Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Primary Intracranial Leiomyoma: A Case Report and Literature Review
Caricato da
candiddreams0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
22 visualizzazioni3 pagineMedical Journal articles
Titolo originale
Intracranial Leiomyoma
Copyright
© © All Rights Reserved
Formati disponibili
PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoMedical Journal articles
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
22 visualizzazioni3 paginePrimary Intracranial Leiomyoma: A Case Report and Literature Review
Caricato da
candiddreamsMedical Journal articles
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 3
CASE REPORT
Primary intracranial leiomyoma: a case report
and literature review
Abdullah E. Ali & Mahmood Fazl & Juan M. Bilbao
Received: 19 April 2006 / Accepted: 26 May 2006 / Published online: 8 August 2006
# Springer-Verlag 2006
Abstract We report a case of primary intracranial leio-
myoma in 29-year-old woman presented with severe
headache. The radiology diagnosis was consistent with
meningioma. However, histologically, the tumor had the
characteristic appearance of benign smooth muscle. This
was confirmed by immunohistochemistry and electron
microscopy. Benign metastasizing leiomyoma was exclud-
ed by thorough imaging. Although rare, leiomyoma should
be considered in the differential diagnosis of well-circum-
scribed intracranial lesion.
Keywords Intracranial
.
Benign
.
Leiomyoma
Abbreviations
AIDS Acquired immunodeficiency syndrome
CNS Central nervous system
CT Computerized tomography
EBV Epstein Barr virus
MRI Magnetic resonance imaging
Introduction
The majority of intracranial extraaxial neoplasms are
meningiomas. Primary intracranial mesenchymal neo-
plasms are extremely rare but when they occur, they should
be classified similarly to their systemic counterparts.
Leiomyomas are benign smooth muscle tumors common
to the uterus. Outside the uterus, leiomyomas have been
reported in almost every organ in the body [9]. In the
central nervous system, they can radiologically mimic
meningiomas [7].
Clinical history
A 29-year-old woman, previously healthy, presented com-
plaining of episodic clusters of severe hemicranial head-
aches for few years. The headache lasted for 10 min and
occurred 1020 times per day. She had no visual symptoms.
On examination, there were no focal findings. Magnetic
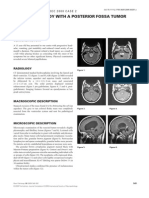
resonance image (MRI) of the brain showed a 1.2-cm
extraaxial lesion in the right frontal fossa floor, medial to
the sphenoid wing (Fig. 1). Although the lesion was small,
surgical resection was done because of the close proximity
to the optic nerve. The patient underwent frontal craniot-
omy. The tumor was attached to the dura. It was carefully
resected and sent to the anatomical pathology for
processing.
Materials and methods
The specimen was fixed in 10% formalin, embedded in
paraffin wax, and cut at 58 m. Immunohistochemical
staining was performed using the avidinbiotin peroxidase
method. The antibodies used in this study were directed
against: smooth muscle actin (1/500, monoclonal, Biogenex),
muscle-specific actin (1/25, monoclonal, Enzo), desmin
(1/100, monoclonal, Biogenex), caldesmon (1/200, mono-
clonal, Dako), collagen IV (1/50, monoclonal, Dako),
Virchows Arch (2006) 449:382384
DOI 10.1007/s00428-006-0252-z
A. E. Ali
:
J. M. Bilbao (*)
Department of Pathology, Sunnybrook Health Science Centre,
2075 Bayview Avenue, Room E-419,
Toronto, ON M4N 3M5, Canada
e-mail: Juan.Bilbao@sunnybrook.ca
M. Fazl
Department of Neurosurgery, Sunnybrook Health Science Centre,
Toronto, ON M4N 3M5, Canada
pancytokeratin (AE1/AE3, 1/300, monoclonal, Dako), epi-
thelial membrane antigen (1/1,200, monoclonal, Dako),
S100 protein (1/1,500, polyclonal, Dako), CD117 (1/400,
polyclonal, Dako), estrogen receptor (1/100, monoclonal,
Novocastra), progesterone receptors (1/100, monoclonal,
Novocastra), CD34 (1/50, monoclonal, Dako), CD30
(1/200, monoclonal, Dako), and Epstein Barr virus (EBV)
(1/100, monoclonal, Dako). The tissue for electron micros-
copy was obtained from the paraffin-embedded material,
routinely processed, stained with uranyl acetate, and exam-
ined in a ZEISS EM 109 electron microscopy.
