Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
02 TabletProcessing
Caricato da
Ian Sanz SequeraDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
02 TabletProcessing
Caricato da
Ian Sanz SequeraCopyright:
Formati disponibili
1
Tablet Processing........................................................................................................................1
Table of Contents ....................................................................................................................1
Process Objective ...................................................................................................................2
Content Uniformity ..............................................................................................................2
Material Composition..........................................................................................................2
Wet Granulation.......................................................................................................................5
Advantages of Wet Granulation ..........................................................................................6
Disadvantages of Wet Granulation......................................................................................7
Dry Mixing ...........................................................................................................................7
Wet Mixing...........................................................................................................................7
Ingredients fir Wet Granulation............................................................................................8
Binders ..........................................................................................................................10
Avicel
PH Microcrystalline Cellulose (MCC) in Wet Granulation .................................10
Wet Milling.........................................................................................................................11
Drying of Granules ............................................................................................................11
Drying of Milling ................................................................................................................14
Direct Compression...............................................................................................................14
Advantage of Direct Compression....................................................................................14
Disadvantages of Direct Compression .............................................................................14
Final Blending....................................................................................................................15
Adhesive mixingFines................................................................................................15
Adhesive mixingCoarse.............................................................................................15
Carrier Blends ...............................................................................................................16
Mixing by Dilution..........................................................................................................16
Dry Granulation .....................................................................................................................16
Advantages of Dry Granulation.........................................................................................19
Disadvantages of Dry Granulation ....................................................................................19
Special Procedures ...........................................................................................................19
Special Procedures: Extrusion-Spheronization.................................................................19
Extrusion Equipment .........................................................................................................20
Liquid Carrier-Based Granulations....................................................................................22
Cushion-Based Blends .....................................................................................................22
Handling of Powders.........................................................................................................22
Containment ......................................................................................................................22
Section 2
Tablet Processing
By Dr. Cecil W. Propst
Table of Contents
The objective of pharmaceutical processing
in manufacturing solid dosage forms is to
prepare the running powder to feed the
tablet press and/or capsule-filling machine.
There are a number of required
characteristics for the running powder, and
many of the requirements vary, depending
on the equipment used to manufacture the
dosage form. Of these properties,
homogeneity of the mix, material flow,
controlled bulk density, and proper lubricity
are the most important. For tablets and
some of the capsule-filling machines, both
compressibility and consolidation
characteristics of the running powder are
also key.
Three main manufacturing methods are
used to prepare the running powder:
Wet granulation
Direct compression
Dry granulation or slugging
Almost all formulations require
supplemental materials (excipients) to be
added to the recipe in addition to the active
ingredient to increase the dosage form size.
and for other essential functions, such as
binding and disintegration. The ingredient
classification used is:
Fillers
Binders
Disintegrants
Lubricants
Wetting agents
Glidants
Antiadherents
Colors and flavors
Preservatives
2
Process Objectives
The choice of excipients greatly depends
on the properties of the drug substance,
the behavior of the product as it is being
processed, and the properties required for
the final dosage form. In order to deliver a
stable, uniform, and effective drug product,
it is essential to know the properties of the
active ingredient, the active ingredient in
combination with the required excipients,
and the requirements of the dosage form,
and then to apply these requirements to
the process.
To better understand these process
objectives, a focus on the general
requirements of the solid dosage form
is required.
Content Uniformity
The content uniformity of the solid dosage
form is controlled by two components:
First, the variation in the weight of the
dosage form, and second, the variation
in the direct distribution in the running
powder.
The dosage weight is controlled mainly
by he bulk density of the running powder.
Variation in flow of the feed material can
vary the weight of fill of the dosage form
only if the flow rate is insufficient to fill the
cavity in the time allowed by the machine
for the filling process. If the powder flow is
fast enough (Figure 1), a reserve of fill time
will be available so that a small change in
flow rate will have little or no effect on the
weight of the final dosage form. Thus, a
process that creates a freely flowing feed
material with a controlled narrow range
bulk density is to be considered ideal
for controlling dosage weight.
Among other things, a shift in the particle
size distribution can change bulk density.
As shown in Figure 2, a change in the
amount of smaller particles (fines) can
cause a dramatic change in the bulk density
of the powder bed. Fines in the context of
this section and for pharmaceutical powders
are defined as passing through a 210-m
screen.
As the amount of fines increases from 0%
to approximately 40%, so does the bulk
density of the feed material (also Figure 2).
This increase in bulk density occurs until
the amount of fines in the mix reaches a
critical content. After this critical amount is
reached, adding more fines causes the
density to decrease.
3
Fines fill the voids present in a powder
bed of larger (coarser) particles (Figure 3).
Once the voids created by the coarse
particles are close to being fully occupied,
the fines begin to displace particles and,
in the displacement, leave behind fine
particles in the space formerly occupied
by he coarse particle. These fine particles
have void spaces between them. As a
result, the bulk density falls due to the
coarse particle displacement. Note that
the maximum bulk density reaches a peak
(Figure 2), which limits the change in bulk
density of the bed. If the process particle
size distribution is controlled at this
maximum, the bulk density will be less
variable.
Figure 1: Flow versus Bulk Density
Flow (g/min)
T
a
b
l
e
t
w
e
i
g
h
t
Bulk density control
4
Figure 2: Effect of Fines on Bulk Density
1.2
1.1
1
0.9
0.8
B
u
l
k
d
e
n
s
i
t
y
g
/
m
L
Fines (%)
0 10 20 30 40 50 60 70 80 90 100
Figure 3: Effect of fines on Voids Created by Coarse Particles
+ =
0% 35%
60%
5
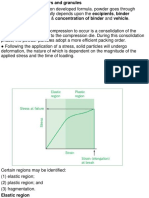
Wet granulation is a process of dry mixing,
wet mixing, and particle size enlargement.
and is a process of particle attachment
(agglomeration). In the most complex
form (Figure 4), it consists of six steps:
1. Dry mixing
2. Wet mixing
3. Milling of the wetted mass
4. Drying
5. Milling of the dried mass
6. Final blending
Figure 4 illustrates the wet granulation
process, in which a planetary mixer is
used or both the dry mixing and wet mixing
steps. A milling step may be required after
Net granulating to reduce the particle size
achieve uniform drying. The tray drier
dries the materials in 4 to 24 hours,
depending on the manner in which the
drier set up and the final liquid content
specification for the dried material. After
the material is dried, a milling step may
be needed to reduce the clusters that form
during drying. A rotating shape V-Mixer is
used in this illustration for final blending.
Figure 4: Wet Granulation
Mix powders
add binder
Mill coarsely
Dry
Mill
Blend and lubricate
Compress or fill
into capsules
Material Composition
The process that prepares the running
powder must provide a uniform chemical
composition. Good mixing is essential, and
segregation during the process should be
prevented.
