Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Tese Nswparte 2
Caricato da
AndrelslDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Tese Nswparte 2
Caricato da
AndrelslCopyright:
Formati disponibili
CHAPTER 1 Introduction
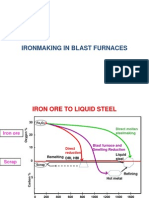
The basic oxygen converter is the main process routes for making steel [1], which
requires a charge of molten iron. The blast furnace is the most important supplier of
molten iron for the steelmaking industry as it is the most efficient ironmaking process in
terms of high productivity, operational reliability and cost competitiveness [2].
However, the blast furnace is a major source of greenhouse gas emissions and also a
large energy consumer. In order to make the blast furnace more sustainable new
technologies [1,3] have been or are being implemented to improve furnace efficiency,
reduce energy consumption and lower the greenhouse gas emissions.
Coke is the most expensive raw material in the blast furnace and its production results
in high levels of greenhouse gas emissions [4]. One way to reduce coke consumption is
to inject supplementary fuels (coal, natural gas, oil) at the tuyere levels of the blast
furnace [5]. These supplementary fuels partially replace coke as a supplier of heat and
reductant gases. However, coke cannot be replaced entirely because it is still needed to
provide both mechanical support to the charge column and act as a permeable bed for
molten iron, slag and gases. Fuel injection implies an increase of the residence time of
coke in the furnace; the mechanical and chemical conditions that coke is subjected to
are much more severe and coke degradation increases[6,7]. Therefore coke quality must
change in order to address the requirements of blast furnace operation with low coke
rate [8].
The Low Temperature Compact Blast Furnace [3] and the Nitrogen Free Blast Furnace
under the European ULCOS (Ultra Low CO
2
Emission Steelmaking) program [1,9] are
two new emerging technologies for blast furnace operation. The Low Temperature
Compact Blast Furnace aim is halving the energy consumption and increase blast
1
Chapter 1 Introduction
furnace efficiency. The goal of the Nitrogen Free Blast Furnace is to drastically lower
greenhouse gas emissions.
The concept of the Low Temperature Compact Blast Furnace is based on lowering the
temperature of the thermal reserve zone. Yagi et al. [3] and Naito et al. [10] have shown
that lowering the temperature of iron oxide reduction from 1000C to 800-900C, using
a highly reactive coke, can improve reduction efficiency and effectively lower the
energy consumption. However, coke gasification at higher rates can lead to a reduction
in coke strength [3], lessening its ability to provide support for the ferrous burden and
permeability for gas and liquids.
The design of the N
2
Free Blast Furnace is to operate nitrogen-free. The oxidizing gas is
a mixture of oxygen with carbon dioxide and carbon monoxide recycled from the
(filtered) furnace top gas. One advantage of this oxy-firing is that oxygen levels can be
increased to approximately 35% without increasing flame temperatures excessively
because carbon dioxide has a higher heat capacity than nitrogen leading to reduced gas
flow in the furnace. However, because of the high carbon oxide levels in the gas stream,
the reaction conditions and thermal profile in the nitrogen-free furnace are expected to
be very different to those in conventional systems.
The fuel injection blast furnace and both Low Temperature Compact Blast Furnace and
the N
2
Free Blast Furnace require cokes of specific quality for efficient operation of the
furnace. Although the operation of the blast furnace is defined by specific parameters
and quality of the raw materials for each technology, a high strength coke is a common
requirement for all these technologies. Strength is the most important quality of coke
because coke provides mechanical support to the charge column, influences gas
distribution in the shaft of the furnace and act as a permeable bed for liquids (molten
iron and slag) and gases.
A poor quality coke significantly impacts the blast furnace operation (Figure 1.1).
Cheng [11] and Gudeneau et al. [12] presented in their reviews the implications on the
blast furnace operation by using a poor quality coke:
2
Chapter 1 Introduction
- reduces permeability in the furnace due to reduction in the mean size of the coke
lumps and increases the size range of the coke lumps;
- changes the top temperature distribution by increasing the wall temperature which
increases the thermal load on the stack refractory;
- severely degrades the bosh and hearth regions triggering flooding;
- long casting times and cut tuyeres;
- produces thermal imbalance and instability in the furnace;
- increases the flue dust levels.
Coke is subjected to degradation as it descends in the furnace due to the action of
factors such as: coke gasification reactions, mechanical load, attrition, thermal stress,
alkali attack, and graphitization. Gasification is one of the most important factors in
coke degradation. At low temperatures (less than 1000C) gasification produces deeper
weakening of the coke lumps [5,13], which increases the formation of fines [14] and
produces fissures in the lumps making their fragmentation easier [7,15]. As temperature
increases gasification of the coke lumps occurs mostly at the surface, which increases
coke abradability, resulting in a reduction in size of the coke lumps [7,15,16].
3
Chapter 1 Introduction
Figure 1.1 The influence of coke quality in the blast furnace [11].
4
Chapter 1 Introduction
5
The coke gasification rate in the blast furnace is controlled by its intrinsic properties and
the operating conditions of the furnace [13,15,17,18,19], in terms of gas composition,
temperature and catalysts. A significant insight into coke gasification has been provided
by previous research. Coke properties such as coke microtexture, porosity and mineral
matter have been reported as indicators of coke reactivity [13,17,18,20,21,22,23,24,25].
Although there is some agreement in the literature in the way coke properties such as
coke microtexture and porosity affects gasification rate, there are still inconsistencies
and less investigated aspects in this area, such as the effect of the inherent catalytic
minerals in coke on reactivity. In order to prepare cokes of suitable quality for each new
technology of operation of blast furnace a very good understanding of the factors that
affect coke reactivity is required. As such, investigation of the less explored coke
properties and the assessment of their influence on coke reactivity are required so that
the most dominant factors affecting coke reactivity can be determined.
CHAPTER 2 Literature Review
2.1 Blast furnace
2.1.1 Overview of the Blast Furnace process coke importance in the furnace
The blast furnace is basically a counter-current reactor; raw materials are charged into
the furnace top, molten products are tapped from the bottom and gases pass from
bottom to the top of the furnace. The raw materials consist of ferrous materials (iron ore
as sinter or pellets), fuel (coke) and fluxes (limestone, dolomite). Other materials, like
coal, oil and natural gas may be co-injected with air through the tuyeres at the base of
the furnace. The reducing gases and heat required for the process are generated at the
bottom of the furnace by combustion of the fuels. These pass upward, counter-current to
the raw materials, exiting at the furnace top after imparting heat and enabling the
required chemical reactions on the raw materials. The main product of the blast furnace
is molten pig iron and the by-products are molten slag and gas. Iron is produced via
direct and indirect reduction of iron oxide. Coke is the main source of carbon, for the
direct reduction, and carbon monoxide, for the indirect reduction.