Results
The tumor was well circumscribed. Microscopically, the
tumor consisted of a bland spindle cell neoplasm composed
of intersecting fascicles of cells with abundant eosinophilic
cytoplasm, elongated cigar-shaped nuclei without promi-
nent nucleoli (Fig. 2). Mitotic activity was very low, and
there was no necrosis. The tumor was immunoreactive to
smooth muscle actin, muscle-specific actin, desmin, cal-
desmon (Fig. 3), and collagen IV, and negative for keratin,
epithelial membrane antigen, S100 protein, CD117, estro-
gen and progesterone receptors, CD34, and CD30. The Ki-
67 stained <1% of tumor cells. Immunohistochemical stain
for EBV was nonreactive. Electron microscopy showed
tumor cells to possess abundant mitochondria and
pinocytotic vesicles. The cytoplasm was filled with
intermediate filaments (Fig. 4). The findings are charac-
Fig. 2 Histology showing bland spindle cell proliferation in fascicles
with abundant eosinophilic cytoplasm. No atypia, increased mitosis,
or necrosis is seen (H&E, 20)
Fig. 3 Immunohistochemistry showing positive caldesmon staining
for smooth muscle cells (20)
Fig. 4 Electron microscopy showing abundant pinocytotic vesicles as
well as cytoplasmic intermediate filaments (12,000)
Fig. 1 MRI scan of the brain showing an extraaxial lesion at the base
of the frontal fossa floor
Virchows Arch (2006) 449:382384 383
teristic of smooth muscle differentiation, and a diagnosis of
leiomyoma was made. The differential diagnosis of benign
metastasizing leiomyoma was entertained but the patient
had negative thoracic and abdominal computerized tomog-
raphy scan as well as gynecological sonography. The final
diagnosis was primary central nervous system (CNS)
leiomyoma.
Discussion
Primary intracranial mesenchymal tumors are uncommon.
Primary CNS smooth muscle tumor is exceedingly rare.
Rhabdomyosarcoma accounts for most primary myogenic
neoplasms of the CNS reported to date [9]. There is a high
incidence of primary CNS smooth muscle neoplasms in
immunocompromised individuals. An association with
EBV had been demonstrated [1, 9]. The first case of
primary intracranial leiomyoma was reported by Kroe et al.
in 1968 [6]. The majority of the reported cases were in the
sellar region [8]. It has been mistaken as meningioma
radiologically [7]. The differential diagnosis of an extra-
axial intracranial lesion would include meningioma,
schwannoma, hemangiopericytoma, and solitary fibrous
tumor. The histology, immunohistochemical profile, and
the electron microscopical findings confirmed the smooth
muscle origin of this tumor. Another rare differential
diagnosis that should not be forgotten, giving the female
gender of the patient, is benign metastasizing leiomyoma
[3]. This was excluded by thorough imaging.
Several hypotheses were suggested for the origin of this
tumor in the CNS. It is presumed that smooth muscle
tumors in the CNS may arise from embryonic rests,
pluripotential mesenchymal cells, perivascular connective
tissue, leptomeninges, or smooth muscle cells of the blood
vessels [6, 10]. A separate study suggested the latter origin
because of the finding of actin inclusions in the proliferat-
ing smooth muscle cells of the adjacent blood vessels
similar to those found in the tumor cells [8].
Leiomyomas were recently added to the list of neoplasms
showing increasing incidence in acquired immunodeficiency
syndrome (AIDS) especially in children [1, 4]. There is an
association between EBV infection and the development of
leiomyoma in those individuals. One report documents a
benign leiomyoma but with EBV positivity in a young
female with AIDS. Her tumor behaved aggressively, and she
died of extensive intracranial disease [2].
To our knowledge this is the seventh case of primary
intracranial leiomyoma [2, 5, 6, 8, 10]. Table 1 summarizes
the clinicopathological data of the reported cases of primary
intracranial leiomyoma. Although rare, leiomyoma should be
considered in the differential diagnosis of a well-circum-
scribed intracranial extraaxial lesion especially at the sellar
or suprasellar regions.
References
1. Bargiela A, Rey JL, Diaz JL (1999) Meningeal leiomyoma in an
adult with AIDS: CT and MRI with pathological correlation.
