Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Melting Points of The Elements C
Caricato da
skruzerTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Melting Points of The Elements C
Caricato da
skruzerCopyright:
Formati disponibili
ELEM ENT S AND CO M PO UNDS
Melting points
G The melting point i sthe poi nt at
whi ch the soli d and liquid phase of a
substance i si n equi li bri um at a gi ven
pressure.
G I n a solid, the parti clesare held i n a
ri gi d structure by the strong forcesof
attracti on that exi st between them.
They vi brate but cannot move
posi ti on. When a soli d i sheated to i ts
melti ng poi nt, the parti clesgai n
suffi ci ent energy to overcome these
forcesof attracti on, and the parti cles
are able to move posi ti on.
G Wi thi n groupsof metalli c elements,
the melti ng poi nt decreasesdown the
group. The converse i strue for non-
metals, where the melti ng poi nt
i ncreasesdown the group.
G Readi ng acrossperiods2 and 3, the
elementsfollow a pattern of metalli c
structure, gi ant covalent structure, and
si mple covalent structure. The melti ng
poi nt i ncreasesunti l a maxi mum i s
reached wi th the element that exi stsas
a gi ant covalent structure.
G The more reacti ve metalsi n group 1
are soft and have low melti ng poi nts.
Transition metals( elementsthat have
an i ncomplete i nner electron
structure) are generally harder and
have hi gher melti ng poi nts.
G The noble gasesexi st assi ngle atoms
wi th only weak forcesof attracti on
between them. Consequently, thei r
melti ng poi ntsare verylow.
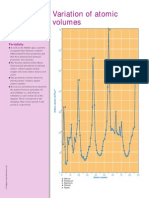
G Usi ng the fi rst si x peri odsmi nusthe
lanthanide series, the di agram
hi ghli ghtsthe element wi th the
hi ghest melti ng poi nt i n a peri od.
E le m e n ts wh o se m e lti n g p o i n ts
a re th e g re a te st i n th e i r p e ri o d
C C a rb o n
S i S i li c o n
V V a n a d i u m
M o M o ly b d e n u m
W T u n g ste n
Melting points of the
elements C
group
group 1
lanthanide series
liquid
melting point
noble gases
period
solid
transition metals
Key words
29
D
i
a
g
r
a
m
V
i
s
u
a
l
I
n
f
o
r
m
a
t
i
o
n
L
t
d
.
2
2
2
7
2
9
9
6
3
4
1
0
3
1
8
0
2
7
0
0
2
4
1
0
1
7
7
2
1
0
6
4
3
9
3
0
4
3
2
8
2
7
1
2
5
4
3
0
4
7
1
1
8
5
2
2
4
6
7
2
6
1
0
2
1
7
2
2
3
1
0
1
9
6
6
1
5
5
4
9
6
2
3
2
1
1
5
6
2
3
2
6
3
1
4
5
0
1
1
4
1
1
2
Z
r
N
b
M
o
T
c
R
u
R
h
P
d
A
g
C
d
I
n
S
n
S
b
T
e
I
X
e
H
f
T
a
W
R
e
O
s
I
r
P
t
A
u
H
g
T
l
P
b
B
i
P
o
A
t
R
n
2
9
7
2
5
3
9
7
6
9
1
5
2
2
R
b
S
r
Y
C
s
B
a
1
6
6
0
1
8
9
0
1
8
5
7
1
2
4
4
1
5
3
5
1
4
9
5
1
4
5
5
1
0
8
3
4
2
0
3
0
9
3
7
8
1
7
2
1
7
1
5
7
T
i
V
C
r
M
n
F
e
C
o
N
i
C
u
Z
n
G
a
G
e
A
s
S
e
B
r
K
r
6
3
8
3
9
1
5
4
1
K
C
a
S
c
2
3
0
0
3
7
0
0
2
7
0
2
1
8
2
2
0
2
4
8
B
C
N
O
F
N
e
9
8
6
4
9
N
a
M
g
1
8
1
1
2
7
8
L
i
B
e
6
6
0
1
4
1
0
4
4
1
1
9
1
0
1
1
8
9
A
l
S
i
P
S
C
l
A
r
2
7
0
H
e
2
5
9
H
1
56 4 3 2
L
a
9
2
1
Potrebbero piacerti anche
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Variation of Melting PointsDocumento1 paginaVariation of Melting PointsskruzerNessuna valutazione finora
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- Variation of Boiling PointsDocumento1 paginaVariation of Boiling PointsskruzerNessuna valutazione finora
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Si UnitsDocumento12 pagineSi UnitsskruzerNessuna valutazione finora
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (894)
- Periodic Table With Masses and NumbersDocumento1 paginaPeriodic Table With Masses and NumbersskruzerNessuna valutazione finora
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Periodic TableDocumento1 paginaThe Periodic Tableskruzer100% (1)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Organizing The ElementsDocumento1 paginaOrganizing The ElementsskruzerNessuna valutazione finora