The blast furnace can be divided into six zones, from the relatively cool zone at the top,
to the intensely hot zone at the bottom: Granular zone (Solid zone), Cohesive zone
(Softening-Melting zone), Active Coke zone, Raceway, Hearth and Deadman (Stagnant
Coke zone) (Figure 2.1).
In the Granular zone the raw materials, layers of coke and iron ore, must ensure a
uniform distribution of the ascending gases. The temperature in this zone is up to
1000C.
6
Chapter 2 Literature Review
The Cohesive zone consists of alternate layers of coke and viscous, semi-fused mass of
slag and iron, through which the ascending gases are unable to flow. The permeable
coke layers, called coke slits, act as gas distributors and permit the gas to flow
horizontally through them. Since most of the gas has to pass through the coke slits, the
slits must be permeable and hence great importance is attached to the resistance of coke
to breakage.
The Active Coke zone contains loosely packed coke, feeding the raceway, and droplets
of iron and slag dripping into deadman and hearth. The temperature in this zone is about
1600C.
The Raceway is the volume around the hearth periphery where coke is burned. The heat
produced by coke burning gives a flame temperature of 1800-2000C.
The loose packed central coke column is called the Deadman zone. The solid coke
maintains an open bed through which the descending liquid iron and slag and ascending
reducing gases can pass. In this zone, for coke to ensure permeability, the coke lumps
must be of a specific mean size and size range and also have enough strength to resist
degradation [11].
The molten iron and slag are cast at regular intervals from the Hearth, where the
temperature is around 1500C.
Based upon the above information the role of coke in the blast furnace can be grouped
into three categories: thermal, chemical and physical as described below:
The thermal role of coke is to provide heat for endothermic reactions and melting of
iron and slag.
The chemical role is to produce carbon monoxide for the reduction of the iron oxides
and to provide carbon for both direct reduction and iron carburisation.
The physical role of coke is: to provide a mechanical support to the charge column,
influence gas distribution in the shaft of the blast furnace and act as a permeable bed,
allowing metal and slag to pass down and the gases to pass up.
7
Chapter 2 Literature Review
Figure 2.1 Schematic of the Blast Furnace.
8
Chapter 2 Literature Review
2.1.2 Coke gasification in the blast furnace
Hot air is blown through the tuyeres into the blast furnace it reacts immediately with
coke to form carbon dioxide.
C + O
2
CO
2
Reaction 2.1
The reaction is strongly exothermic and supplies much of the energy necessary to
reduce iron oxides to metallic iron. Because carbon dioxide is unstable in the presence
of coke at temperatures over 1000C it is converted to carbon monoxide [5,6] according
to the reaction below:
CO
2
+ C 2CO
Reaction 2.2
This reaction is known as the Boudouard reaction, gasification reaction or solution loss
reaction. The Boudouard reaction provides the reducing agent for the iron oxides. The
tuyere gases, which consist mainly of carbon monoxide, nitrogen and small amounts of
steam [5,6], leave the combustion zone and pass up through the burden to the top of the
furnace.
Above the cohesive zone wustite (FeO) is reduced to iron by carbon (direct reduction) at
temperatures greater than 1000C (Reaction 2.3) and carbon monoxide (indirect
reduction) at temperatures lower than 1000C (Reaction 2.4) [5]. Carbon dioxide
generated by the indirect reduction gasifies the lumps of coke.
FeO + C Fe + CO Reaction 2.3
FeO + CO Fe + CO
2
Reaction 2.4
Due to the endothermic nature of the direct reduction reaction and coke gasification
reactions the temperature of the ascending gases decreases. As the gases ascend in the
furnace, the concentration of carbon monoxide decreases because of indirect reduction
of wustite, which produces carbon dioxide. Another source of carbon dioxide is
9
Chapter 2 Literature Review
reduction of magnetite (Fe
3
O
4
) and hematite (Fe
2
O
3
) by carbon monoxide in the Solid
zone of the blast furnace (Reactions 2.5 and 2.6, respectively).
Fe
3
O
4
+ CO 3FeO + CO
2
Reaction 2.5
3Fe
2
O
3
+ CO 2Fe
3
O
4
+ CO
2
Reaction 2.6
About 70% of the iron oxides are reduced by indirect reactions with carbon monoxide
and hydrogen (in a small contribution) before the cohesive zone (1250-1300C);
whereas the remaining 30% of FeO is reduced via the direct reduction with coke within
the cohesive zone [14]. The gases that exit from the top of the conventional furnace are
mostly formed of 20-30% CO, 10-20% CO
2
and the rest is nitrogen [5]. Biswas [5]
divided the blast furnace along its height into three main zones based upon the
temperature and type of reaction that occurs within these zones, namely Preheating
Zone, Thermal Reserve Zone and Direct Reduction and Melting Zone (Figure 2.2).
The total amount of solution loss in the blast furnace is mainly determined by the
carbon dioxide concentration which itself is controlled by the availability of oxygen
from iron oxides [15]. Goleczka and Tucker [26] and Barnaba [14] assumed that coke
gasification in the blast furnace due to solution loss is about 20-30% and 25%,
respectively.
Hatano et al. [16] created a mathematical model to find the temperature range at which
the solution loss reaction occurs in the blast furnace. They believe that the temperature
where gasification starts is affected by coke size, coke reactivity and the alkali content
in the coke ash. They consider the beginning temperature of the zone of solution loss
reaction is the temperature at which the reaction rate is 410
-5
gg
-1
min
-1
. This
temperature increases with increasing coke size, decreasing coke reactivity and
decreasing content of alkali in the coke ash. The maximum temperature in the zone of
solution loss reaction is 1400C. Van der Velden et al. [18] and Hutny et al. [6]
assumed that the reaction of coke with carbon dioxide in conditions similar to those in
blast furnace begins at temperatures around 900C and Gill et al. [25] believe that the
solution loss reaction becomes significant at a temperature range about 950-1100C.
10
Chapter 2 Literature Review
Figure 2.2 A theoretical diagram of temperature distribution of gas and solids
along the height of the blast furnace and the chemical reactions within
these zones [5].
11
Chapter 2 Literature Review
The gasification rate increases with temperature from 1000-1200C whether in pure
carbon dioxide or gas mixtures [7,18,26]. In pure carbon dioxide the rate of reaction
increases when the temperature is increased to 1300C [16]. However, Van der Velden
et al. [18] observed that the gasification rate in gas mixtures similar to those present in
the blast furnace reached a maximum at 1200C after wich the reaction rate decreased.