Neuroradiology 41(9):696698
2. Bette K, Kleinschmidt-DeMasters BK, Mierau GW (1998)
Unusual dural and skull-based mesenchymal neoplasms: a report
of four cases. Hum Pathol 29(3):240245
3. Goyle KK, Moore DF Jr, Garrett C (2003) Benign metastasizing
leiomyomatosis: case report and review. Am J Clin Oncol 26
(5):473476
4. Karpinski NC, Yaghmai R, Barba D (1999) Case of the month:
March 1999a 26 year old HIV positive male with dura based
masses. Brain Pathol 9(3):609610
5. Kim SH, Youm JY, Song SH, Kim Y, Song KS (1999) Primary
intracranial leiomyoma. Case illustration. J Neurosurg 90(1):171
6. Kroe DJ, Hudgins WR, Simmons JC (1968) Primary intrasellar
leiomyoma. J Neurosurg 29(2):189191
7. Lai PH, Yang CF, Huang CH (1998) Primary intracranial
leiomyoma. Neuroradiology 40(4):238241
8. Lin SL, Wang JS, Huang CS (1996) Primary intracerebral
leiomyoma: a case with eosinophilic inclusions of actin filaments.
Histopathology 28(4):365399
9. Rosai J (2004) Rosai and Ackermans surgical pathology, 9th edn.
Mosby, St. Louis, MO
10. Thierauf P, Weiland H (1978) Intracranial leiomyoma. Med Welt
29(33):12121280
Table 1 Reported cases of primary intracranial leiomyoma
Author Age Sex Site Size
(cm)
Presentation EBV
status
HIV
status
1 Kroe et al. [6] 68 F Intrasellar 2.53.0 Visual defect NA NA
2 Thierauf and Weiland [10] Child F Suprasellar 45 Visual defect NA NA
3 Lin et al. [8] 20 F Left superior temporal and
insular gyri
321 Limb numbness
and pain
NA NA
4 Bette et al. [2] 56 F Intra- and suprasellar 3.2 Visual defect NA NA
5 Bette et al. [2] 34 F Cavernous sinus 1.20.8 Right-sided sixth nerve
palsy
Positive Positive
6 Kim et al. [5] 40 F Temporoparietal convexity 74.54 Headache NA NA
7 Present case 29 F Right frontal lobe floor 1.2 Headache Negative NA
NA Not available
384 Virchows Arch (2006) 449:382384
Potrebbero piacerti anche
- Charlotte Gerson - The Gerson Way - Healing Prostate & Testicular CancerDocumento24 pagineCharlotte Gerson - The Gerson Way - Healing Prostate & Testicular Cancerjilotepec100% (3)
- The Vitamin You Need To Prevent Prostate CancerDocumento3 pagineThe Vitamin You Need To Prevent Prostate Cancercafjnk100% (1)
- Statistical Approach in HematologyDocumento33 pagineStatistical Approach in HematologycandiddreamsNessuna valutazione finora
- Statistical Approach in HematologyDocumento33 pagineStatistical Approach in HematologycandiddreamsNessuna valutazione finora
- Oral Examination: Dr. Kristina Corazon L. RoblesDocumento52 pagineOral Examination: Dr. Kristina Corazon L. RoblesKristina RoblesNessuna valutazione finora
- MANI Quality Control in Hematology AnalysersDocumento65 pagineMANI Quality Control in Hematology Analyserscandiddreams100% (1)
- Validation Cell AnalyzersDocumento45 pagineValidation Cell AnalyzerscandiddreamsNessuna valutazione finora
- Intramedullary Spinal Cord TumorsDocumento7 pagineIntramedullary Spinal Cord TumorsmutalimNessuna valutazione finora
- Osteomyelitis Case StudyDocumento41 pagineOsteomyelitis Case StudyJohn Bernard Ting Tizon100% (2)
- Advances in Prostate Cancer - G. Hamilton (Intech, 2013) WW PDFDocumento700 pagineAdvances in Prostate Cancer - G. Hamilton (Intech, 2013) WW PDFdvisionNessuna valutazione finora
- Current Practice of Gleason Grading of Prostate Carcinoma: ReviewarticleDocumento8 pagineCurrent Practice of Gleason Grading of Prostate Carcinoma: ReviewarticlecandiddreamsNessuna valutazione finora