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Structure of Some Ionic CrystalsDocumento1 paginaStructure of Some Ionic CrystalsskruzerNessuna valutazione finora
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Variation of Atomic NumbersDocumento1 paginaVariation of Atomic NumbersskruzerNessuna valutazione finora
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (587)
- The MoleDocumento1 paginaThe MoleskruzerNessuna valutazione finora
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (265)
- Solar SystemDocumento1 paginaSolar SystemskruzerNessuna valutazione finora
- Planet CompositionDocumento1 paginaPlanet CompositionskruzerNessuna valutazione finora
- Planetary Density, Size, and AtmosphereDocumento1 paginaPlanetary Density, Size, and AtmosphereskruzerNessuna valutazione finora
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Luminescence Atomic StructureDocumento1 paginaLuminescence Atomic StructureskruzerNessuna valutazione finora
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- Measuring The Charge On The ElectronDocumento1 paginaMeasuring The Charge On The ElectronskruzerNessuna valutazione finora
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- Investigating The Electron 1Documento1 paginaInvestigating The Electron 1skruzerNessuna valutazione finora
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Investigating The Electron 2Documento1 paginaInvestigating The Electron 2skruzerNessuna valutazione finora
- Geiger and Marsden's ApparatusDocumento1 paginaGeiger and Marsden's ApparatusskruzerNessuna valutazione finora
- Investigating The Electron 2Documento1 paginaInvestigating The Electron 2skruzerNessuna valutazione finora
- Energy Levels HydrogenDocumento1 paginaEnergy Levels HydrogenskruzerNessuna valutazione finora
- Determination of Avogadro's ConstantDocumento1 paginaDetermination of Avogadro's ConstantskruzerNessuna valutazione finora
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Calculate The Molecular Mass of CompoundsDocumento1 paginaCalculate The Molecular Mass of CompoundsskruzerNessuna valutazione finora
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2219)
- Crystal Structure of Metals Lattice Structure 0Documento1 paginaCrystal Structure of Metals Lattice Structure 0skruzerNessuna valutazione finora
- Atomic Volumes of The ElementsDocumento1 paginaAtomic Volumes of The ElementsskruzerNessuna valutazione finora
- Crystal Structure of Metals Efficient PackingDocumento1 paginaCrystal Structure of Metals Efficient PackingskruzerNessuna valutazione finora
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Boiling Points of The Elements CDocumento1 paginaBoiling Points of The Elements CskruzerNessuna valutazione finora
- Atomic Emission Spectrum HydrogenDocumento1 paginaAtomic Emission Spectrum HydrogenskruzerNessuna valutazione finora
- Cathode Ray OscilloscopeDocumento1 paginaCathode Ray OscilloscopeskruzerNessuna valutazione finora
- Atomic StructureDocumento1 paginaAtomic StructureskruzerNessuna valutazione finora
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (119)
- Atomic MassDocumento1 paginaAtomic MassskruzerNessuna valutazione finora
- Feel The Burn: Communal Living Kansas in HeatDocumento16 pagineFeel The Burn: Communal Living Kansas in HeatThe University Daily KansanNessuna valutazione finora
- Offers Promises and Decisions WorksheetDocumento3 pagineOffers Promises and Decisions WorksheetMICHELE DIONE BERNES TORRESNessuna valutazione finora
- 5in5 and PCADocumento4 pagine5in5 and PCAKelvin GilesNessuna valutazione finora
- Ultimate Exercise Cheat Sheet - TricepsDocumento13 pagineUltimate Exercise Cheat Sheet - TricepsAHMED EMADNessuna valutazione finora
- PEH Supplementary ModuleDocumento2 paginePEH Supplementary ModuleMarc Morris Bautista MancenidoNessuna valutazione finora