They concluded that the decrease of carbon dioxide concentration in the gas as the
temperature increases has a much stronger effect on the reaction rate than increasing
temperature in the furnace. Negro et al. [15] also considered that the total amount of
solution loss in the blast furnace is largely determined by the carbon dioxide
concentration which itself is controlled by the availability of oxygen from iron oxides.
2.2 Coke characterisation
Metallurgical coke can be described as a porous solid material comprising an organic
part, which is mainly carbon and small amounts of sulfur, nitrogen, hydrogen and
oxygen, and an inorganic part (approximately 10%). The organic part of the coke is
characterised by two properties namely microtexture and microstructure, which were
defined by Coin [27]:
Microstructure refers to the physical and spatial relation of the coke material,
that is, porosity, pore sizes, pore wall thickness etc., whereas Microtexture
refers to the nature of the carbon in the coke, its crystallite development, degree
of optical anisotropy etc. [classified by optical microscopy]
The characteristics of the organic and inorganic components of the coke will be
discussed in the following sections.
12
Chapter 2 Literature Review
2.2.1 Coke microtexture
Microtexture is a description of the organic matter given by optical microscopy. Under
optical microscope examination, the coke microtexture is classified as anisotropic or
isotropic carbon. When isotropic carbon is exposed to polarised light it does not produce
variation in the wavelength of the reflected polarised light; it originates from coal
macerals which did not fuse during carbonisation process (fusinite and macrinite) and
fused vitrinite from low rank coals [27]. The anisotropic carbon causes variation in the
wavelength of the reflected polarized light and characterises the macerals fused during
carbonisation.
The anisotropic microtexture is itself classified by its size, shape and form of the
textural unit. Both the nomenclature and classification of the anisotropic carbon vary
between different laboratories (Figure 2.3). Since there is a large variation of the
definitions of anisotropy between different groups of researchers, it is important to be
aware of these differences when the results are compared.
The rank of the parent coal affects the microtexture of the product coke. Low rank coals
make cokes with predominantly isotropic microtexture. As the rank of the coal increases
different types of anisotropic microtexture such as fine, medium and coarse mosaic
units and flow types, gradually replace the isotropic microtexture in the coke [28,29].
Cokes made from high rank coals do not usually contain significant amounts of both
isotropic and fine mosaic microtextures [21,28]. The microtexture of metallurgical coke
is characterised by a high proportion of mosaic microtexture [30,31]. These
microtextural variations are illustrated in Figure 2.4.
Although optical microscopy is a widely used technique to characterize cokes it
nevertheless has limitations because of its low resolution (approximately 0.3m [32]).
Transmission Electron Microscopy (TEM) is another imaging technique used for coke
microtexture characterisation. TEM examination supplements optical microscopy by
examination on a size scale not reachable by optical techniques (down to 0.8 nm) [33].
13
Chapter 2 Literature Review
Figure 2.3 Nomenclature and classification of the anisotropic carbon by different
laboratories [27].
14
Chapter 2 Literature Review
(a) (b) (c)
Figure 2.4 Coke microtextures: a) Isotropic (I) and Very Fine Mosaic (VF); b)
Very Fine Mosaic (VF) and Fine Mosaic (F) and c) Medium Mosaic
(M) and Flow-like anisotropic microtexture (FA) [21].
A description of coal and coke microtexture using TEM was given by Oberlin and
Rouzaud in several studies [34,35,36]. They concluded that coke microtexture consists
of polyaromatic basic structural units (BSU) with size about 1 nm, formed by
polyaromatic layers (4 to 10 rings) isolated or stacked by 2 or 3. The BSUs are ordered
in stacked planes of the aromatic layers, named molecular orientation domains (MOD)
or local molecular orientation (LMO) [28,34]. A graphic representation of coke
microtexture is shown in Figure 2.5. Inside the molecular orientation domains (MODs)
the polyaromatic basic structural units (BSUs) are either misoriented or locally
orientated in parallel [37].
Coke microtexture is characterised by MODs of different sizes, varying from 5 nm to a
few micrometers [28]. The isotropic microtexture observed using optical microscopy is
discriminated by TEM into eight categories as a function of MOD size (Figure 2.6). The
optically anisotropic microtexture is also differentiated by TEM into crumpled lamellae
and planar lamellae. The quantification of coke microtexture is represented by a
frequency histogram of MODs categories.
15
Chapter 2 Literature Review
Figure 2.5 Schematic presentation of MOD [38].
Figure 2.6 Classification of Molecular Orientation Domains [36].
16
Chapter 2 Literature Review
X-ray diffraction is another technique that characterises the atomic level structure of the
carbonaceous materials. The X-ray diffraction (XRD) pattern of a carbonaceous
material such as coke shows diffuse peaks that correspond to (002), (100) and (110)
reflections of graphite and strong low-angle scattering [39]. The diffuse peaks of (002),
(100) and (110) reflections indicate the presence of small graphite-like domains [39].
The non-crystalline carbon (amorphous carbon) forms the background intensity of X-
ray diffraction pattern.
The crystalline structure of graphite consists of flat polycondensed aromatic layers,
which are known as lamellae, ordered in parallel to form a crystallite [36] (Figure 2.7).
The graphite crystallite is usually layered in the form ABABAB. The X-ray diffraction
(XRD) pattern of graphite is dominated by the position of the (002), (100) and (110)
reflections. The (002) reflection indicates the stacking of the aromatic layers and both
the (100) and (110) reflections correspond to the two-dimensional lattices of the
aromatic layers. The crystallographic parameters which can be determined using the
XRD pattern are both the height (L
c
) and length (L
a
) of the crystallite and the interlayer
spacing (d) (Figure 2.7). The crystallite height and the distance between lamellae in the
coke depend on the rank of the parent coal; as the coal rank increases the crystallite
height increases and the distance between lamellae decreases [36].
The mean crystallite size can be determined using the Scherrer equation [40]:
Bcos
K
L
c/a
= Equation 2.1
Where K is a constant depending on the reflection plane (0.89 for (002) band and 1.84
for (110) band [41]), is the wavelength of the incident radiation, B is the width of the
peak at half-maximum intensity and is the peak position.
17
Chapter 2 Literature Review
Figure 2.7 A schematic picture of a crystallite of graphite [42].
2.2.2 Coke microstructure
Cokes have pores with a wide range of sizes, from less than 1 nanometre to several
hundred microns [43]. A classification of the pore structure is given by Dubinin [44]:
micropores (the effective radii from 0.5-0.6 nm to 1.3-1.4 nm), mesopores (the effective
radii between 1.5-1.6 nm and 100-200 nm) and macropores (the effective radii over
100-200 nm). Total porosity includes the empty spaces left between the different carbon
microtextures (each MOD forms a pore wall Figure 2.5) [37], large pores
(macropores) formed due to release of volatile matter during carbonisation and the
fissures produced by internal stress in the coke [23]. SEM images of cokes show that
pores greater than 0.1 - 5 microns are of different shapes such as circular, elliptical,
rectangular, triangular and slit-like pore sections [43]. Also blind pores have been
observed.
