 269
(1979).
8. T. Deb Roy and A. K. Majumdar, J. Metals 33" 42(1981).
9. J. H. Grevet, J. Szekely and N. EI-Kaddah, I nt. J. Heat Afass
Transfer 25(4),487 (1982).
10. Y. Sahai and R. 1. L. Guthrie, Metall. Trans. I3B, 193(1982).
11. Q. He, Y. Pen, T. C. Hsiao, W. Zhao, L. Hu and P. Liu, Proc.
Shenyang Symp. on I njection Metallurgy & Secondary Refining of
Steel, Shenyang, September 1984,pp. 98-113.
12. E. Steinmetz, H. Wilhelmi, W. Wimmer andJ. Imo, Arch. Eisenhzitt-
Wes.54, 19(1983).
13. F. Oeters, H.-C. Dromer and J. Kepura, Scaninject III, Lulea,
Sweden, paper 7(1983).
14. Y. Sahai and R. 1. L. Guthrie, Metall. Trans. I3B, 203(1982).
15. S. Schneider, H.-C. Dromer, J. Mietz and F. Oeters, Proc. 6th
Japan- Germany Seminar, Tokyo, May 1984,pp. 1/12(1984).
16. G. Ebneth and W. Pluschkell, Proc. 6th Japan- Germany Seminar,
Tokyo, May 1984,pp. 25/37 (1984).
17. J.-C. He, S. Asai and 1. Muchi, Tetsu- to- Hagane' 70, 1590(1984).
18. H.-C. Dromer, J. Mietz, F. Oesters and S. Schneider, Proc. Shen-
yang Symp. on I njection Metallurgy &Secondary Refining of Steel,
Shenyang, September 1984,pp. 114--128.
-- model calculations
measured data
from 40 t ladle
II
50 100
time [s]
150 200
Fig. 13. Transient response of dimensionless concentration for
a40-tonne steel melt.
REFERENCES
1. J. Szekelyand N. EI-Kaddah, I ron Steel maker 11, 22(1984).
2. K. Berner and E. H. K. Miiller, Arch. EisenhzittWes. 40, 829
(1969).
J. MIETZ and F. OETERS: MIXING OF STEEL MELTS
27
19. D. Mazumdar and R. I. L. Guthrie, Metall. Trans. 16B, 83 (1985).
20. R. Matway, H. Henein, R. J. Fruehan and J. Isaacs, Proc. 4th
Process Tech. con!, Vol. 4, pp. 39-43, Chicago Meeting, April 3-
9,1984. Iron and Steel Society of AIME, NewYork.
21. T. Lehner, G. Carlsson and T. C. Hsiao, Scaninject II, Lulea,
Sweden, paper 22 (1980).
22. J. Mietz and F. Oeters, Steel Res. 58,446 (1987).
23. K. Nakanishi, J. SzekelyandC. W. Chang, I ronmaking Steelmaking
2, 115 (1975).
24. M. Sano and K. Mori, Scaninject III, Lulea, Sweden, paper 6
(1983). ,
25. K. Nakanishi, T. Fujii and J. Szekely, I ronmaking Steelmaking
2(3), 193 (1975).
26. G. Carlsson, W. T. Xiu and M. Bramming, I ronmaking Steel-
making 11(2), 95 (1984).
27. O. Haida, T. Emi, S. Yamada and F. Sudo, Scaninject II, Lulea,
Sweden, paper 20 (1980).
28. T. Lehner, in Proc. Symp. on Ladle Treatment of Carbon Steel,
McMaster University, Hamilton, Canada, 1979, paper 7 (1979).
29. O. Haida and J. K. Brimacombe, Scaninject III, Lulea, Sweden,
paper 5 (1983).
30. U. P. Sinha and M. J. McNallan, Metall. Trans. 16B, 850 (1985).
31. M. Sano and K. Mori, Trans. I ron Steel I nst. Japan 23, 169 (1983).
32. V. Krujelskis and F. Mucciardi, Proc. 4th Process Tech. ConJ.,
Chicago Meeting, April 3-9, 1984, Vol. 4, pp. 33-38. Iron and Steel
Society of AIME.
33. L. W. Helle, JI . S. Afr. I nst. Min. Metall. 81, 329 (1981).
34. A. Ferretti, V. Giordano and M. Podrini, Boll. tee. Finsider 361,
209 (1977).
35. G. G. K. Murthy, S. P. Mehrotra and A. Ghosh, Proc. 6th I nt.
I ron and Steel Congr. Washington Meeting, April 6-9, 1986, Vol.
5, Book 3, pp. 401-407. Iron and Steel Society of AIME.
36. T. Maruyama, N. Kamishima and T. Mizushina, J. chem. Eng
Japan 17(2), 120 (1984).
37. H. Wilhelmi, E. Forster and J. Pietzka, inProc. 6th Japan- Germany
Seminar, Tokyo, May 1984, pp. 50/61 (1984).