- Teaching Care Plan - Open CholecystectomyDocumento4 pagineTeaching Care Plan - Open CholecystectomyWyen CabatbatNessuna valutazione finora
- Drugs Acting On Respiratory SystemDocumento52 pagineDrugs Acting On Respiratory SystemIrwan M. Iskober100% (4)
- Molecular Biology of Human Brain Tumors: November 2017Documento39 pagineMolecular Biology of Human Brain Tumors: November 2017Aleksandar Dimovski100% (1)
- Combined Set of Kaplan 900 and High Frequency Words PDFDocumento17 pagineCombined Set of Kaplan 900 and High Frequency Words PDFcandiddreams100% (2)
- Mean Normal Prothombin Time (MNPT)Documento10 pagineMean Normal Prothombin Time (MNPT)candiddreamsNessuna valutazione finora
- 1009 Case 2Documento4 pagine1009 Case 2willygopeNessuna valutazione finora
- Follicular Carcinoma of Thyroid Presenting As Brain MetastasisDocumento3 pagineFollicular Carcinoma of Thyroid Presenting As Brain MetastasisArif Susilo RahadiNessuna valutazione finora
- 0709 Case 2Documento4 pagine0709 Case 2willygopeNessuna valutazione finora
- Neurofibroma, Schwannoma or A Hybrid Tumor of The Peripheral Nerve Sheath?Documento4 pagineNeurofibroma, Schwannoma or A Hybrid Tumor of The Peripheral Nerve Sheath?As AsNessuna valutazione finora
- E - Uniform Round Tumour Cells With Small Nucleus and Clear Vacuolated CytoplasmDocumento16 pagineE - Uniform Round Tumour Cells With Small Nucleus and Clear Vacuolated CytoplasmAien LeeNessuna valutazione finora
- Laryngeal Plasmacytoma in Kahlers Disease: A Case ReportDocumento6 pagineLaryngeal Plasmacytoma in Kahlers Disease: A Case ReportIJAR JOURNALNessuna valutazione finora
- 1208 Case 1Documento2 pagine1208 Case 1willygopeNessuna valutazione finora
- Stomeo 2009Documento5 pagineStomeo 2009elvirNessuna valutazione finora
- Jkms 20 687 PDFDocumento4 pagineJkms 20 687 PDFDamodara KumaranNessuna valutazione finora
- Abdominal Wall Extraskeletal Ewing Sarcoma - Case ReportDocumento3 pagineAbdominal Wall Extraskeletal Ewing Sarcoma - Case ReportInternational Organization of Scientific Research (IOSR)Nessuna valutazione finora
- Epithelioid Hemangioendothelioma of The Infundibular-Hypothalamic Region: Case Report and Literature ReviewDocumento6 pagineEpithelioid Hemangioendothelioma of The Infundibular-Hypothalamic Region: Case Report and Literature ReviewcarlosNessuna valutazione finora
- Ccaass Cclliinniiq Quuee //ccaassee Rreeppo OrrttDocumento4 pagineCcaass Cclliinniiq Quuee //ccaassee Rreeppo OrrttCamil ChouairyNessuna valutazione finora
- Cerebellopontine Angle Epidermoid CYST: Case ReportDocumento3 pagineCerebellopontine Angle Epidermoid CYST: Case ReportTamajyoti GhoshNessuna valutazione finora
- 5 5 5 Pontine Atypical Neurocytoma Case Report 副本Documento8 pagine5 5 5 Pontine Atypical Neurocytoma Case Report 副本singhNessuna valutazione finora
- Primary Extraskeletal Ewing Sarcoma of The Sinonasal Tract - A Rare Case Report and Review of The LiteratureDocumento7 paginePrimary Extraskeletal Ewing Sarcoma of The Sinonasal Tract - A Rare Case Report and Review of The LiteraturemohammadsayfooNessuna valutazione finora
- Vol-1, Issue-1 Prospective Study of Peripheral Nerve Tumors Over A Period of 2 YearsDocumento4 pagineVol-1, Issue-1 Prospective Study of Peripheral Nerve Tumors Over A Period of 2 YearsijhrmimsNessuna valutazione finora
- 1208 Case 2Documento4 pagine1208 Case 2willygopeNessuna valutazione finora
- Colreavy 2000Documento6 pagineColreavy 2000Yi-Hung SungNessuna valutazione finora