Coke microstructure is developed during the carbonisation. Its formation is affected by
the rank of the parent coal, fluidity, the amount of reactive macerals and coking rate
[45]. For instance, low rank coals (high-volatile coals) with good fluidity make cokes
18
Chapter 2 Literature Review
with high porosity (55-60 vol%) and mean pores size larger than 200 m, whereas
medium rank coals produce cokes less porous (<54%) and smaller pore size (about 125
m) [46].
2.2.3 Mineral matter
The mineral matter content in coke is typically around 8-12% [47]. The mineralogical
composition of coke is different to that of the parent coal. During carbonisation some
minerals decompose and also reactions between minerals occur.
Minerals identified in metallurgical cokes by previous studies are quartz (SiO
2
), iron
oxides, mullite (Al
6
Si
2
O
13
), fluorapatite (Ca
5
(PO
4
)3F), pyrrhotite (Fe
1-x
S), brookite
(TiO
2
), anatase (TiO
2
), cristobalite (SiO
2
), alkali feldspars ((K,Na)(AlSi
3
O
8
)) and
aluminosilicates [23,48,49]. A recent study made on eleven metallurgical cokes from
different international sources identified the following additional minerals: akermanite
(Ca
2
MgSi
2
O
7
), anorthite ((Ca,Na)(Si,Al)
4
O
8
), calcium iron oxide (CaFe
2
O
4
), diopside
(CaMgSi
2
O
6
), fayalite ((Fe,Mg)
2
SiO
4
), gehlenite (Ca
2
Al
2
SiO
7
), metallic iron, oldhamite
(CaS) and rutile (TiO
2
) [50].
The aluminosilicates present in cokes are formed due to decomposition of clays such as
kaolinite, illite, montmorillonite and chlorite, during carbonisation [48,51]. Quartz,
fluorapatite, anatase and brookite are minerals that originate from the parent coals and
are relatively unaffected by the coking process. Alkali feldspars have been identified in
cokes by Mahoney et al. [48] and also in the bituminous coals [23] but they are affected
to some extent by the carbonisation process.
Some minerals have been identified to have an impact on the size of the anisotropic
microtexture formed in cokes. Gray and Champagne [52] and Gill et al. [25] observed in
some cokes that the anisotropic carbon around some clays and pyrite is of smaller size
than that in similar areas that lack minerals.
19
Chapter 2 Literature Review
2.3 Coke gasification
2.3.1 Gas-solid reactions
Gasification of a porous particle involves several steps: transport of the reactant gas to
the particle surface, diffusion of the reactant inside the particle through the pores to the
reaction sites, reaction between gas and solid and elimination of the products [53,54,55].
The way that coke is gasified can be divided into three different regimes depending on
the step that limits the reaction rate, namely, chemical kinetics (regime I), pore diffusion
(regime II) and gas phase mass transfer (regime III).
Regime I Chemical kinetics
At low temperature the rate of chemical reaction is lower than the rate of diffusion of
the reactant gases on the external surface and through the pore structure of the particle.
The gasification rate is determined by the rate of chemical reaction on the coke surface
or the intrinsic reactivity. Intrinsic reactivity is the reaction rate per unit area of pore
surface without any mass transfer restriction. In regime I, the bulk density of the coke
decreases but the size of the particle remains constant during gasification. The
activation energy measured is the true activation energy [56].
The activation energy for the reaction of metallurgical coke with carbon dioxide under
chemically controlled conditions, measured in previous studies, were typically in the
range of 215-240 kJ/mol [56,57,58,59].
Regime II Pore diffusion
As the particle temperature is increased gasification becomes limited by diffusion of the
reactant or product through the coke pore structure. The diffusion rate of gases is
influenced by bulk flow diffusion and pore size distribution [60]. In other words, the
collisions between gas molecules in the pores (molecular diffusion) or collisions of gas
molecules with the pore walls (Knudsen diffusion) affect the diffusion rate. The
activation energy decreases to half the true activation energy [56].
20
Chapter 2 Literature Review
Regime III Gas phase mass transfer
In this regime the physical process of mass transfer to the external surface of the particle
controls the reaction rate. The rate of conversion depends on particle size, gas
composition and a little on temperature. The slight dependence with temperature is a
result of low activation energy in this regime, which is close to zero. As the reaction
proceeds the coke particle becomes smaller and smaller but the density of the particle
remains constant.
An Arrhenius plot of the reaction rate as a function of temperature is used to indicate the
transition from a chemically controlled reaction to a gas phase mass transfer controlled
reaction (Figure 2.8). The zones I, II and III indicate the chemically controlled, pore
diffusion controlled and mass transfer controlled reactions. Two intermediary zones a
and b shows the transition between ideal regimes, where both regimes I and II and
both regimes II and III coexist, respectively.
The transition temperature between regimes depends on the reaction parameters such as
gas flow rate, gas type, pressure and particle size, and some intrinsic coke properties
such as coke porosity (size and type of the pores) and the concentration of active sites of
coke particle [24,43,56,61,62]. For instance Regime I is favoured by low temperature,
low pressure, small particle size, low concentration of active sites, high gas flow rate
and high porosity of coke particle. Harris and Smith [59] concluded that at 800C
gasification with CO
2
of coke with particle size between 0.2 and 2.0 mm and the
reactant gas parameters such as flow-rate and partial pressure ranging 500-1000 mlmin
-
1
and 0.1-1 atm, respectively, occurs under Regime I conditions; the activation energy
measured for metallurgical coke was 216 kJ mol
-1
.
21
Chapter 2 Literature Review
Figure 2.8 Ideal representation of the three controlling zones of carbon
gasification, where E
a
is the apparent activation energy and E
t
is the
true activation energy [53].
2.3.2 Mechanism of reaction
The mechanism of the gasification reaction is based on the ability of carbon to remove
an oxygen atom from a carbon dioxide molecule and retain it on certain sites by
chemical bonding [61]. The oxygen is then released as carbon monoxide and a new
atom of carbon is exposed to carbon dioxide. It is generally agreed that coke
gasification reaction follow the oxygen-exchange mechanism [24,42,56,61]:
C
f
+ CO
2
CO + C(O) Reaction 2.7
C(O) CO + C
f
Reaction 2.8
22
Chapter 2 Literature Review
where C
f
is a free carbon active site, C(O) is the chemisorbed oxygen and i
1
, j
1
and j
3
are
the rates constants for forward and reverse reactions. The reversible reaction (Reaction
2.7) is a fast reaction [24]. The desorption step (Reaction 2.8) is slow and its rate
controls the overall of gasification reaction [24,63,64].