- LAFD - Wellness and Fitness Exercise Manual (2004) 63p R20090725DDocumento63 pagineLAFD - Wellness and Fitness Exercise Manual (2004) 63p R20090725DfilzovocNessuna valutazione finora
- Reflection of Physical EducationDocumento6 pagineReflection of Physical Educationapi-235007312Nessuna valutazione finora
- Warm-Up Exercises For Table TennisDocumento12 pagineWarm-Up Exercises For Table TennisEmma OmictinNessuna valutazione finora
- T - Anh 12 - de Thi Hs Gioi - 5Documento8 pagineT - Anh 12 - de Thi Hs Gioi - 5lamthienphong13Nessuna valutazione finora
- FITNESS TRAINING: AEROBIC, ANAEROBIC AND THERAPEUTIC EXERCISESDocumento26 pagineFITNESS TRAINING: AEROBIC, ANAEROBIC AND THERAPEUTIC EXERCISESShimmering MoonNessuna valutazione finora
- Grade 9 4th QuarterDocumento3 pagineGrade 9 4th QuarterRenlieLegaspiNessuna valutazione finora
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- Mass XL Workout Plan by Guru Mann PDFDocumento4 pagineMass XL Workout Plan by Guru Mann PDFSaptarshi BiswasNessuna valutazione finora
- Handout No. 1 in Physical Education and Health 11: Co Qah + Melc LWDocumento8 pagineHandout No. 1 in Physical Education and Health 11: Co Qah + Melc LWjingNessuna valutazione finora
- University of Montreal Track Test: Marker Cones Measuring TapeDocumento2 pagineUniversity of Montreal Track Test: Marker Cones Measuring TapeMimiMichelleMichaelNessuna valutazione finora
- Habits - Mark MansonDocumento25 pagineHabits - Mark MansonKeerthivasanNessuna valutazione finora
- Collins1993 Are Adaptations To Combined Endurance and Strength Training Affected by The Sequence of TrainingDocumento8 pagineCollins1993 Are Adaptations To Combined Endurance and Strength Training Affected by The Sequence of TrainingKatherine Nicole Díaz DelgadoNessuna valutazione finora
- Course Syllabus in (P.E. 112 - Movement Competency)Documento8 pagineCourse Syllabus in (P.E. 112 - Movement Competency)Fernando BornasalNessuna valutazione finora
- 9875 0 Grade2EJanuaryWeek1Documento1 pagina9875 0 Grade2EJanuaryWeek1Niharika DeNessuna valutazione finora
- Final - Pathfit-2 I.M.Documento142 pagineFinal - Pathfit-2 I.M.Shopee PhilippinesNessuna valutazione finora
- Physical Activity, Leadership Training and Adventure SportsDocumento20 paginePhysical Activity, Leadership Training and Adventure SportsAKSH NAGARNessuna valutazione finora
- How To Stay in Here and NowDocumento11 pagineHow To Stay in Here and NowJohn OxNessuna valutazione finora
- Assignment 2Documento2 pagineAssignment 2ShahidMahfoozNessuna valutazione finora
- Optimize Paragraph StructureDocumento7 pagineOptimize Paragraph StructureJacqueline Gregorio RamosNessuna valutazione finora
- John Flais - Where The Rubber Hits The Mat - Protoversion 1Documento138 pagineJohn Flais - Where The Rubber Hits The Mat - Protoversion 1SteveNessuna valutazione finora
- Chapter Iii: Flexibility Training Lesson 1 What Is Flexibility?Documento39 pagineChapter Iii: Flexibility Training Lesson 1 What Is Flexibility?Cheryll BogtaeNessuna valutazione finora
- Current Golf Performance Literature and Application To TrainingDocumento11 pagineCurrent Golf Performance Literature and Application To TrainingdesignkebasNessuna valutazione finora
- Grade 8 Health Physical EducationDocumento2 pagineGrade 8 Health Physical Educationapi-364536997Nessuna valutazione finora
- 3:2 Diminishing Intervals: My Account (/My-Account/)Documento3 pagine3:2 Diminishing Intervals: My Account (/My-Account/)Leonardo ZaraNessuna valutazione finora
- Progress Test 1Documento9 pagineProgress Test 1Ольга100% (1)
- A Testing Battery For The Assessment of Fitness in Soccer PlayersDocumento12 pagineA Testing Battery For The Assessment of Fitness in Soccer Playersbolebs14Nessuna valutazione finora