- Surgical Neurology International: Unusual Growth Pattern of A MeningiomaDocumento3 pagineSurgical Neurology International: Unusual Growth Pattern of A MeningiomaJamie NicholsNessuna valutazione finora
- Spinal Tumors ArticleDocumento5 pagineSpinal Tumors ArticleNouman Safdar AliNessuna valutazione finora
- Spinal Tumors ArticleDocumento5 pagineSpinal Tumors ArticleMariaLakhaniNessuna valutazione finora
- X BibliDocumento7 pagineX BibliBJ CarminatorNessuna valutazione finora
- International 2Documento4 pagineInternational 2daffunkmadansyahNessuna valutazione finora
- Solitary Plasmacytoma of The Thoracolumbar Spine A Rare Cas - 2022 - InterdisciDocumento5 pagineSolitary Plasmacytoma of The Thoracolumbar Spine A Rare Cas - 2022 - Interdiscib00403007Nessuna valutazione finora
- Cancer-5 (HSS 2305) - F2022Documento10 pagineCancer-5 (HSS 2305) - F2022zahra.lalani14Nessuna valutazione finora
- Small Round Cell Tumors of Soft Tissue and BoneDocumento13 pagineSmall Round Cell Tumors of Soft Tissue and BoneAdriana Gabriela Ugarte MacíasNessuna valutazione finora
- 1109 Case 2Documento4 pagine1109 Case 2willygopeNessuna valutazione finora
- Tonsil TumorDocumento4 pagineTonsil TumorafrisiammyNessuna valutazione finora
- DR SARMA'S DERMPATH: Dermal Nerve Sheath Myxoma. Internet J Dermatol 2007: Vol 5:2Documento4 pagineDR SARMA'S DERMPATH: Dermal Nerve Sheath Myxoma. Internet J Dermatol 2007: Vol 5:2Deba P SarmaNessuna valutazione finora
- Pinacetal Primary Uterine AngiosarcomaDocumento3 paginePinacetal Primary Uterine AngiosarcomaIlincaNessuna valutazione finora
- Komatsu 2020Documento5 pagineKomatsu 2020Felipe RohamNessuna valutazione finora
- Net Cu Meta in HipofizaDocumento6 pagineNet Cu Meta in HipofizaLaura PredescuNessuna valutazione finora
- Alveolar Soft Part SarcomaDocumento20 pagineAlveolar Soft Part SarcomaAffan Akbar TalamiNessuna valutazione finora
- Tumefactive Demyelinating Lesions: Nine Cases and A Review of The LiteratureDocumento9 pagineTumefactive Demyelinating Lesions: Nine Cases and A Review of The LiteraturePS NeuroNessuna valutazione finora
- BMJ Case Reports-2014-Yi-bcr-2013-202851Documento2 pagineBMJ Case Reports-2014-Yi-bcr-2013-202851mkfireNessuna valutazione finora
- Herlina Eka ShintaDocumento1 paginaHerlina Eka ShintaBayu SetiaNessuna valutazione finora
- Cerebral Edema Associated With MeningiomaDocumento3 pagineCerebral Edema Associated With MeningiomaEstefania Ramirez CardonaNessuna valutazione finora
- PosterDocumento1 paginaPosterRaif RizqullahNessuna valutazione finora
- Keywords: Pilomatrixoma, Skin Nodule, Fine Needle Aspiration CytologyDocumento4 pagineKeywords: Pilomatrixoma, Skin Nodule, Fine Needle Aspiration CytologyachmadaNessuna valutazione finora
- Abdollahi Et AlDocumento5 pagineAbdollahi Et AlMarc DaouNessuna valutazione finora
- Meningeal Solitary Fibrous Tumor: Jong-Myong Lee, M.DDocumento3 pagineMeningeal Solitary Fibrous Tumor: Jong-Myong Lee, M.DFerry TebeNessuna valutazione finora
- Case Report: Iatrogenic Intraspinal Epidermoid CystDocumento3 pagineCase Report: Iatrogenic Intraspinal Epidermoid CystWilda HanimNessuna valutazione finora
- Cerebellar Hemangioblastoma-A Rare EntityDocumento10 pagineCerebellar Hemangioblastoma-A Rare EntityJay LeheriNessuna valutazione finora
- Case Report 2Documento6 pagineCase Report 2ancillaagraynNessuna valutazione finora
- Case Report 2022Documento5 pagineCase Report 2022Reyes Ivan García CuevasNessuna valutazione finora