With these objectives in mind, consider the
three major processes used to prepare the
running powder:
Wet granulation
Direct compression
Dry granulation or slugging
Wet Granulation
6
Now the material is ready for the dosage
form manufacturing unit. The wet
granulation process of Figure 4 illustrates
a very complex process arrangement.
A simpler process (Figure 5) employs a
single-step fluid bed wet granulator to do
the dry mixing, wet massing, and drying
steps all in one unit. Milling is not usually
required when a fluid bed is used to wet
granulate. A finishing mixer, such as the
V-Mixer, is needed after fluid bed drying
to blend the lubricant, color, and flavor,
as required, into the running powder.
The wet granulation process binds the
primary particles of the dry mix together
to form an enlarged particle called a
granule. These granules are actually
enlarged primary particle agglomerates
and are usually rounded, and therefore,
free flowing.
Advantages of Wet Granulation
Physical characteristics of the drug
are usually not important.
The coalescing of particles locks in
blend uniformity.
A wide variety of powder materials can
be processed into a uniform mix with
improved flow.
Optimum fill density can be achieved
by adjusting the process to create the
optimum final particle size distribution.
Compressibility and consolidation are
improved via the choice of the correct
binder and the moisture content of the
granules.
Dissolution is modified through
hydrophilization to improve wetting
or, with the choice of more insoluble
binders, to obtain a modified release
pattern.
Dust and segregation tendencies are
reduced.
Figure 5: Single-Step Fluid Bed Wet Granulator
air flow
a
i
r
f
l
o
w
v
e
r
t
i
c
a
l
g
r
a
d
i
e
n
t
horizontal gradient
7
Disadvantages of Wet Granulation
Large number of process steps; each
step requires qualification, cleaning,
and cleaning validation.
Long process time, particularly for
drying.
High labor and manufacturing costs.
Some material loss during processing.
Problems associated with heat and
solvent sensitive drugs.
Capital requirements for extra building
space and equipment.
Upon aging, dissolution from granules
can be slowed after tableting.
Assay problems may occur for low
dosage drugs due to incomplete
extraction if the active ingredient is
complexed by the binder, or adsorbed
onto one of the other excipients.
Still no exact way to determine
granulation endpoint (torque, power
consumption, etc.).
Dry Mixing
When wet granulating, the components
in the mix can be milled to a fine powder.
and even micronized if necessary, prior to
mixing. Micronized powders can be blended
for the dry mix, then the particle size
enlarged by agglomeration in the wet
massing step.
This fine powder mixture can be blended
into a homogeneous mass with a great deal
of mechanical agitation (and/or pressure).
Once mixed, these fine powder dispersions
segregate with difficulty, and thus tend to
remain mixed. Many granulators, such as
extruders and instantizers, need to be fed
with metered materials or a dry mix. Other
granulators, such as conventional fluid
beds, require that the active ingredient be
added in the spray to obtain good content
uniformity for certain low-dose formulations.
Most other granulators can perform the
dry mix step in the same vessel as the
wet massing vessel (e.g., a high shear
granulator).
Wet Massing
Water or other solvents and/or binder
solutions are added slowly over an
extended time period (or all at once) to
the dry powder. This is done with the
mixing to blend the liquid into the powder.
Adequate mixing time must be provided
after addition of the liquid to allow the
granules to develop.
The structure of the developed granule
(Figure 6) can vary depending on the
process chosen. Such flexibility allows
for a strategy to be developed to obtain
the desired density, porosity, texture, and
dissolution pattern of the granule formed.
Some of the key characteristics of granule
development include:
Figure 6: Granule Structure Development
Dry mix Pendular Funicular Capillary Kneaded capillary
Increasing granulating solution With pressure
8
Liquid contentthe capillary granule
contains the greatest amount of liquid
per unit mass.
Amount of surface liquid changes as
the granulation process develops
usually the funicular phase and the
overwet capillary phase have the
greatest amount of surface liquid.
Porosity is highest in the dry mix
followed by the pendular granule;
porosity of the granule is lowest in
the kneaded capillary.
Torque generated on the agitator will
be greatest during the overwet capillary
phase; also, a peak in torque often
occurs at the completion of the funicular
phase, just upon entering the capillary
phase.
Texture can be made soft (melt in the
mouth) using pendular granules; the
capillary and kneaded capillary forms
tend to be gritty.
Dissolution is generally fastest with
higher porosity granules; however, if a
disintegrant is added to the dry mix,
the problems of dense capillary granule
disintegration can be minimized.
Consolidation is essentially developed
in the granule by establishing a plane of
deformation in the binder film used as
the granulation binder; establishing the
proper distribution of this film, along with
the dispersion of the nonconsolidatable
components, is important.
Friability or dust generation during
further processing is reduced as the
process approaches the
capillary/kneaded capillary phase.
In most cases, percent of fines on
milling is least with the capillary/kneaded
capillary granule.
The structure of the granules that are
formed are dependent on the amount of
added liquid, the time allowed to granulate,
and the type of granulator used.
As shown in Figure 7, the fluid bed tends to
produce the pendular form, and can yield a
funicular arrangement. High shear tends to
yield funicular granules, but also can make
capillary and kneaded capillary granules.
Tumbling granulators tend to make capillary
granulations. Extruders are usually set up
to make kneaded capillary structured
granules. It is important to avoid adding
too much granulating solution or running a
high shear granulator beyond (or sometimes
even into) the kneaded capillary phase.
Both can result in a very dense matrix.
The mass can be doughlike and become
very difficult, if not impossible, to process
further. In such cases, screens may clog
in the wet phase mill, dried granules can
be too hard, compression forces necessary
for tableting can be high, and disintegration
of the granules may be prolonged.
9
Figure 7: Granule Form Dependent Upon Mixing/Granulation Equipment
Pendular Funicular Capillary Kneaded capillary
Mixing Equipment
Fluid bed
High shear
Tumble mixer
Extruder
Resulting granule forms
Pendular, funicular
Pendular, funicular, capillary
Capillary
Kneaded capillary
Figure 8: Effect of Surface Tension on Granule Strength and Density
Alcohol
%
Surface
Tension
dynes/cm
Granule
Strength
kp
Density
g/mL
0
10
30
100
72.8
50.2
35
22.3
18.9
13.7
10.3
5.5
1
0.985
0.964
0.79
10
Ingredients for Wet Granulation
Other than the drug material itself, the wet
binder is the most important ingredient in
the wet granulation process. The choice of
the binder will influence the granule size,
hardness, wetting, disintegration, and
consolidation characteristics.
The surface tension of the binding solution
controls the granule strength in the wet
phase and, therefore, the particle size of
the granule (Figure 8). As a result, changes
in surface tension can be employed to
control the particle size of the granulation
produced.