38. S. Asai, T. Okamoto, J.-C. Heand I. Muchi, Trans. I ron SteelI nst.
Japan 23, 43 (1983).
39. K. Narita, T. Makino, H. Matsumoto and K. Ogawa, Tetsu- to-
Hagane 69(3), 392 (1983).
40. J. C. Billington and K. Gregory, Radex Rdsch. 1/2, 368 (1981).
41. S. T. Johansen and T. A. Engh, Scand. J. Me tall. 14, 214 (1985).
42. S. Schneider and F. Oeters, Steel Res. (1989).
43. O. Levenspiel, Chemical Reaction Engineering. J. Wiley, NewYork
(1972).
Potrebbero piacerti anche
- Biomass As Blast Furnace Injectant - Considering AvailabilityDocumento10 pagineBiomass As Blast Furnace Injectant - Considering AvailabilityakshukNessuna valutazione finora
- A Gibbs Energy Minimization Method For CDocumento13 pagineA Gibbs Energy Minimization Method For CakshukNessuna valutazione finora
- Part-1 Kinetics of ZNS RoastingDocumento9 paginePart-1 Kinetics of ZNS RoastingakshukNessuna valutazione finora
- AISTech 2019 Successful Use Case Applications of Artificial Intelligence in The Steel IndustryDocumento14 pagineAISTech 2019 Successful Use Case Applications of Artificial Intelligence in The Steel IndustryakshukNessuna valutazione finora
- The Extractive Metallurgy of Zinc - by Roderick J. Sinclair: January 2006Documento4 pagineThe Extractive Metallurgy of Zinc - by Roderick J. Sinclair: January 2006akshukNessuna valutazione finora
- Part-2 Kinetics of ZNS RoastingDocumento7 paginePart-2 Kinetics of ZNS RoastingakshukNessuna valutazione finora
- Dri Update May - 2021Documento31 pagineDri Update May - 2021Vivek RanganathanNessuna valutazione finora
- Aparajita Stotra From Visnudharmottara P PDFDocumento7 pagineAparajita Stotra From Visnudharmottara P PDFakshukNessuna valutazione finora
- Urban Et Al. De-P Startegies Iron Steel Tech 2015Documento12 pagineUrban Et Al. De-P Startegies Iron Steel Tech 2015akshukNessuna valutazione finora
- Final Master ProgrammeDocumento9 pagineFinal Master ProgrammeakshukNessuna valutazione finora
- Overview of The Applications of Thermodynamic Databases To Steelmaking ProcessesDocumento31 pagineOverview of The Applications of Thermodynamic Databases To Steelmaking ProcessesakshukNessuna valutazione finora
- Steelmaking Technology For The Last 100 Years TowaDocumento32 pagineSteelmaking Technology For The Last 100 Years TowaHari BudiartoNessuna valutazione finora
- Physicochemical Aspects of Reactions in Ironmaking and Steelmaking Processes PDFDocumento21 paginePhysicochemical Aspects of Reactions in Ironmaking and Steelmaking Processes PDFakshukNessuna valutazione finora
- Ironmaking and Steelmaking PDFDocumento466 pagineIronmaking and Steelmaking PDFakshukNessuna valutazione finora
- Ironmaking and Steelmaking PDFDocumento466 pagineIronmaking and Steelmaking PDFakshukNessuna valutazione finora
- Iron and SteelmakingDocumento139 pagineIron and SteelmakingRajatSehgalNessuna valutazione finora
- NumericsAndOpenFOAMv7 LimitedDocumento155 pagineNumericsAndOpenFOAMv7 LimitedakshukNessuna valutazione finora
- Copper Extraction Mass Balance ProblemsDocumento6 pagineCopper Extraction Mass Balance ProblemsakshukNessuna valutazione finora
- Fe and Cu Smelting PDFDocumento6 pagineFe and Cu Smelting PDFakshukNessuna valutazione finora
- Manuscript IJCMR PDFDocumento35 pagineManuscript IJCMR PDFakshukNessuna valutazione finora
- The Phosphorus Reaction in Oxygen Steelmaking - Thermodynamic Equi PDFDocumento211 pagineThe Phosphorus Reaction in Oxygen Steelmaking - Thermodynamic Equi PDFakshukNessuna valutazione finora
- Kinetics of Fundamental Reactions Pertinent To Steelmaking PDFDocumento16 pagineKinetics of Fundamental Reactions Pertinent To Steelmaking PDFakshukNessuna valutazione finora
- 02-Stoichiometric CalculationsDocumento47 pagine02-Stoichiometric CalculationsHandayani KesumadewiNessuna valutazione finora
- Climate Change and MigrationDocumento9 pagineClimate Change and MigrationakshukNessuna valutazione finora
- Climate Change and Migration PDFDocumento287 pagineClimate Change and Migration PDFakshukNessuna valutazione finora
- 19660004844Documento244 pagine19660004844akshukNessuna valutazione finora