The product of reaction, carbon monoxide, has been identified by previous studies as an
inhibitor of gasification [61,65]. The retardation of the gasification rate occurs due to
chemisorption of carbon monoxide onto the active carbon sites.
The reaction rate (R) of the two stages process follows a Langmuir-Hinshelwood type
equation [24,56,61]:
( )
2
2
3 2
1
1
CO CO
CO
P k P k
P k
R
+ +
= Equation 2.2
k
1
= i
1
c,
3
1
2
j
j
k = ,
3
1
3
j
i
k =
where k
1
, k
2
and k
3
are the rate constants, P refers to the partial pressures of CO and
CO
2
and c represents the total available active carbon sites. The rate constants are
directly related to the temperature. As temperature increases k
1
increases and both k
2
and k
3
decrease [24,60]. The nature of the coke described by the active carbon sites and
impurities that catalyse or inhibit coke gasification are the coke intrinsic properties that
affect the rate constants [24].
Previous studies showed that only a part of the total surface of the pores participates in
the reaction, namely the active surface area. Ergun and Mentser [61] and Laurendeau
[56] defined the active sites as sites formed by irregularities of the surface which are
able to chemisorb a gas phase through electron transfer. Carbon edges, dislocations,
inorganic impurities and oxygen functional groups are considered active sites [56].
23
Chapter 2 Literature Review
2.3.3 Reaction rate measurement
The gasification rate of carbonaceous samples is usually measured by the mass loss of
the sample during the reactivity test divided by the initial mass of sample [56,65]
(Equation 2.3):
dt
dW
W
1
R
0
= Equation 2.3
where, R is the reaction rate and W
0
is the initial mass of the dry ash-free sample.
Radovic et al. [66] expressed the reaction rate as a function of the mass of carbon at
time t (instantaneous mass of carbon) (Equation 2.4):
dt
dW
W
1
R = Equation 2.4
where, R is the reaction rate and W is the mass of the dry ash-free sample at time t.
Carbon conversion is defined by the initial mass and the mass of sample at time t
(Equation 2.5):
0
0
W
W W
X
= Equation 2.5
where, X is the fractional carbon conversion and W
0
is the initial mass of sample.
Equation 2.4 becomes:
dt
dX
) X 1 (
1
R
= Equation 2.6
The advantage of Equation 2.6 comparing to Equation 2.3 is that the former allows the
calculation of the reaction rate at any moment and it can be used to calculate intrinsic
reaction rate (the reaction rate at time t is divided by the total surface area at time t),
whereas the latter gives only the variation of carbon mass during the reactivity test.
24
Chapter 2 Literature Review
2.4 Factors influencing coke gasification
Coke properties such as microtexture, microstructure and mineral matter are the main
factors that affect gasification rate. Coke properties depend on the properties of the
parent coal such as coal rank, maceral composition and mineral matter, and
carbonization conditions. In this section the effect of coke properties on gasification rate
and also the influence of coal properties and carbonization conditions on coke properties
will be presented.
2.4.1 Coke microtexture
Both isotropic and anisotropic coke microtextures react with carbon dioxide but they
react at different rates. Several studies indicate that isotropic microtexture is more
reactive with carbon dioxide than anisotropic microtexture [18,22,23,67,68,69,70].
Figure 2.9 shows coke microtexture after reaction with carbon dioxide. The isotropic
microtexture, which originated from inert macerals (see section 2.5.1), was more
affected by gasification than the anisotropic microtexture.
As shown in section 2.2.1, the anisotropic microtexture is classified as a function of the
size, shape and form of the textural unit. The reactivity of different classes of
anisotropic microtexture varies [21,30,31,45]. Flow type anisotropic microtexture and
coarse mosaic showed a strong resistance to carbon dioxide attack. The medium mosaic
was consumed in small proportions whereas the fine mosaic was the most reactive.
25
Chapter 2 Literature Review
Figure 2.9 Gasification of coke microtexture [18].
This reduced reactivity of the anisotropic microtexture compared to the isotropic
microtexture may be explained by either a lower surface area of the carbon available for
reaction or lower intrinsic reactivity (the reaction rate per unit area of pore surface in the
absence of any mass transfer restriction [71]) of anisotropic carbon [31] due to a lower
number of active carbon sites. As the most reactive carbon atoms are located at the
edges of the lamellae not on the layer planes (basal planes) [53], the density of
accessible layer edges depend on MOD size. Therefore the smaller MOD size the
greater the free edge density [22,28,34,45]. Moreover the reactivity of the carbon active
sites located on different edges such as armchair and zig-zag is different [72].
Kashiwaya and Ishii [41] designed an experiment to observe the difference in reactivity
of carbon atoms located on the basal plane and the edges of the polyaromatic layers.
They measured the crystallite height (L
c
) and crystallite length (L
a
) of a metallurgical
coke at different temperatures under inert gas (Ar) and reactive gas (mixtures of Ar-CO-
CO
2
) (Figure 2.10). Crystallite height was affected only by temperature and no
significant difference between the L
c
of both the annealed and reacted cokes, implying
that the reaction is very slow on the basal plane. The crystallite length of both annealed
and reacted cokes also showed an increase with temperature but the reacted coke had a
26
Chapter 2 Literature Review
lower L
a
than the annealed coke at similar temperatures, indicating that the reaction
occurs at the edges of the polyaromatic layers.
Figure 2.10 Crystallite size (L
c
and L
a
) of a metallurgical coke function of
temperature under inert gas (Ar) and reactive gas (Ar-CO-CO
2
)
[41].
Feng et al. [73] determined the crystallite size of a char sample during reaction with
carbon dioxide at constant temperature (800C) up to 90% carbon conversion.
Crystallite height did not change significantly below approximately 60% carbon
conversion but it decreased at greater conversion levels, whereas crystallite length
decreased during gasification, even at an early stage. They assumed that initially the
reaction occurs predominantly at the edges of the polyaromatic layers, which implies a
decrease of the crystallite length, and only at later stages of reaction the entire
polyaromatic layers are consumed.
Coke samples removed from the raceway region of the blast furnace showed that the
flow type anisotropic microtexture is selectively consumed [15,74,75]. This behaviour
may be explained by the alkali effect or abrasion. Flow type anisotropic microtexture
reacts readily with K and Na to form an intercalated compound due to the regular
27
Chapter 2 Literature Review
arrangement of carbon layers creating micro-fissures which increases the reactivity with
carbon dioxide. The low mechanical strength of flow type anisotropic microtexture
enhances its abradability.