In fluid bed processing, which uses
atomization spraying (nebulization),
surface tension is not as important a
parameter as viscosity in controlling the
particle size of the granules. Viscosity is
the controlling parameter when using
atomizers. Viscosity controls droplet size,
and droplet size controls the granule size
developed in the fluid bed.
Binders
The role of the binder is paramount to
the development of the structure and
performance of the granulation. Linear
polymers are ideal in that they can be
used at low concentrations. With some
residual moisture present, the polymer
can perform the essential role of plastic
deformation and bonding during tableting.
Branched binder structures, such as high
bloom strength gelatins, have a tendency
to create harder granules and generally
require higher concentrations to achieve
the same particle size. Since Section 4 is
devoted to binders, the discussion here
will be limited to the processing aspects
of the binder.
The hydration level of the binder must
be uniform from batch to batch. This is
especially critical with branched polymers,
such as starch and many of the natural
gums. If the extent of hydration is variable,
this can contribute to changes in particle
size and density between batches.
Controlling the duration and temperature
of heating in preparing the granulation
fluid is important.
Liquid distribution is the key to the success
of the wet massing step. Either atomization
or slow pouring and sufficient mixing time is
required to obtain proper distribution of the
granulation fluid.
Avicel
PH Microcrystalline Cellulose (MCC)
in Wet Granulation
Major advantages provided by Avicel
PH
101 and PH-102 in the wet granulation
process of tablet making are:
Rapid, even wetting
- Wicking action thoroughly distributes
the granulating fluid throughout the
powder bed.
Control of wet mass consistency
- Large surface area and adsorptive
capacity provide a wider range of
liquid addition without overwetting.
Less screen blocking
- Good wet mass consistency aids in
trouble-free screening.
Uniform, rapid drying
- Promotes rapid release and
evaporation of liquid from the wet
granulation.
Content uniformity control of water-
soluble drugs
- Regulates active ingredient variation
in all particles of the granulation.
Wet binder-like activity
- Auxiliary wet binder promotes harder
granules with less fines.
Use of Avicel
MCC in postaddition step
provides:
- Excellent binding
- High hardness at low pressures
- Low friability
- Good disintegration
As a wet-massing adjunct only, the total
quantity of MCC is added to the initial dry
blend. Preferably, one half is incorporated
into the wet mass and the remainder into
the final blend. This postaddition of Avicel
PH is most useful for the assurance of
trouble-free tableting when compression is
sensitive to variations in raw materials or
processing.
Wet Milling
A wet milling step may be necessary after
the wet massing step to reduce the particle
size of the wet granule in preparation for
drying. If milling is needed, it will result in
cleavage of the pendular bonds (Figure 9)
and separation of the firmer-structured
granules (called granulytes). This opens
the clusters, which are fairly dry, without
exposing the capillary water included in
the small granulytes.
Plugging of the screen and build-up in
the mill are problems for many types of
equipment. The best mills place the
grinding zone of the mill as close to the
retention screen as possible. Two such
designs are featured in the oscillating
granulator (Figure 10) and cone mill
(Figure 11).
The variable speed of the blades of the
cone mill allow for a variable impact
velocity, which can be adjusted to reduce
breakage of the capillary particle, therefore
allowing cleavage at the pendular bridge
and press particles into the screen. The
mill zone is formed by the blade and the
screen, and when the granule is small
enough, it can be immediately screened.
Drying of Granules
Caution is needed in drying because of
the potential decomposition and chemical
migration that can occur during the process.
The high temperature and high solvent
content present at the beginning of the
drying process may lead to hydrolysis. At
the end of the drying process, dehydration
reactions can possibly occur.
The air temperature and humidity of the
oven controls the rate of drying. As a
minimum, the temperature and time of
drying should be controlled. Although the
ambient humidity of the air is important,
it has limited impact on most drying
operations, as room temperature air is
heated to 60C to 80C during the drying
process. Migration of dissolved ingredients
can occur during drying. Wicking agents,
such as microcrystalline cellulose, can be
used to reduce and even prevent migration.
Migration can occur between particles or
within a single particle from the core of the
particle to the surface. Changes in granule
consistency and surface characteristics can
result in changes in porosity and binding
strength. Migration can deposit drug at
the surface, and affect the structure of the
tablet during compression, and generate
dust from the surface during handling.
11
12
Figure 9: Wet Milling
Direction of
milling force
Figure 10: Oscillating Granulator
Compression and
transportation
13
Figure 11: Cone Mill
Figure 12: End-Point Control
Direction of particle travel
End-point control of drying is important. The
residual moisture in the granule aids in the
plastic deformation characteristics of the
particle during compression. If too dry, a
granule will be created that will brittle-
fracture excessively when compressed on
a tablet press. If too wet, the granules will
be sticky and may bind to the tooling.
The correct end point can be obtained by
as simple a technique as applying a given
inlet temperature to the process for a given
length of time. Another approach (Figure
12) tracks the wet bulb temperature and
compares it with the product temperature.
For example, a temperature difference (T)
of 35C between the wet bulb thermometer
reading and that of the product can be set
to stop the dryer at a specific time (e.g.,
40 minutes), or it can be set to continue
drying for a specific time after the product
temperature begins to rise. The drying
process is stopped when either a T of
35C is reached (primary control), or at
40 minutes beyond the point where the
product temperature rise is detected
(secondary control).
Inlet temperature
T
e
m
p
e
r
a
t
u
r
e
(
C
)
Dry
bulb
Wet
bulb
Product moisture content
Product
temperature
Drying time (min)
5 20 40 60
60
40
20
T
1
T
Advantages of Direct Compression
Economy in labor, time, equipment
operational energy, and space.
Problems due to heat and moisture
eliminated.
Greater physical stability provided;
hardness and porosity changes less
with time when direct compression is
broadly compared to wet granulation
systems.
Extraction of the drug from the dosage
form is not inhibited during the assay
procedure (polymer binding).
Choice of ingredients allows the
formulator to improve or retard
dissolution rate.
Disadvantages of Direct Compression
Critical nature of the raw materials;
need for greater quality control in
purchasing to assure batch uniformity.
Difficulty obtaining dense hard tablets
for high-dose drugs.
Nonhomogenous distribution of lowdose
drugs due to segregation after blending
(content uniformity).
Sensitivity of direct compression
"running" blends to overlubrication.
Limitations in color variations.
Need for assisted feed and
precompression for some high-dose
drugs.
Need for commensurate particle size or
particle size distribution between drug
and excipients.
The final moisture content of the dried
granulation is an important consideration
for both granulation stability and granulation
compactability. For crystalline binders (sugar,
corn syrup, dextrose), the moisture content
generally must be below 1% to prevent
sticking, yet greater than 0.4% to allow for
compactability. For polymer-type binders
(PVP, HPMC, gelatin), the finished moisture
content is usually higher (at 0.8% or greater)
to create a plastic-deforming particle.
Dry Granule Milling
Milling of the dried granulation is not
always necessary. Particle sizes larger than
2000 m (10 mesh) are usually considered
too large for tableting purposes.
