Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Acid Base
Caricato da
blue_l1Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Acid Base
Caricato da
blue_l1Copyright:
Formati disponibili
ACI D BASE REACTI ONS
Q.1 Give one of the major products of the reaction between acetic acid
(CH
3
CO
2
H, pKa ~ 4.5) and formic acid (HCO
2
H, pKa ~ 3.5)
(A) (B) (C) (D)
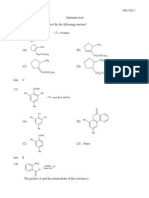
Q.2 Which of the following structures are correct ?
(a) (b) (c) (d)
(A) a, b (B) b, c (C) a, d (D) b, d
Q.3 For the following two acid base reactions, which is true ?
I CH
3
CH
2
+ CH
3
NH
2
CH
3
CH
3
+ CH
3
NH
pKa = 35 pKa = 50
II F
+ H
2
O HF + HO
pKa = 15.7 pKa = 3.2
(A) I is favored to the right, II is favored to the left
(B) I is favored to the left, II is favored to the right
(C) I is favored to the right, II is favored to the right
(D) I is favored to the left, II is favored to the left
Q.4 From the following reactions
HC ? CH + LiNH
2
? NH
3
+ HC ? CLi
H
2
O + CH ? CLi ? HC ? CH + LiOH
predict which of the following orders regarding the acid strength
is correct.
(A) HC ? CH > H
2
O > NH
3
(B) HC ? CH > NH
3
> H
2
O
(C) H
2
O > HC ? CH > NH
3
(D) H
2
O > NH
3
> HC ? CH
Q.5 The correct mechanism of reaction between formaldehyde and boron
trifluoride given by-
(A)
H
H
C
C +
BF
3
BF
3
:O:
O
?
? H
H
(B)
H
H
C
C + BF
3
BF
3
:O:
O
?
?
H
H
(C)
H
H
C
C +
BF
3
BF
3
:O:
O
?
?
H
H
(D)
H
H
C
C + BF
3
BF
3
:O:
O
?
?
H
H
Q.6 What is true about the following equilibrium ?
H
?
K
?
+ H
3
C H
H
H
+ H
3
C C
?
K
?
(A) It will be almost completely shifted to the left.
(B) It will be almost completely shifted to the right.
(C) The equilibrium constant is very close to one.
(D) The equilibrium constant is zero.
Q.7 What is the ranking of the equilibrium constants for the following
reactions ?
(A)
(B)
(A) A > B > 1 (B) 1 > A > B (C) A > 1 > B (D) B > 1 > A
Q.8 Identify each species in the following equilibrium according to the
code :
SA = stronger acid; SB = stronger base;
WA = weaker acid; WB = weaker base
The pK
a
of (CH
3
)
2
NH is 36; the pK
a
of CH
3
OH is 15.2.
CH
3
OH + (CH
3
)
2
NK l
1 2
1 2 1 2 1 2
(A) WA WB (B) WB WA (C) SA SB
(D) SB SA (E) WA WA
Q.9 Referring to the following equilibrium (R = alkyl group)
RCH
2
CH
3
+ RC?C:
RCH
2
CH
. . ?
2 + RC?CH
(A) K < 1; the equilibrium would lie to the left.
(B) K > 1; the equilibrium would lie to the right.
(C) K = 1; equal amounts of all species would be present
(D) Not enough information is given; the structure of R must be
known.
Q.10 Which of the following produces a significant amount of acetylide
ion on reaction with acetylene ?
(A) Conjugate base of CH
3
OH (pK
a
= 16)
(B) Conjugate base of H
2
(pK
a
= 35)
(C) Conjugate base of H
2
O (pK
a
= 16)
(D) Both (a) and (c)
ANSWER KEY
Q.No. 1 2 3 4 5 6 7 8 9 10
Ans. C C A C B A C C A B
SOLUTI ONS (ACI D BASE REACTI ON)
Ans.1 pK
a
?
1
acidity
; HCOOH acts as an acid while CH
3
COOH acts as on
base.
Ans.2 Above are sodium salt produced by neutralization and first
neutrilization always takes plack at most acidic H.
Ans.3 Equilibrium is favoured in that direction in which weak acid & weak
base are formed.