Kerkkonen [51] observed on cokes sampled from a quenched blast furnace that the
isotropic carbon gasified more in the lumpy zone, while mosaic carbon was removed in
a higher proportion in the raceway. He believed that the removal of the mosaic carbon is
due to evaporation of the silicates causing mechanical weakening of the carbon
structure.
2.4.2 Coke microstructure
Porosity of coke, defined by the volume percentage, morphology and size distribution of
the pores, has a major influence on coke strength. Coke strength decreases as the
porosity volume increases [7,31,46].
The rate of reaction of coke with carbon dioxide is determined not only by intrinsic
reactivity of carbon but also by pore accessibility [45,71]. Pore characteristics and pore
surface area play an important role when the reaction rate is slow (regime I conditions)
[76]. The reactivity increases as the surface area increases [45].
Szekely and Aderibigbe [60] observed the behaviour of pores during coke gasification
at 1000C and P
CO2
/P
CO
=0.5 (Figure 2.11). At early stages of reaction the size of pores
increases causing an increase in surface area. As gasification proceeds the pores are
further enlarged and coalesce resulting in a reduction of surface area. They observed the
reduction of surface area after approximately 40% carbon conversion. A similar
development of surface area (measured by nitrogen) of a coke sample during reactivity
test at 1100C and 100% CO
2
was reported by Kawakami et al. [77], but the decrease of
surface area was observed as early as 25% conversion. The decrease of surface area at
different carbon conversion was probably due to different properties of the cokes used
in these studies and also the reaction conditions. Turkdogan et al. [43] believes that with
increasing reaction temperature surface area decreases due to incomplete internal
28
Chapter 2 Literature Review
reaction. Szekely and Aderibigbe [60] also observed that the total pore volume for each
pore size from 10 nm to 100 m increased during gasification up to approximately 50%
burn-off.
Figure 2.11 Variation of surface area (N
2
adsorption) and porosity during
gasification [60].
Patrick and Walker [46] correlated coke reactivity not only with porosity volume but
also with pore size, number of pores and pore wall size. They concluded that reactivity
increases with increasing volume of the pores, mean pore size and number of pores, and
decreasing pore wall size.
Vogt et al. [7] observed that coke gasification reaction controlled by kinetics affects
coke strength due to abrasion and also fragmentation of the coke lump as fissures are
opened during gasification. Van der Velden et al. [18] observed that the surface of the
cracks developed during further coking was gasified preferentially compared to the pore
surfaces. In addition, coke crushing may affect coke reactivity and stability by
introducing more fissures with reactive surfaces. Thus caution is recommended when
comparing reactivity of crushed coke to uncrushed coke.
29
Chapter 2 Literature Review
Kerkkonen et al. [23] and Sakawa et al. [78] did not find a distinct connection between
porosity and reactivity of coke with carbon dioxide. This suggests that gasification is
probably more influenced by a certain range of pore size than the total porosity.
Kawakami et al. [77] concluded from the variation of the pore size distribution of a coke
during gasification that the reaction occurs mainly on the surface of the pores smaller
than 1 m. Also, Kerkkonen et al. [23] found that the porosity was lowered by
increasing the content of inertinite in the parent coal. Moreover, Kerkkonen et al. [23]
and Sakawa et al. [78] observed an increase of the reaction rate with increasing
inertinite content, so they concluded that the rate was affected more by the amount of
inertinite in the parent coal than the porosity.
Total porosity and pore size distribution are usually determined using mercury
porosimetry, which measures the relationship between pressure and effective volume of
the sample and is used to determine porosity and pore size distribution. Pore surface
area is determined by both nitrogen and carbon dioxide adsorption onto the surface of
the sample. Nitrogen measures surface area of both mesopores and some micropores
[79] whereas carbon dioxide measures surface area of micropores [80] (see Chapter 4).
The total surface area is commonly used to normalise the reaction rate in order to
remove the effect of surface area, but several studies [66,77,81,82,83] have shown that
active surface, the surface area of the coke exposed to gas that actually reacts with
carbon dioxide, would be more appropriate. The ratio Active surface area/Total surface
area decreases as the carbon conversion increases up till 20%; after 20% carbon
conversion the ratio becomes constant [81,83]. However, there is no standard method
for determining the active surface area. The most commonly used methods for the
measurement of the active surface area, namely Gravimetric and Temperature-
programmed desorption (TPD) methods, introduce errors in determination of active
surface area due to the presence of some physisorbed oxygen. The physisorbed oxygen
reacts with carbon and the reaction product, carbon dioxide, also reacts with carbon
during its removal through the pores [82]. Also, the measurement of the active surface
area is influenced by different parameters such as temperature and pressure [84], which
makes difficult the comparison of the data between different studies. Moreover, the
30
Chapter 2 Literature Review
determination of active surface area is suitable only for samples free of mineral matter
because minerals introduce active sites [66,72,85,86].
However, Miura et al. concluded [87] that carbon content and pore surface area do not
indicate the reactivity of char when catalysts are present. Kyotani et al. [88] also
observed no obvious correlation between surface area and chars reactivity. They believe
the effect of catalysis by mineral matter is more dominant than surface area.
2.4.3 Mineral matter
Minerals in coke can enhance coke degradation in different ways:
- Some of the minerals catalyse the gasification;
- The oxides in the mineral matter are reduced inside the coke lump by the available
carbon at high temperature (above 1400C) [25];
- Iron bearing minerals (such as hematite, siderite, pyrite), sulphur and MgO can
reduce the size of the anisotropic microtexture around them during coking [89];
- Large mineral particles increase the coke gasification rate by formation of cracks
around them during coking, allowing carbon dioxide to penetrate the coke pieces
more easily [23];
- The aluminosilicates increase their volume when they decompose to form slag; this
weakens the internal structure of the coke lumps in the active zone of the blast
furnace [51].
The ash yield from the proximate analysis of the coke was found to be a good indicator
of coke reactivity by Gill et al. [25] and Vogt et al. [67] in their study; coke reactivity
increased as ash content increased. However Duval et al. [22] did not observe any
influence of ash yield on coke reaction rate. They concluded that coke microtexture was
more dominant than the mineral impurities in controlling coke reactivity.
Ash composition has been considered an important parameter in coke reactivity
[20,25,70]. For instance total iron oxide from the ash analysis was considered a fairly
good predictor of coke reactivity [89] [13,90]; reactivity of the cokes increased as the
31
Chapter 2 Literature Review
concentration of Fe
2
O
3
in the coke increased. Calcium and potassium oxides are also
known to act as catalysts in the gasification process [18]. The catalytic activity of
different oxides is dissimilar. Gill et al. [25] classified the elements that affect
gasification as it follows: Si=Al < Mg < Fe < Ca < Na < K.