The ideal process from a capital and
operational cost basis is direct
compression. This is, at most, a two-step
process involving screening and/or milling
and final mixing.
An effective excipient binder is needed
and should have good compression and
consolidation properties as a dry additive,
even at low concentrations (< 30%) in the
formulation.
Good adhesive properties in the dry form
are a combination of a rough and porous
surface combined with a van der Waal's
and/or a hydrophilic bonding mechanism
to attach the active ingredient(s) to the
excipient. This feature is needed to assure
good mixing of drug and excipients and
to prevent segregation.
14
Direct Compression
Avicel
PH MCC is one of the most useful
excipients available for direct-compression
tableting. Its strong bonding properties and
capacity for dry binding other materials have
made MCC a standard to which other direct
compression excipients are often compared.
MCC performs many functions in the direct
compression process:
Strong, dry binder
High dilution potential
High tablet hardness at low punch
pressures
Low tablet friability
Excellent tablet disintegration
Flow aid
Inherent lubrication
Antiadherent
Final Blending
Final blending is usually done in a cone or
V-type blender (Figure 13). To obtain the
final blend, it is necessary to consider the
objectives of the mixing steps:
To achieve drug content uniformity
15
To obtain uniformity of flow and bulk
density
To effect distribution of lubricant, color,
and surface active agents
To reduce or eliminate segregation
Adhesive mixingFines
Particles smaller than 50 m are candidates
for mixing by aggregation (Figure 13).
Particles of 10 m or smaller have a
tendency to selfadhere easily. This adhesion
means that, if all the cohesive attachments
between like materials are severed and
converted to unlike attachments, a very
effective mix can be generated that is less
prone to segregation. For example, a
particulate binding of the type A-A and B-B
(like to like) can be broken and rejoined to
form an A-B and A-B type (unlike to unlike).
Blending a formulation containing particles
of Avicel
PH-101 MCC (50 m in size) with
other particles of similar size produces a
product which is less prone to segregate. In
addition, most modern tablet presses and
encapsulating machines can use such finer
particle size powders in the feed system.
Figure 13: Final Blending
Blend and lubricate
Compress or
fill into capsules
Mill
ingredients into a formulation. Equal
volumes of the drug and a fine-sized
particle excipient, such as microcrystalline
cellulose, are mixed. This first step can
include milling of the drug with the excipient
to assure the removal of aggregates. This
premix is combined with an additional
volume of excipient, usually equal to the
total volume of the premix, and the
combination is mixed further. This
progression of mixing by dilution with a
fine particle excipient allows for an energetic
dispersion of a concentrate of drug into a
matrix or premix, which is then blended with
the remaining materials in subsequent steps.
An example of mixing by dilution involves
spraying of the solution of a drug onto
an absorbing substance. The drug being
sprayed is spread over a large mass of
excipient, which can absorb the liquid
and attach the drug. Avicel
PH MCC is
an example of a good absorber. Avicel
PH
will absorb the liquid and the drug, and the
mixture can then be further blended into a
formulation. This method is used to obtain
a more uniform distribution of the drug.
The third process for making the "running"
powder for tableting or encapsulation is
the dry granulating process (Figure 14).
This process requires five steps:
Mixing
Roller compaction
Milling
Screening
Final blending
The process is continuous and no heat or
moisture are applied, yet the particle size
of the mixture is increased.
16
Adhesive mixingCoarse
Blending of powders when the particle size
of the active ingredient is 200 m or larger
presents the problem of lack of significant
surface adhesion. A mix, when achieved,
can result in a blend that may separate.
The adhesive surface force between
particles is not strong enough to prevent
separation. Therefore, coarse blends
(particle ingredients generally larger
than 200 m) are usually restricted to
formulations with high doses of active
ingredients. To reduce segregation, the
porosity of the bed should be minimized
and vibration exposure reduced.
Carrier Blends
This type of blend is characterized by fine
particles attached to larger particles called
carriers. For maximum effect, the smaller
particles should be less than 10 m in size.
The fines attached to coarse particles are
called ordered units. The larger particles
are greater than 90 m in size, and have
pores and/or indentations in the surface
to aid in the attachment of the smaller
particles. This attachment is another
adhesive attachment mechanism similar
to the fine-fine attachment.
Mechanical stability is an important property
of powder mixtures. An ordered unit (in
this case, fine particles adhering to larger
carrier particles) can separate in two ways.
First, the smaller particles can lose their
attachment and separate. Second, the
ordered units can be of varying size due
to the varying size of the carrier particles
and can therefore separate.
Mixing by Dilution
Mixing by dilution is often used to
incorporate very potent low-dose active
Dry Granulation
17
The roller compactor and tablet press
perform the same functions. Material
is fed to the rollers and the powder is
compressed. A dwell time under pressure
allows for consolidation of the feed into a
sheet or flakes. The product then exits the
compression zone and is released from the
rolls. The roller compactor lacks an
upper punch to hold the materials in the
compression zone, called the nip area
(Figure 15). Air moves up against the
powder flow. The faster the rollers turn,
the more material is compressed, and
the greater the volume of air is displaced.
Figure 14: Roller Compactor
Gear
Air Air
Product
R
e
c
y
c
l
e
Subsequent milling process
Material characteristics
Particle size distribution, compressibility,
and consolidation characteristics must
be considered. It is important to keep
in mind that certain materials, such as
lactose (which compact by a brittle-
fracture mechanism), will be less
compactable if too high a pressure is
used during initial roller compression.
With other materials, such as ibuprofen,
the elasticity can be reduced by applying
higher pressures in roller compression.
These surface attachments are usually
weak, therefore the granules/sheets
produced are fragile. Upon milling, a
significant amount of dust is usually
generated, which is removed by screening
and is recycled back through the roller
compaction system. This feature is
needed to assure good mixing of drug
and excipients and to prevent segregation.
18
Figure 15: Nip area (Region) of Roller Compactor
The material must be properly gripped
by the roller. Accumulation of most larger
particles can occur in the nip area. This
can lead to excessive pressures being
generated for short times and banging of
the rollers as they open to allow material
to flow through. Machining of pockets or
gripping indentations in the rollers can
reduce the tendency for larger particles
to be retained. It is apparent that the
major controls are:
Pressure applied
The time under pressure and the
amount of pressure applied is of primary
importance; it is controlled by roller
speed and the height of the nip area.
The nip area (Figure 15) expands as
more gripping force or friction is applied
by the feed screw on the roller surface.
The friction between the material being
fed and the roller prevents the material
from slipping on the roller surface, and
thus, draws the material into the high
pressure area.
Release
region
Feed pressure
Roll
Nip angle
11.6
19
Advantages of Dry Granulation
Permits mechanical handling without
loss in mix quality.
Eliminates the problems due to heat
and moisture.