- Adrenal Neuroblastoma With Bone Marrow Metastasis in Anadult: A Case Report and Review of The LiteratureDocumento5 pagineAdrenal Neuroblastoma With Bone Marrow Metastasis in Anadult: A Case Report and Review of The LiteratureIJAR JOURNALNessuna valutazione finora
- AsianJNeurosurg102126-4310714 115827 PDFDocumento3 pagineAsianJNeurosurg102126-4310714 115827 PDFSucipto HartonoNessuna valutazione finora
- Olfactory Groove MeningiomaDocumento16 pagineOlfactory Groove Meningiomaclaudio RivasNessuna valutazione finora
- Tumorpediatricoembrionalcerebelo PDFDocumento8 pagineTumorpediatricoembrionalcerebelo PDFRafael Mujica OreNessuna valutazione finora
- Emb Oj 2009327 ADocumento14 pagineEmb Oj 2009327 Athemoonwalker2014Nessuna valutazione finora
- EN + EosinofiliaDocumento3 pagineEN + EosinofiliaAna Flavia BaptistaNessuna valutazione finora
- DOI: 10.1515/jbcr-2015-0170: Case ReportDocumento4 pagineDOI: 10.1515/jbcr-2015-0170: Case ReporttaufikolingNessuna valutazione finora
- 0209 Case 2Documento4 pagine0209 Case 2willygopeNessuna valutazione finora
- Cervical Sympathetic Chain Ganglioneuroma: Case Report and Review of LiteratureDocumento4 pagineCervical Sympathetic Chain Ganglioneuroma: Case Report and Review of LiteratureIOSR Journal of PharmacyNessuna valutazione finora
- Accuracy of Computerised Tomography in Diagnosis of Brain Tumours in ChildrenDocumento3 pagineAccuracy of Computerised Tomography in Diagnosis of Brain Tumours in ChildrenVidinikusumaNessuna valutazione finora
- Magnetic Resonance Imaging of Meningiomas: A Pictorial ReviewDocumento10 pagineMagnetic Resonance Imaging of Meningiomas: A Pictorial ReviewKevin EdroNessuna valutazione finora
- Ca EndometriumDocumento10 pagineCa Endometriumoktaviany810Nessuna valutazione finora
- Atlas of Clinical Cases on Brain Tumor ImagingDa EverandAtlas of Clinical Cases on Brain Tumor ImagingYelda ÖzsunarNessuna valutazione finora
- 7 Restaurants Worth Visiting in KolkataDocumento5 pagine7 Restaurants Worth Visiting in KolkatacandiddreamsNessuna valutazione finora
- 2010 BaxterDocumento7 pagine2010 BaxtercandiddreamsNessuna valutazione finora
- Polymorphous Breast CA.Documento6 paginePolymorphous Breast CA.candiddreamsNessuna valutazione finora
- NeuroendoDocumento8 pagineNeuroendocandiddreamsNessuna valutazione finora
- Best Television SeriesDocumento37 pagineBest Television SeriescandiddreamsNessuna valutazione finora
- MGCTDocumento11 pagineMGCTcandiddreamsNessuna valutazione finora
- Hepatic Collision TumourDocumento6 pagineHepatic Collision TumourcandiddreamsNessuna valutazione finora
- MesotheliomaDocumento7 pagineMesotheliomacandiddreamsNessuna valutazione finora
- Lung OsteomaDocumento4 pagineLung OsteomacandiddreamsNessuna valutazione finora
- LeiomyomaDocumento3 pagineLeiomyomacandiddreamsNessuna valutazione finora
- Male Breast CA.Documento6 pagineMale Breast CA.candiddreamsNessuna valutazione finora
- Meningioma SDocumento10 pagineMeningioma ScandiddreamsNessuna valutazione finora
- Hyperplastic Gastric PolypDocumento5 pagineHyperplastic Gastric PolypcandiddreamsNessuna valutazione finora
- Gastric CancerDocumento8 pagineGastric CancercandiddreamsNessuna valutazione finora
- AtherosclerosisDocumento8 pagineAtherosclerosiscandiddreamsNessuna valutazione finora
- Eye AstrocytomaDocumento5 pagineEye AstrocytomacandiddreamsNessuna valutazione finora
- Clonality Analysis in Hematolymphoid Malignancies: DR Jay MehtaDocumento65 pagineClonality Analysis in Hematolymphoid Malignancies: DR Jay MehtacandiddreamsNessuna valutazione finora