Kerkkonen et al. [23] and Samaras et al. [91] concluded that the elemental composition
of the ash cannot be used to predict the reactivity of coke, but minerals present in the
coke were considered more suitable indicators of coke reactivity than ash chemistry. For
instance transition metals, their oxides, alkali and alkaline earth metal compounds
exhibit catalytic activity during gasification [13,72].
Metallic iron is considered a very efficient catalyst of gasification [92,93]. During
gasification metallic iron in contact with carbon dioxide is oxidised. Reactivation of
metallic iron as a catalyst could be achieved by introducing hydrogen or carbon
monoxide for a specific length of time [92,93,94]. Figure 2.12 shows a substantial
increase of reaction rate after purging the sample with carbon monoxide and hydrogen
alternatively. Tanaka [95] also observed reduction of wustite to -iron after 5 minutes of
treatment with CO at 780-800C of a char sample. However the catalytic effect of
metallic iron could not be revived beyond about 60% mass loss after the sample was
treated in 100% H
2
[93].
32
Chapter 2 Literature Review
Figure 2.12 Reactivation of metallic iron during gasification by CO and H
2
[92].
Magnetite was not considered by Walker et al. [92] to be a catalyst, but Price et al. [89]
observed an increase in coke reactivity with increasing magnetite content. Also an in-
situ XRD study for the iron-catalysed CO
2
gasification of carbon made by Ohtsuka et al.
[96] showed a high catalytic activity of magnetite; gasification was enhanced in the area
where the only iron compound present was magnetite.
Wustite exhibits a higher activity than magnetite for gasification with carbon dioxide
[96]. Walker et al. [92] identified wustite as a catalyst but with lower activity than
metallic iron.
Pyrrhotite was considered by Vandezande [97] to be a catalyst of the gasification
reaction. He observed in some cases using optical microscopy that pyrrhotite appear to
33
Chapter 2 Literature Review
create tunnels through the coke particle. Hu et al. [98] showed that oxidation of
pyrrhotite under a carbon dioxide atmosphere produces iron oxide. The iron oxide form
depends on the oxygen partial pressure. It can be concluded that pyrrhotite catalyses
gasification due to formation of iron oxide. Moreover, Price et al. [89] performed a
reactivity rest on a coke made from a coal doped with pyrite. Pyrrhotite is the most
likely mineral formed from pyrite during carbonization although some metallic iron can
be also formed. The coke from the doped coal was more reactive than the untreated one.
Apatite [89], quartz [23] and feldspar [23,89] were found not to affect the gasification
reaction rate. Potassium and sodium bound in alkali feldspars did not show any catalytic
activity in the tests performed by Price et al. [89]. Fayalite is assumed not to catalyse
gasification. A mixture of coke with excess of SiO
2
treated at 1000C converted iron
oxide to a form (most likely fayalite) that did not catalyse gasification [92].
The other minerals identified hitherto in the cokes such as mullite, akermanite,
anorthite, calcium iron oxide, diopside, gehlenite, oldhamite, rutile, brookite and anatase
have not been investigated for their potential to be catalysts, but they have been
expected to catalyse gasification.
Alkali metals such as potassium and sodium are associated with aluminosilicates in an
unexchangeable form and they are believed to be catalytically inactive [99]. Iron,
calcium, magnesium, potassium and sodium included in montmorillonite and illite in
coal have only a slight effect on coke reactivity and only then because they may release
some of these elements [23].
The amount of catalytic minerals is an important factor that determines coke reactivity.
Walker et al. [92] believe that even traces amounts of catalyst (less than 1 ppm) are able
to affect the reaction rate. The degree of dispersion of the catalyst is another important
factor that controls reactivity [72,100]. Lindert and Timmer [90] and Tanaka et al. [95]
observed an increase of coke reactivity to carbon dioxide as the dispersion of metallic
iron increased.
34
Chapter 2 Literature Review
Another aspect of the behaviour of the catalytic minerals during gasification is sintering
[72]. During gasification, carbon is consumed around the catalytic mineral particles
which allow them to become mobile and agglomerate to form large particles. This
suggests that the activity of the catalyst diminishes.
Huang et al. [101] prepared chars from vitrinite- and inertinite-enriched fractions from a
low rank coal (34% volatile matter). The carbonised vitrinite-enriched fraction was
more reactive than the inertinite-enriched fraction but after demineralisation the former
was less reactive. The surface area of the micropores and mesopores of the inertinite-
enriched fraction was greater than that of the vitrinite-enriched fraction. Also no
anisotropy was observed in any of the samples. They concluded that the catalytic effect
of the mineral matter on the reaction rate is greater than the surface area. A similar
observation was made by Czechowski and Kidawa [102] in their study. They assumed
that the greater concentration of the elements Ca, K and Na in the ash of the carbonised
vitrinite-enriched fraction is responsible for its higher reactivity compared to that of the
inertinite-enriched fraction.
Mechanisms of catalysis
The catalyst may affect gasification in several ways [92]:
- It may affect both steps of the oxygen-exchange mechanism of the gasification
reaction by either changing the number of active sites or lowering the overall
activation energy of the reaction;
- It may induce pits in the carbon basal plane and expose additional edge planes for
reaction;
- It may bypass the oxygen-exchange mechanism of the gasification reaction
completely.
Two mechanisms of catalysis of the gasification reaction have been proposed: the
oxygen-transfer mechanism and the electron-transfer mechanism.
35
Chapter 2 Literature Review
The oxygen-transfer mechanism also known as the spill-over mechanism is the most
accepted [72,88,92,94]. The mechanism can be applied either for metallic iron or iron
oxide and proceeds in the following manner: carbon dioxide dissociates onto the
catalyst surface and the oxygen atom is chemisorbed on the catalyst surface, then the
oxygen atom is transferred to an adjacent carbon site. However, the mechanism may not
follow exactly the steps mentioned above [88]. The catalysis of carbon gasification by
both metallic iron and iron oxide is described by Reaction 2.9-11 [92] and Reaction
2.11-13 [88], respectively; where C
f
represents the active carbon site.
x Fe + y CO
2
Fe
x
(O)
y
+ y CO Reaction 2.9
Fe
x
(O)
y
+ y C
f
x Fe + y C(O) Reaction 2.10
C(O) C
f
+ CO Reaction 2.11
Fe
x
O
y
+ CO
2
Fe
x
O
y+1
+ CO Reaction 2.12
C
f
+ Fe
x
O
y+1
C(O) + Fe
x
O
y
Reaction 2.13
C(O) C
f
+ CO Reaction 2.11
The electron-transfer mechanism is based on the ability of transitional metals to accept
electrons and influence the distribution of electrons in the aromatic layers [92]. Figure
2.12 shows two types of distribution of the electrons may occur at a carbon active site.