Improves flow of powders by increasing
particle size.
Decreases the elastic recovery for
certain compounds, thereby increasing
final compactability.
Facilitates extraction of drug from
dosage form during analysis (shows less
tendency to interfere due to polymer
binding).
Disadvantages of Dry Granulation
High amount of recycle or reprocessing
(dissolution, friability, batch control).
Possible loss in tablet compressibility in
the material during roller compacting.
Particle erosion and segregation during
finished mixing and handling (content
uniformity).
Limitations in color variety.
Special Procedures
Many predosage form manufacturing
processes can be considered specialized
and can be employed to generate a very
special product to be used for the running
powder. These processes include:
1. Extrusion-spheronization
2. Liquid carriers-based granulation
3. Cushion-based blends
Special Procedures: Extrusion-
Spheronization
A unique way to make spherical particles is
to combine an extruder with a spheronizing
machine. The spheronizer, or marumerizer,
(Figure 16), was the first machine used for
this extrusion-spheronization application.
It produces dense spherical particles from
fine powders.
The formulation requires special materials
which must be plastically deformable when
wet, but not sticky. Microcrystalline cellulose
(Avicel
PH) is the most popular ingredient
for this purpose, and is used at levels of at
least 20% in such formulations.
Basically, the process consists of producing
roughly cylindrical pellets from the plastic
mass of the mixed powders using water or
other solvents, sometimes in the presence
of a binder. The mass is extruded through a
radial screen or axial die plate by means of
a screw feeder. The larger diameter-denser
pellets are made using an axial extruder.
Spheres as small as 0.5 mm are made with
a radial screen extruder.
The pellets, once formed, are placed into
the spheronizer. The base plate of the
spheronizer spins to create friction and
a bumping action, which break down the
pellets until the length-to-diameter ratio
is approximately the same, thus forming
spheres. Total spheronization process
times range from 30 seconds to 5 minutes
or longer.
The centrifugal force created by the base
plate aggressively carries the deposited
feed pellets from the center of the machine
to the wall, then rolls them up the wall. This
action creates an annulus ring of continually
moving and rolling material.
20
Figure 16: Spheronizer (Marumerizer)
Charge chute
Fines discharge
Rotating shift
Product
discharge
Discharge
chute
Friction plate
Jacket
Scraper
Spacer
The action also forces the rounder, smaller
particles toward the interior of the roll, while
the larger, more elongated particles tumble
near the surface, facing the friction plate
as it rolls under the ring. Interparticulate
frictional forces, plus those between
particles and the moving base, spheronize
and increase the density of the particles.
The product size distribution is usually
extremely uniform, and the output can be
as great as 2000 kg/hour.
Extrusion Equipment
To spheronize a material, a preformed
particle is needed. The extrusion method
involves the application of pressure to a
wet mass until it flows through a defined
opening (orifice) in a metal plate. It is
obvious that the technique controls two
dimensions of an agglomerate. The width
is determined by the size of the orifice,
while the length is controlled by the
characteristics of the material and the
rheology imparted to the particle by the
process. Because the orifice defines the
cross-sectional geometry, extrudate length
is usually the only dimensional variable.
Two types of screw extrusion devices are
used widely:
Axial
Radial
The lower-pressure radial extruder (Figure
17) uses a transport screw to move the
material along the screen. The material
exits the die holes as it is spread across
the screen. As a result, smaller diameter,
softer extrudate particles can be produced.
An axial extruder (Figure 18) is operated
at a higher pressure and forms denser
particles. This design utilizes a transport
section to move the material forward, and
often a compression zone (having
progressively closer screw flights) to
increase the density of the material.
21
Figure 17: Twin Screw Radial Extruder
Bearing
Sprocket
Gear
Oil bath
Screen
Figure 18: Axial Extruder
Gear
Hopper
Screw
Die
plate
Extrusion
zone
Compression
zone
Feed
zone
Handling of Powders
Segregation of the active ingredient during
the handling process (Figure 19) must be
controlled to assure proper product
performance. It can be prevented by one
of two mechanisms:
Containment
Attachment (aggregation)
Both of these mechanisms treat the
particles as a group. Containment holds
the group as a set volume of particles,
while attachment creates a particle cluster
by some sort of adhesive force. The
containment mechanism is the more
important of the two.
Containment
The set volume of contained particles
could be the powder filled into a capsule,
into a vial, in front of a blade in a ribbon
blender, or in the plug grouping of a dense
phase conveyor. Because the particles are
not allowed very much movement with
respect to each other when transferred,
their tendency to segregate from each
other is reduced.
The closer the process is to containing
the individual particle and allowing only
groups to move, the less tendency there
is for segregation. Some containment
trends include:
Liquid Carrier-Based Granulations
Many granulations are made with the idea
of distributing a liquid into or onto a carrier
particle (see Mixing by Dilution). Adding a
granulating and/or coating step can seal
the particle/liquid mixture as well as modify
the redissolution of the drug substance.
A taste-masking example might involve
the granulation of the absorbed drug
onto Avicel
PH MCC with a pH-activated
polymer such as Aquacoat
CPD (cellulose
acetate phthalate). This massing and
polymer barrier would prevent the
dissolution of the drug in the mouth during
chewing, yet would release the drug in the
stomach or duodenum upon exposure to
the correct pH.
Cushion-Based Blends
Several products in the marketplace are
made as coated extended-release or
delayed-release beads in a tablet matrix.
The compression process is used to create
the dosage form. The rupture of the bead
coating in compression must be avoided.
Low-compression pressures must be
applied during the tableting process. The
formation of the tablet at low pressures,
with little (if any) internal friction, is needed.
Dry binders, such as Avicel
PH MCC, are
used with a waxy material, such as PEG
8000, to obtain a reasonable tablet
structure while applying low levels of
pressure.
22
to charge into a container. The bag
weight, as it is filled, is supported to
allow gradual entry. Also, once the
bag is filled, it is tied very tightly to
discourage bed expansion.
Moving mixed materials into bins,
rather than pneumatically or by screw
conveyor.
Low-porosity powder beds, which
reduce the segregation tendency of
finer particles percolating through pores
in the particle bed.
Charging into plastic deflated bags; the
force of the material moving into the
open bag is more of a containment
system than simply allowing the material
23
Figure 19: Active Ingredient Variation During Processing
Dry mix Wet mix Drying Handling Tableting
+0.5%
-0.5%
M
i
x
n
e
s
s
(
s
%
)
FMC logo, Avicel, Ac-Di-Soltrademarks of FMC Corporation.