- Single-Step Ironmaking From Ore To Improve EnergyDocumento134 pagineSingle-Step Ironmaking From Ore To Improve EnergyakshukNessuna valutazione finora
- BREF - Iron and Steel Production PDFDocumento621 pagineBREF - Iron and Steel Production PDFRajeev SinghNessuna valutazione finora
- Virtual+steelmaking Steeluniversity ENDocumento16 pagineVirtual+steelmaking Steeluniversity ENakshukNessuna valutazione finora
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (120)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- SCC-C ManualDocumento28 pagineSCC-C ManualGian Paul Ramos AcostaNessuna valutazione finora
- Formulas and CalculationsDocumento14 pagineFormulas and CalculationsNaomi Lucana MuñozNessuna valutazione finora
- مصادر الطاقة التقليديةDocumento3 pagineمصادر الطاقة التقليديةMohamed Al-OdatNessuna valutazione finora
- Dse-0018.2 6pt-Ii enDocumento2 pagineDse-0018.2 6pt-Ii enAnonymous PJP78mSxNessuna valutazione finora
- Chemical Equilibrium in 1 Shot Me PDFDocumento71 pagineChemical Equilibrium in 1 Shot Me PDFShivansh UppalNessuna valutazione finora
- Science8 Q3 SLM1Documento15 pagineScience8 Q3 SLM1ANGEL MANGLICMOTNessuna valutazione finora
- 5 6154255657731096609Documento6 pagine5 6154255657731096609Yoko HamaNessuna valutazione finora
- Suco - Pressure Monitoring CatalogDocumento66 pagineSuco - Pressure Monitoring Catalogjason_meyer_14Nessuna valutazione finora
- ME8391 IQ 03 - by LearnEngineering - inDocumento24 pagineME8391 IQ 03 - by LearnEngineering - inelabalajiNessuna valutazione finora
- 3 Chemistry End of Year Assessment Answer KeyDocumento5 pagine3 Chemistry End of Year Assessment Answer KeyMylene Heraga100% (1)
- Zeroth, 1st and 2nd Laws of ThermodynamicsDocumento9 pagineZeroth, 1st and 2nd Laws of ThermodynamicsYomi BrainNessuna valutazione finora
- Paper Scale OnepetroDocumento15 paginePaper Scale OnepetroRani JuliariniNessuna valutazione finora
- Separator Test PDFDocumento6 pagineSeparator Test PDFNizar AliNessuna valutazione finora
- National Senior Certificate: Grade 11Documento18 pagineNational Senior Certificate: Grade 11Mahlatsi LegodiNessuna valutazione finora
- Grade - 7 Scholarship Exam SyllabusDocumento13 pagineGrade - 7 Scholarship Exam Syllabusstale cakeNessuna valutazione finora
- Year 8 308Documento8 pagineYear 8 308Insyirah AfiqahNessuna valutazione finora
- 2020-21 Ap 08 PS Tqa emDocumento31 pagine2020-21 Ap 08 PS Tqa emsrikanth PosaNessuna valutazione finora
- Flow Meter SpecificationDocumento2 pagineFlow Meter SpecificationDan4ChristNessuna valutazione finora
- Isochronal Test3Documento36 pagineIsochronal Test3Veviet pomataNessuna valutazione finora
- Exercise - 3.1: NCERT Solutions For Class 9 Science Chapter 3 Atoms and MoleculesDocumento12 pagineExercise - 3.1: NCERT Solutions For Class 9 Science Chapter 3 Atoms and MoleculesMannat MadanNessuna valutazione finora
- PBS & TBDocumento3 paginePBS & TBRajan BhandariNessuna valutazione finora
- Group 3 Grade Three Detailed Lesson PlanDocumento10 pagineGroup 3 Grade Three Detailed Lesson PlanRicell Joy RocamoraNessuna valutazione finora
- Exercise 2Documento2 pagineExercise 2heyjude0Nessuna valutazione finora
- Foxboro CFT50 Digital Coriolis Mass Flow TransmitterDocumento4 pagineFoxboro CFT50 Digital Coriolis Mass Flow TransmitterPhaniNessuna valutazione finora
- Rock Breakage ExplosivesDocumento12 pagineRock Breakage ExplosivesRONELNessuna valutazione finora
- AP Chem SyllabusDocumento10 pagineAP Chem SyllabusColin ManNessuna valutazione finora
- Nr-220802-Chemical Engineering Thermodynamics - IDocumento8 pagineNr-220802-Chemical Engineering Thermodynamics - ISrinivasa Rao GNessuna valutazione finora
- MatterDocumento22 pagineMatterRon GruellaNessuna valutazione finora
- APG-710-H2 Hydrogen Analysis Module: AppliedDocumento2 pagineAPG-710-H2 Hydrogen Analysis Module: AppliedCarlos MarquezNessuna valutazione finora
- 2022 MENG 1001 Engineering Thermodynamics I In-Course ExamDocumento3 pagine2022 MENG 1001 Engineering Thermodynamics I In-Course ExamElisha DanielNessuna valutazione finora