Ans.4 HC ? CH + LiNH
2
l NH
3
+ HC ? CLi
SA SB wA wB
H
2
O + CH ? CLi l HC ? CH + LiOH
SA SB wA wB
Ans.5 It is an Lewis Acid-Base Reaction
BF
3
= Lewis Acid
H C H
O
||
= Lewis Base
Head of the arrow is pointing towards BF
3
& tail is present on the
oxygen atom of the H C H
O
||
which donates its lone pair..
Ans.6 A reaction is feasible if
strong Acid + strong Base ? ? ? weak Acid + weak Base
Ans.7 In (A) equilibrium is shifted to the forward direction so K > 1.
In (B) equilibrium is shifted to the backward direction so K < 1.
Ans.8 pK
a
?
1
acidity
;
? CH
3
OH is stronger acid than (CH
3
)
2
NH.
* Conjugate base of weaker acid is strong
(CH
3
)
2
NH ? ? ? (CH
3
)
2
NK
weak acid strong base
Ans.9 On the left side of reaction, weak acid and weak base are formed so
equilibrium is shifted to backward direction so K < 1.
Ans.10 Conjugate base of H
2
is
H
?
.
CH?CH +
H
?
CH?C
?
+ H
2
SA SB wB wA
Reaction is favoured to the right.
Conjugate base of CH
3
OH is CH
3
C
?
.
CH?CH + CH
3
O
?
CH?C
?
+ CH
3
OH
wA wB SA SB
Reaction is favoured to the left.
Conjugate base of H
2
O is O
H
?
.
CH?CH + O
H
?
CH?C
?
+ H
2
O
wA wB SB SA
Reaction is favoured to the left.
Potrebbero piacerti anche
- P. Joy: Class Test - 3Documento1 paginaP. Joy: Class Test - 3Uday Prakash SahuNessuna valutazione finora
- Aromatic Compounds 13thDocumento15 pagineAromatic Compounds 13thRaju SinghNessuna valutazione finora
- Chapter 2, Acids and BasesDocumento13 pagineChapter 2, Acids and BasesSheree Jones FinleyNessuna valutazione finora
- This Test Contains A Total of 15 Objective Type Questions. Each Question Carries 1 Mark. There Is NO NEGATIVE MarkingDocumento8 pagineThis Test Contains A Total of 15 Objective Type Questions. Each Question Carries 1 Mark. There Is NO NEGATIVE MarkingvarunkohliinNessuna valutazione finora
- CH 18 Solutions ManualDocumento67 pagineCH 18 Solutions ManualAhmad FauzanNessuna valutazione finora
- Acidicity Basicity & H - Bonding Tautomerism (Q.B.) 12thDocumento16 pagineAcidicity Basicity & H - Bonding Tautomerism (Q.B.) 12thAritra Lahiri100% (1)
- Chemistry Sheet Hacked - 3Documento11 pagineChemistry Sheet Hacked - 3manasgandhi684Nessuna valutazione finora
- Chapter13 (11th Ed) Practice ProblemsDocumento22 pagineChapter13 (11th Ed) Practice ProblemslianahajjNessuna valutazione finora
- Bansal Classes Organic Chemistry Study Material For IIT JEEDocumento477 pagineBansal Classes Organic Chemistry Study Material For IIT JEEAditya Kavuluri40% (5)
- Solved Multiple Choice Questions IE by NKB - PDF 116788864Documento15 pagineSolved Multiple Choice Questions IE by NKB - PDF 116788864Pranav SharmaNessuna valutazione finora
- Organic Chemistry 7th Edition Bruice Test BankDocumento10 pagineOrganic Chemistry 7th Edition Bruice Test Bankmelissa100% (21)
- WBJEE 2014 Chemistry Question Paper With SolutionsDocumento15 pagineWBJEE 2014 Chemistry Question Paper With SolutionsLokesh Kumar50% (2)
- Notes Chapter 887Documento87 pagineNotes Chapter 887notime ReactionNessuna valutazione finora
- JEE Main Chemistry Previous Year Questions With Solutions On EquilibriumDocumento5 pagineJEE Main Chemistry Previous Year Questions With Solutions On EquilibriumConimicNessuna valutazione finora
- EQUILIBRIUM Practice PaperDocumento4 pagineEQUILIBRIUM Practice PapersandysrilakshmiNessuna valutazione finora