1998 FMC Corporation. All rights reserved. RS
Potrebbero piacerti anche
- Pharmaceutical Analysis (Lab) Bulk/Tap/Apparent Density and Angle of ReposeDocumento17 paginePharmaceutical Analysis (Lab) Bulk/Tap/Apparent Density and Angle of ReposemasorNessuna valutazione finora
- Flow charts of pharmaceutical quality control tests for different dosage formsDa EverandFlow charts of pharmaceutical quality control tests for different dosage formsNessuna valutazione finora
- 1B Compiled Lab ReportsDocumento68 pagine1B Compiled Lab ReportsmasorNessuna valutazione finora
- Oral Solid Dosage FormsDocumento26 pagineOral Solid Dosage FormsAl-Homam SalahNessuna valutazione finora
- Tablet Technology StepsDocumento9 pagineTablet Technology StepsVikas JhawatNessuna valutazione finora
- PHT 261Documento54 paginePHT 261Tara Chand KhatriNessuna valutazione finora
- Dry Granulation by Roller CompactionDocumento24 pagineDry Granulation by Roller CompactionwaelNessuna valutazione finora
- Powders & Granules TextDocumento12 paginePowders & Granules Textabdullah2020Nessuna valutazione finora
- Experiment No: Date: Determination of Bulk Density, True Density and PorosityDocumento6 pagineExperiment No: Date: Determination of Bulk Density, True Density and PorosityVishwajeet Ghorpade67% (3)
- MillingDocumento26 pagineMillingHussein Al-jmrawiNessuna valutazione finora
- Pharmaceutical Powders, Blends, Dry Granulations, and Immediate-Release TabletsDocumento9 paginePharmaceutical Powders, Blends, Dry Granulations, and Immediate-Release TabletsOgunjimi Abayomi Tolulope50% (2)
- IPQC Test of TabletsDocumento24 pagineIPQC Test of TabletsSk Anam100% (1)
- Pravin B. Awate (M.Pharm, Pharmaceutics) Assistant Professor Rdcop, BhorDocumento15 paginePravin B. Awate (M.Pharm, Pharmaceutics) Assistant Professor Rdcop, BhorPravin AwateNessuna valutazione finora
- Physical PharmacyDocumento13 paginePhysical PharmacyPatrocinio Wilzen Grace L.Nessuna valutazione finora
- Physics of Tablet Comp ActionDocumento25 paginePhysics of Tablet Comp ActionPraanav DaNessuna valutazione finora
- Quality Control Test of Pharmaceutical Solid Dosage FormDocumento31 pagineQuality Control Test of Pharmaceutical Solid Dosage FormMoriyom Akther77% (22)
- Design and Analysis of Powder Mixing Ribbon Blender - A ReviewDocumento5 pagineDesign and Analysis of Powder Mixing Ribbon Blender - A ReviewjohnsonregoNessuna valutazione finora
- Powder 1Documento71 paginePowder 1afsana mamedovaNessuna valutazione finora
- Scholars Research Library: A Review: On Scale-Up Factor Determination of Rapid Mixer GranulatorDocumento16 pagineScholars Research Library: A Review: On Scale-Up Factor Determination of Rapid Mixer GranulatorSartha KumarNessuna valutazione finora
- Pravin B. Awate (M. Pharm Pharmaceutics) Assistant Professor Rdcop, BhorDocumento27 paginePravin B. Awate (M. Pharm Pharmaceutics) Assistant Professor Rdcop, BhorPravin AwateNessuna valutazione finora
- Powder Mixing ReportDocumento12 paginePowder Mixing ReportKhaledAhmedNessuna valutazione finora
- LABORATORY 4: Preparation of Tablets and Factors Influencing Tablets QualitiesDocumento10 pagineLABORATORY 4: Preparation of Tablets and Factors Influencing Tablets QualitiesSara JuitaNessuna valutazione finora
- PharmaceutisDocumento36 paginePharmaceutismohamedNessuna valutazione finora
- Roller CompactionDocumento14 pagineRoller CompactionAddi Rashe'dNessuna valutazione finora
- PDF ManualDocumento52 paginePDF ManualAnil Singh Rajput100% (1)
- By Chetana ModiDocumento30 pagineBy Chetana ModiGadi Srinath ReddyNessuna valutazione finora
- Ten Things You Need To Consider When Choosing and Installing A Roller Press SystemDocumento8 pagineTen Things You Need To Consider When Choosing and Installing A Roller Press SystemMani MaranNessuna valutazione finora
- Powders and GranulesDocumento4 paginePowders and GranulesMXLTR100% (1)
- Content Uniformity of Direct Compression TabletsDocumento12 pagineContent Uniformity of Direct Compression Tabletsanggi yudhatamaNessuna valutazione finora
- Malvern3 PDFDocumento6 pagineMalvern3 PDFGururaj VasudevamurthyNessuna valutazione finora
- Particle Size ReductionDocumento13 pagineParticle Size ReductionMarcel MrcNessuna valutazione finora
- Tech Library Poster11Documento1 paginaTech Library Poster11Pépé TechopathamNessuna valutazione finora
- Milling and Size ReductionDocumento6 pagineMilling and Size ReductionYasir MahmoodNessuna valutazione finora
- Industrial Lab ManualDocumento98 pagineIndustrial Lab Manualosama2010bNessuna valutazione finora
- Quality ControlDocumento34 pagineQuality ControlNathalie OmeroNessuna valutazione finora
- PMPS Spring 2005 MicronDocumento4 paginePMPS Spring 2005 MicronJorge DominguezNessuna valutazione finora
- Tablet and Capsule ManufacturingDocumento28 pagineTablet and Capsule ManufacturingShibaprasad DandapatNessuna valutazione finora
- 2020 09 Hosseinzadeh Population and GranulationDocumento8 pagine2020 09 Hosseinzadeh Population and GranulationHQ EntertainmentNessuna valutazione finora
- Mixing of Solids in Different Mixing DevicesDocumento11 pagineMixing of Solids in Different Mixing DevicesKhevym Escobar DzxNessuna valutazione finora
- Mixing-Powders & Granules: Chapter 6 of Text - PG 168-88Documento75 pagineMixing-Powders & Granules: Chapter 6 of Text - PG 168-88Larry JeffNessuna valutazione finora
- Pharma ProcessDocumento16 paginePharma ProcessDeepakraj BansalNessuna valutazione finora
- Effect of Size Reduction On The Extraction of Palm Kernel OilDocumento41 pagineEffect of Size Reduction On The Extraction of Palm Kernel OilAppeal MatthewNessuna valutazione finora
- Solid Incrementation ReportDocumento5 pagineSolid Incrementation ReportCami TamayoNessuna valutazione finora
- Optimum Tablet Press Optimization - Machine Versus GranulationDocumento10 pagineOptimum Tablet Press Optimization - Machine Versus GranulationbookulNessuna valutazione finora
- GranulationDocumento32 pagineGranulationSantosh Payghan100% (1)