- Apocrine Breast LesionsDocumento7 pagineApocrine Breast LesionscandiddreamsNessuna valutazione finora
- BreastDocumento3 pagineBreastcandiddreamsNessuna valutazione finora
- TMH PBS PresentationDocumento61 pagineTMH PBS PresentationcandiddreamsNessuna valutazione finora
- Gujral FCMDocumento102 pagineGujral FCMcandiddreamsNessuna valutazione finora
- Normal Hematolymphoid TissuesDocumento182 pagineNormal Hematolymphoid TissuescandiddreamsNessuna valutazione finora
- CA OvaryDocumento2 pagineCA Ovaryyainakata9Nessuna valutazione finora
- 221 812 1 PBDocumento64 pagine221 812 1 PBeeeejayNessuna valutazione finora
- Pleuralna KapaDocumento8 paginePleuralna KapazixdiddyNessuna valutazione finora
- MRI and CT Insufficiency Fractures of Pelvis and Proximal FemurDocumento17 pagineMRI and CT Insufficiency Fractures of Pelvis and Proximal FemureosfieldNessuna valutazione finora
- Intestinal TuberculosisDocumento15 pagineIntestinal Tuberculosisalfaz lakhaniNessuna valutazione finora
- Meckel's Diverticulum: Clinical Features, Diagnosis and ManagementDocumento7 pagineMeckel's Diverticulum: Clinical Features, Diagnosis and Managementbayu pamungkasNessuna valutazione finora
- 2.0 Review of LiteratureDocumento14 pagine2.0 Review of LiteraturePankaj KushwahNessuna valutazione finora
- Ang Title Ibutang Sa Babaw Gid - 1 Page para Sa Cover PageDocumento6 pagineAng Title Ibutang Sa Babaw Gid - 1 Page para Sa Cover PageAries Lorrien Balarito AndeaNessuna valutazione finora
- B SC Counselling Psychology - VI Sem. Core Course - Health PsychologyDocumento66 pagineB SC Counselling Psychology - VI Sem. Core Course - Health PsychologySindura SadanandanNessuna valutazione finora
- Human Body Modeling ANSYS SoftwareDocumento53 pagineHuman Body Modeling ANSYS Softwarej_c_garcia_d100% (1)
- DataDocumento207 pagineDatamikhael muaneoNessuna valutazione finora
- Pernicious Anemia in Young: A Case Report With Review of LiteratureDocumento5 paginePernicious Anemia in Young: A Case Report With Review of LiteratureeditorijmrhsNessuna valutazione finora
- +lymphoedema 150118110815 Conversion Gate02Documento24 pagine+lymphoedema 150118110815 Conversion Gate02Oussama ANessuna valutazione finora
- PAPER (ENG) - Swallowing Disturbance Questionnaire For Detecting DysphagiaDocumento5 paginePAPER (ENG) - Swallowing Disturbance Questionnaire For Detecting DysphagiaAldo Hip NaranjoNessuna valutazione finora
- MSDS - Crude OilDocumento6 pagineMSDS - Crude OilHerman HbNessuna valutazione finora
- MIMS Doctor August 2015 RGDocumento48 pagineMIMS Doctor August 2015 RGDika MidbrainNessuna valutazione finora
- Pathophysiology of SyncopeDocumento2 paginePathophysiology of SyncopeErwin Chiquete, MD, PhDNessuna valutazione finora
- 1101 FullDocumento8 pagine1101 FullAprilia R. PermatasariNessuna valutazione finora
- Insert - Elecsys CEA.07027079500.V5.EnDocumento4 pagineInsert - Elecsys CEA.07027079500.V5.EnVegha NedyaNessuna valutazione finora
- ACR Appropriateness Criteria JaundiceDocumento15 pagineACR Appropriateness Criteria Jaundicesitoxpadang ceriaNessuna valutazione finora
- The Role of Momma 042522Documento18 pagineThe Role of Momma 042522Glaizel NicolasNessuna valutazione finora
- Nej Mo A 1905287Documento12 pagineNej Mo A 1905287Ari KurniawanNessuna valutazione finora
- Toxic Pathologic 2Documento19 pagineToxic Pathologic 2Anonymous Xmb6QQvRNessuna valutazione finora