- 70 Practice Problems For CH 7Documento10 pagine70 Practice Problems For CH 7ULFA TUFFAHATINessuna valutazione finora
- 12 TH Chem P82023Documento5 pagine12 TH Chem P82023Yashvir KillzNessuna valutazione finora
- 30 QuestionDocumento6 pagine30 QuestionsohitNessuna valutazione finora
- HC Docx1Documento13 pagineHC Docx1ayushsekhariNessuna valutazione finora
- Alkyl and Aryl Halide TestDocumento6 pagineAlkyl and Aryl Halide TestSoren Sharma50% (6)
- A CIDITYDocumento17 pagineA CIDITYApex InstituteNessuna valutazione finora
- Ebook Chemical Principles The Quest For Insight 7Th Edition Atkins Test Bank Full Chapter PDFDocumento66 pagineEbook Chemical Principles The Quest For Insight 7Th Edition Atkins Test Bank Full Chapter PDFJaniceMarqueznxed100% (13)
- Chemical Principles The Quest For Insight 7th Edition Atkins Test BankDocumento45 pagineChemical Principles The Quest For Insight 7th Edition Atkins Test Bankwadeperlid9d98k100% (27)
- Organic Chemistry 7th Edition Bruice Test BankDocumento10 pagineOrganic Chemistry 7th Edition Bruice Test BankCarolHutchinsonmrwjn100% (14)
- Chemistry For Today General Organic and Biochemistry Hybrid Edition 8Th Edition Seager Test Bank Full Chapter PDFDocumento32 pagineChemistry For Today General Organic and Biochemistry Hybrid Edition 8Th Edition Seager Test Bank Full Chapter PDFelise.green301100% (14)
- Chemistry For Today General Organic and Biochemistry Hybrid Edition 8th Edition Seager Test Bank 1Documento33 pagineChemistry For Today General Organic and Biochemistry Hybrid Edition 8th Edition Seager Test Bank 1charleslopezqxstcfgbka100% (28)
- Problems For Chapter 1 & 2 ANSWERS: 2xH 2 2xN 10 O 6Documento6 pagineProblems For Chapter 1 & 2 ANSWERS: 2xH 2 2xN 10 O 6JibrilAttawarahNessuna valutazione finora
- Chemical Principles The Quest For Insight 7th Edition Atkins Test BankDocumento38 pagineChemical Principles The Quest For Insight 7th Edition Atkins Test Bankgarrywolfelsjftl100% (15)
- Jee 2022, 100Q, Advanced Sprint, Organic ChemistryDocumento70 pagineJee 2022, 100Q, Advanced Sprint, Organic ChemistrySarvjeet Singh KalsiNessuna valutazione finora
- XN3lz Std12ChemistryCBSEModel TestQP FinalDocumento8 pagineXN3lz Std12ChemistryCBSEModel TestQP FinalPRAKASH .ENessuna valutazione finora
- 1 AllDocumento18 pagine1 AllMarcos ViníciusNessuna valutazione finora
- VMC TestDocumento17 pagineVMC TestTushar AgrawalNessuna valutazione finora
- 11th - Chemistry MaterialDocumento12 pagine11th - Chemistry Materialprathiksha6660Nessuna valutazione finora
- Chapter 1 Org ChemDocumento18 pagineChapter 1 Org ChemBheaBylRiveraNessuna valutazione finora
- Exercise-01 Check Your Grasp: O CH HO HODocumento29 pagineExercise-01 Check Your Grasp: O CH HO HOHet PrajapatiNessuna valutazione finora
- CHM 1321 Assignment 4 AnswersDocumento12 pagineCHM 1321 Assignment 4 AnswersSara YuenNessuna valutazione finora
- Acids - Bases (AP MC)Documento7 pagineAcids - Bases (AP MC)Habiba AbdeenNessuna valutazione finora
- Chemistry Final ExamDocumento4 pagineChemistry Final ExamIpshita pathakNessuna valutazione finora
- Ionic Equilibrium (Assignment 2)Documento19 pagineIonic Equilibrium (Assignment 2)Agriye KambojNessuna valutazione finora