- Internal MixerDocumento5 pagineInternal MixerFaisal IqbalNessuna valutazione finora
- MEP Expert Guide Sieving enDocumento52 pagineMEP Expert Guide Sieving enM. Ali ParvezNessuna valutazione finora
- Unit 3 Size ReductionDocumento33 pagineUnit 3 Size ReductionM.sathishkumarNessuna valutazione finora
- HistDocumento32 pagineHistSakshi LodhiNessuna valutazione finora
- Granulation Process Basic UnderstandingDocumento3 pagineGranulation Process Basic UnderstandingRainMan75Nessuna valutazione finora
- Spiral Jet MillDocumento8 pagineSpiral Jet MillĐức Linh100% (1)
- Solid Liquid Separation - Centrifugal FiltrationDocumento12 pagineSolid Liquid Separation - Centrifugal FiltrationAminEsmaeiliNessuna valutazione finora
- Disolusi Ketoprofen3Documento12 pagineDisolusi Ketoprofen3Teddy KeccowNessuna valutazione finora
- Powder Dosage Form Part IIDocumento13 paginePowder Dosage Form Part IIEman AzizNessuna valutazione finora
- Tablet Manufacturing: Industrial Pharmacy 5 Class 1 SemesterDocumento23 pagineTablet Manufacturing: Industrial Pharmacy 5 Class 1 SemesterShirzad HasanNessuna valutazione finora
- Komar University: of Science and TechnologyDocumento18 pagineKomar University: of Science and TechnologyAsma GhazyNessuna valutazione finora
- Skill Development of Tablet PressDocumento29 pagineSkill Development of Tablet PressSakshi LodhiNessuna valutazione finora
- Pharmaceutical Analysis 2008PHM Laboratory Manual - 2014 REPORTDocumento22 paginePharmaceutical Analysis 2008PHM Laboratory Manual - 2014 REPORTTales FernandoNessuna valutazione finora
- MICROMERITCSDocumento11 pagineMICROMERITCSWaqar Khan AttariNessuna valutazione finora
- Formulation Development and Evaluation of New AlbeDocumento12 pagineFormulation Development and Evaluation of New AlbeDương TrungNessuna valutazione finora
- Journal of Drug Delivery and Therapeutics: Role of Naturally Isolated Pear Starch On The Bioavailability of FamotidineDocumento6 pagineJournal of Drug Delivery and Therapeutics: Role of Naturally Isolated Pear Starch On The Bioavailability of FamotidineyunitaknNessuna valutazione finora
- Bioavailability of DrugsDocumento17 pagineBioavailability of DrugsLodhi MohasinNessuna valutazione finora
- Development of Nanostructured Lipid Carriers Loaded With Corosolic Acid An Efficient Carrier For Antidiabetic Effects PDFDocumento5 pagineDevelopment of Nanostructured Lipid Carriers Loaded With Corosolic Acid An Efficient Carrier For Antidiabetic Effects PDFVidyavardhini UshirNessuna valutazione finora
- Starch CapsulesDocumento6 pagineStarch Capsuleskunalprabhu148100% (1)
- Chemistry of Pectin and Its Pharmaceutical Uses: A Review: January 2003Documento24 pagineChemistry of Pectin and Its Pharmaceutical Uses: A Review: January 2003Madalina CalcanNessuna valutazione finora
- Dissolution Equipment PDFDocumento48 pagineDissolution Equipment PDFRajesh AkkiNessuna valutazione finora
- Bioav 3Documento264 pagineBioav 3Sabiruddin Mirza DipuNessuna valutazione finora
- Group 28 - PharmaceuticalsDocumento49 pagineGroup 28 - PharmaceuticalsRehan TyagiNessuna valutazione finora
- Advanced Pharmaceutical Analysis: Hod: Dr. C. Sreedhar Presented By: Kshitiz K. GaundDocumento36 pagineAdvanced Pharmaceutical Analysis: Hod: Dr. C. Sreedhar Presented By: Kshitiz K. GaundIndiraNessuna valutazione finora
- SwitraDocumento6 pagineSwitradrbhaveshpNessuna valutazione finora
- Chapter 7 Summ.& Concl.Documento2 pagineChapter 7 Summ.& Concl.SAI ASSOCIATENessuna valutazione finora
- ECA Dissolution TestingDocumento6 pagineECA Dissolution Testinghosein bagheriNessuna valutazione finora
- The Maximal Electroshock Seizure (Mes) Model in The Pre Clinical Assessment of Potential New Anti Epileptic DrugsDocumento6 pagineThe Maximal Electroshock Seizure (Mes) Model in The Pre Clinical Assessment of Potential New Anti Epileptic DrugsewqewqewqewqewqNessuna valutazione finora
- Simulate SubQ - The Methods and The MediaDocumento17 pagineSimulate SubQ - The Methods and The MediaLaudaaNessuna valutazione finora
- Sustained Release Drug Delivery SystemDocumento8 pagineSustained Release Drug Delivery Systemsyed fahadNessuna valutazione finora
- Ash Michael Ash Irene Handbook of Pharmaceutical Additives PDFDocumento2.995 pagineAsh Michael Ash Irene Handbook of Pharmaceutical Additives PDFivanlawms6745100% (1)
- Report On PreformulationDocumento9 pagineReport On PreformulationH FaithNessuna valutazione finora
- Efficacy of Agents To Prevent and Treat Enteral Feeding Tube ClogsDocumento5 pagineEfficacy of Agents To Prevent and Treat Enteral Feeding Tube Clogsmariosan81Nessuna valutazione finora
- Quality by Design (QBD) - Complete ReviewDocumento9 pagineQuality by Design (QBD) - Complete ReviewDrSajithChandranNessuna valutazione finora
- DEC Study in Formulation DevelopmentDocumento9 pagineDEC Study in Formulation Developmentfad12345Nessuna valutazione finora
- Ritalin ADocumento8 pagineRitalin ALeonardo David Dearo SimonettiNessuna valutazione finora
- Drug Stability and DegradationDocumento15 pagineDrug Stability and DegradationPuspa DasNessuna valutazione finora
- Critical Materrial Attributes in QBD TIPADocumento47 pagineCritical Materrial Attributes in QBD TIPAsurapolNessuna valutazione finora
- Pharmaceutical Dosage Forms Answer Key BLUE and PINK PACOPDocumento115 paginePharmaceutical Dosage Forms Answer Key BLUE and PINK PACOPAlexios Deimos (alxios)Nessuna valutazione finora
- Dosage Form UspDocumento30 pagineDosage Form UspDeepakNessuna valutazione finora
- Pharmacology Lab ManualDocumento73 paginePharmacology Lab ManualRonald Darwin58% (12)
- Didanosine PDFDocumento6 pagineDidanosine PDFFilda SetyaNessuna valutazione finora
- Development and Characterization of Orally Disintegrating Tablets Containing A Captopril-Cyclodextrin ComplexDocumento19 pagineDevelopment and Characterization of Orally Disintegrating Tablets Containing A Captopril-Cyclodextrin ComplexValentina AnutaNessuna valutazione finora