- Which of The Following Has The Highest Boiling Point?: OH OH OH OH (A) (B) (C) (D)Documento7 pagineWhich of The Following Has The Highest Boiling Point?: OH OH OH OH (A) (B) (C) (D)Yarys Yau100% (1)
- GOC Sheet PDFDocumento55 pagineGOC Sheet PDFAayush KharbandaNessuna valutazione finora
- CHEMISTRY-24 - 13th Paper-I SOLUTIONDocumento6 pagineCHEMISTRY-24 - 13th Paper-I SOLUTIONRaju SinghNessuna valutazione finora
- Carbonyl Compounds SheetDocumento133 pagineCarbonyl Compounds Sheetadityavaish739Nessuna valutazione finora
- Acids Bases and Salts Review W KeyDocumento19 pagineAcids Bases and Salts Review W KeyJosephine Charles HoNessuna valutazione finora
- Practice Chapter 18 Carboxylic AcidsDocumento0 paginePractice Chapter 18 Carboxylic AcidsRochelle BartiletNessuna valutazione finora
- Aromatic Compound SheetDocumento71 pagineAromatic Compound Sheetadityavaish739Nessuna valutazione finora
- Organic Chemistry-JeeDocumento33 pagineOrganic Chemistry-JeeRamesh Babu GarlapatiNessuna valutazione finora
- Quiz Organic 1Documento6 pagineQuiz Organic 1ronakgupta332005Nessuna valutazione finora
- Class 12 - HHDocumento74 pagineClass 12 - HHgujjarvikram123456Nessuna valutazione finora
- CLASS 12 Chemistry-PQDocumento24 pagineCLASS 12 Chemistry-PQJeremiah ShibuNessuna valutazione finora
- Chemistry PQDocumento13 pagineChemistry PQAman SilayachNessuna valutazione finora
- 2024 Set 2Documento23 pagine2024 Set 2Manab GhoshalNessuna valutazione finora
- Vidymandir TestDocumento16 pagineVidymandir TestArshil Khan100% (1)
- CadDocumento8 pagineCadRamesh Babu GarlapatiNessuna valutazione finora
- University of Buea CHM201 ExamsDocumento6 pagineUniversity of Buea CHM201 Exams2peterlimanNessuna valutazione finora
- Kvs Sample Paper Chemistry Page 2 - 6Documento5 pagineKvs Sample Paper Chemistry Page 2 - 6Rohan BaghelNessuna valutazione finora
- Chap 1 AssignDocumento7 pagineChap 1 AssignJianqi NiHao ChenNessuna valutazione finora
- Multiple Choice Exam Review - WITH ANSWERSDocumento13 pagineMultiple Choice Exam Review - WITH ANSWERSgizeNessuna valutazione finora
- Practice Makes Perfect in Chemistry: Acids, Bases, and SaltsDa EverandPractice Makes Perfect in Chemistry: Acids, Bases, and SaltsNessuna valutazione finora
- Practice Makes Perfect in Chemistry: Oxidation-ReductionDa EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionValutazione: 5 su 5 stelle5/5 (1)
- Paras Prateek Assignment A10658Documento4 pagineParas Prateek Assignment A10658Paras PrateekNessuna valutazione finora
- Scientific Reason For Drying of River SARASWATIDocumento11 pagineScientific Reason For Drying of River SARASWATIParas PrateekNessuna valutazione finora
- Scientific Reason For Drying of River SARASWATIDocumento11 pagineScientific Reason For Drying of River SARASWATIParas PrateekNessuna valutazione finora
- Architects' AddressesDocumento8 pagineArchitects' AddressesParas PrateekNessuna valutazione finora
- NASA-ADI Industiral Design Trophy 2014-15Documento5 pagineNASA-ADI Industiral Design Trophy 2014-15Paras Prateek0% (1)
- Asset Management Council 1401Documento31 pagineAsset Management Council 1401Paras Prateek100% (1)
- List of Important Questions From H C Verma and I E Irodov For IIT JEE PreparationDocumento3 pagineList of Important Questions From H C Verma and I E Irodov For IIT JEE PreparationParas Prateek100% (1)