- The Story of the World, Vol. 1 AudiobookDa EverandThe Story of the World, Vol. 1 AudiobookValutazione: 4.5 su 5 stelle4.5/5 (3)
- Dumbing Us Down: The Hidden Curriculum of Compulsory SchoolingDa EverandDumbing Us Down: The Hidden Curriculum of Compulsory SchoolingValutazione: 4.5 su 5 stelle4.5/5 (497)
- How to Talk to Anyone: Learn the Secrets of Good Communication and the Little Tricks for Big Success in RelationshipDa EverandHow to Talk to Anyone: Learn the Secrets of Good Communication and the Little Tricks for Big Success in RelationshipValutazione: 4.5 su 5 stelle4.5/5 (1135)
- Weapons of Mass Instruction: A Schoolteacher's Journey Through the Dark World of Compulsory SchoolingDa EverandWeapons of Mass Instruction: A Schoolteacher's Journey Through the Dark World of Compulsory SchoolingValutazione: 4.5 su 5 stelle4.5/5 (149)
- Summary: Trading in the Zone: Trading in the Zone: Master the Market with Confidence, Discipline, and a Winning Attitude by Mark Douglas: Key Takeaways, Summary & AnalysisDa EverandSummary: Trading in the Zone: Trading in the Zone: Master the Market with Confidence, Discipline, and a Winning Attitude by Mark Douglas: Key Takeaways, Summary & AnalysisValutazione: 5 su 5 stelle5/5 (15)
- Summary: The Laws of Human Nature: by Robert Greene: Key Takeaways, Summary & AnalysisDa EverandSummary: The Laws of Human Nature: by Robert Greene: Key Takeaways, Summary & AnalysisValutazione: 4.5 su 5 stelle4.5/5 (30)
- Stoicism The Art of Happiness: How the Stoic Philosophy Works, Living a Good Life, Finding Calm and Managing Your Emotions in a Turbulent World. New VersionDa EverandStoicism The Art of Happiness: How the Stoic Philosophy Works, Living a Good Life, Finding Calm and Managing Your Emotions in a Turbulent World. New VersionValutazione: 5 su 5 stelle5/5 (51)
- The 16 Undeniable Laws of Communication: Apply Them and Make the Most of Your MessageDa EverandThe 16 Undeniable Laws of Communication: Apply Them and Make the Most of Your MessageValutazione: 5 su 5 stelle5/5 (73)
- How to Improve English Speaking: How to Become a Confident and Fluent English SpeakerDa EverandHow to Improve English Speaking: How to Become a Confident and Fluent English SpeakerValutazione: 4.5 su 5 stelle4.5/5 (56)
- Learn Spanish While SleepingDa EverandLearn Spanish While SleepingValutazione: 4 su 5 stelle4/5 (20)
- Summary: Dotcom Secrets: The Underground Playbook for Growing Your Company Online with Sales Funnels by Russell Brunson: Key Takeaways, Summary & Analysis IncludedDa EverandSummary: Dotcom Secrets: The Underground Playbook for Growing Your Company Online with Sales Funnels by Russell Brunson: Key Takeaways, Summary & Analysis IncludedValutazione: 5 su 5 stelle5/5 (2)
- Cynical Theories: How Activist Scholarship Made Everything about Race, Gender, and Identity―and Why This Harms EverybodyDa EverandCynical Theories: How Activist Scholarship Made Everything about Race, Gender, and Identity―and Why This Harms EverybodyValutazione: 4.5 su 5 stelle4.5/5 (221)
- Learn Japanese - Level 1: Introduction to Japanese, Volume 1: Volume 1: Lessons 1-25Da EverandLearn Japanese - Level 1: Introduction to Japanese, Volume 1: Volume 1: Lessons 1-25Valutazione: 5 su 5 stelle5/5 (17)
- Summary: I'm Glad My Mom Died: by Jennette McCurdy: Key Takeaways, Summary & AnalysisDa EverandSummary: I'm Glad My Mom Died: by Jennette McCurdy: Key Takeaways, Summary & AnalysisValutazione: 4.5 su 5 stelle4.5/5 (2)
- Summary: The 5AM Club: Own Your Morning. Elevate Your Life. by Robin Sharma: Key Takeaways, Summary & AnalysisDa EverandSummary: The 5AM Club: Own Your Morning. Elevate Your Life. by Robin Sharma: Key Takeaways, Summary & AnalysisValutazione: 4.5 su 5 stelle4.5/5 (22)
- Rooted in Joy: Creating a Classroom Culture of Equity, Belonging, and CareDa EverandRooted in Joy: Creating a Classroom Culture of Equity, Belonging, and CareNessuna valutazione finora
- Summary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisDa EverandSummary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisValutazione: 5 su 5 stelle5/5 (3)
- Why Smart People Hurt: A Guide for the Bright, the Sensitive, and the CreativeDa EverandWhy Smart People Hurt: A Guide for the Bright, the Sensitive, and the CreativeValutazione: 3.5 su 5 stelle3.5/5 (54)
- Make It Stick by Peter C. Brown, Henry L. Roediger III, Mark A. McDaniel - Book Summary: The Science of Successful LearningDa EverandMake It Stick by Peter C. Brown, Henry L. Roediger III, Mark A. McDaniel - Book Summary: The Science of Successful LearningValutazione: 4.5 su 5 stelle4.5/5 (55)
- Follow your interests: This will make you feel better about yourself and what you can do.: inspiration and wisdom for achieving a fulfilling life.Da EverandFollow your interests: This will make you feel better about yourself and what you can do.: inspiration and wisdom for achieving a fulfilling life.Nessuna valutazione finora
- Little Soldiers: An American Boy, a Chinese School, and the Global Race to AchieveDa EverandLittle Soldiers: An American Boy, a Chinese School, and the Global Race to AchieveValutazione: 4 su 5 stelle4/5 (25)
- Summary: Greenlights: by Matthew McConaughey: Key Takeaways, Summary & AnalysisDa EverandSummary: Greenlights: by Matthew McConaughey: Key Takeaways, Summary & AnalysisValutazione: 4 su 5 stelle4/5 (6)
- Follow The Leader: A Collection Of The Best Lectures On LeadershipDa EverandFollow The Leader: A Collection Of The Best Lectures On LeadershipValutazione: 5 su 5 stelle5/5 (122)
- Taking Charge of ADHD: The Complete, Authoritative Guide for ParentsDa EverandTaking Charge of ADHD: The Complete, Authoritative Guide for ParentsValutazione: 4 su 5 stelle4/5 (17)
- Financial Feminist: Overcome the Patriarchy's Bullsh*t to Master Your Money and Build a Life You LoveDa EverandFinancial Feminist: Overcome the Patriarchy's Bullsh*t to Master Your Money and Build a Life You LoveValutazione: 5 su 5 stelle5/5 (1)