The catalyst is believed to induce type (b) distribution (Figure 2.12b) of the electrons,
which requires less energy to break the carbon-carbon bonds to release a CO molecule
implying a lower activation energy for desorption of carbon monoxide. The position of
the catalyst can be anywhere on the plane with the carbon active site.
36
Chapter 2 Literature Review
(a) (b)
Figure 2.12 Distribution of electrons in the aromatic rings (a) not affected by
catalyst and (b) influenced by catalyst [92].
2.5 Factors influencing coke properties
Coke properties are affected by a number of factors, namely coal properties such as coal
rank, maceral composition and coal fluidity on the one hand and coke oven conditions
on the other hand. In this section the influence of coal properties and carbonization
conditions on coke properties will be discussed.
2.5.1 Coal properties
The coals most suitable for the preparation of metallurgical cokes are bituminous coals
characterized by a carbon content between 80-90% and vitrinite reflectance (R
0
mean)
values between 0.6 to 1.6%.
37
Chapter 2 Literature Review
2.5.1.1 Coal rank
The coal rank can be assessed according to the volatile matter yield from heated coal,
carbon content or vitrinite reflectance. The coal rank increases with increasing carbon
content, increasing vitrinite reflectance and decreasing volatile matter yield.
Coal rank is a major determinant of coke microtexture [21]. The coal becomes more
aromatic and more polymerised as the rank increases, therefore the size and content of
anisotropic carbon increases [29,31]. Hirsch [36] created a model of the coal structure
as function of rank, using X-ray diffraction (Figure 2.13). The model has three types of
structure namely open structure, liquid structure and anthracitic structure. The
open structure is a very porous system occurring in low rank coals with carbon
contents of up to 85% (Figure 2.13 a). The lamellae are relatively randomly orientated
in all directions and are connected by amorphous material. The liquid structure is
attributed to medium rank coals or bituminous coals (the carbon range from about 85 to
91%). The porosity of the liquid structure is very low and the lamellae are better
orientated than in the previous structure forming crystallites of two or three combined
lamellae (Figure 2.13 b). The anthracitic structure is characteristic of high rank coals
with carbon contents of over 91%. The degree of lamellae orientation is the highest of
all structures and the amorphous material has disappeared (Figure 2.13 c). Takagi et al.
[103] have shown that the crystallite height (L
c
) of a number of coals of different rank
(60-90 % C, dry ash free base) increased as the coal rank increased (L
c
varied between
0.71-1.89 nm). Moreover, Lu et al. [104] reported an increase of both crystallite height
(L
c
) and crystallite length (L
a
) as the coal rank increased form high volatile bituminous
coal to semi-anthracite.
As coal rank increases the ordering in coke microtexture increases. Coals with open
structure make poorly ordered cokes whereas cokes from coals with anthracitic
structure have the greatest ordering. Many studies have shown that coal rank is one of
the main factors influencing coke reactivity because coal rank has an important role in
the formation of coke microtexture [26,29,67,105]. Low rank coals make cokes with
small MOD with size about 5 nm, whereas cokes from high rank coals are characterised
by large MOD with size about 20 m [67]. However, the carbon crystallite size (L
c
and
38
Chapter 2 Literature Review
L
a
) of chars prepared from coals of different rank (81.9 91.3 % C, dry ash free basis)
showed little influence of coal rank, although the crystallite size of the parent coals was
significantly affected by the rank [106].
There are different opinions regarding the rank of the coals that make cokes with the
highest resistance to carbon dioxide attack. Toshimitsu et al. [107], Vogt et al. [67] and
Koba and Ida [68] consider coals with vitrinite reflectance approximately R
0
=1.4%
make cokes with minimum reactivity. But, Graham and Wilkinson [17] believe the
reactivity of coke to carbon dioxide is at a minimum when the parent coal has a vitrinite
reflectance about 1.25%.
Figure 2.13 Schematic representation of coal structure made by Hirsch [36].
39
Chapter 2 Literature Review
2.5.1.2 Macerals
During coalification the original plant material is transformed into three main organic
groups of the coal defined by the optical microscopy as macerals, namely vitrinite,
liptinite (formerly called exinite) and inertinite. Van Krevelen [36] presented a brief
description of these macerals. He assumed that vitrinite is a product of coalification of
woody tissue. It acts as a binder surrounding the other macerals and mineral matter and
is very brittle [47]. Liptinite has a greater hydrogen content than vitrinite. It is the
maceral group that becomes the most fluid during the coking process. Macerals from the
inertinite group contain less hydrogen than vitrinite. During the carbonisation process a
small amount of the inertinite macerals fuse, the other part remains practically
unchanged. Inertinite is rich in carbon, poor in hydrogen and volatile matter, hard and
brittle [47].
During carbonisation macerals that are fusible like vitrinite, exinite and a part of the
inertinite form the reactive maceral derived component (RMDC) in the coke. Inertinite
that does not fuse yields the inert maceral derived component (IMDC). Fusible
macerals form anisotropic microtexture and small amounts of isotropic microtexture
whereas non-fusible macerals form isotropic microtexture only.
The distribution and size of macerals in coals control the development of coke
microtexture [17,21]. The inert maceral derived component must be in a certain
proportion so that strong coke cell walls can be formed. Coals containing fine inertinite
particles form coke with larger anisotropic microtexture and thicker walls than similar
rank coals with fewer or coarser inertinite particles. Coarse IMDC limits the anisotropy
size because the space between them is narrow and the domains cannot grow [52].
Homogenous distribution of macerals and minerals in coal form coke with a more
uniform structure than coals that have alternate layers of variable maceral concentration
[52].
40
Chapter 2 Literature Review
Because fusibility of inertinite can change the microtextural composition of coke a
small number of researchers have tried to identify the factors that affect its fusibility and
classify the inertinites in terms of their ability to fuse.
Diessel [108] proposed a classification of inertinite by its behaviour during
carbonisation. The first category is of highly reactive inertinite, which produces cokes
with mosaic to flow anisotropy and variable pore size. The inertinite reflectance of this
class before carbonisation varies from 1.0 to 1.5%. Moderately reactive inertinite is
the second class. The inertinite shows weak plasticity and the coke product has basic
anisotropy, small degassing pores and the inertinite reflectance before carbonisation
varies from 1.5 to 1.8%. Non-reactive inertinite of small size is the third category. It
strengthens the coke due to a good integration into the reactive derived maceral
component (RDMC). Non reactive inertinite of large size is the last category. Coke is
weakened by this material because of a poor integration into the reactive derived
maceral component (RDMC).
The inertinite reflectance that is the boundary between fusible and non-fusible inertinite
is shown in Figure 2.14; the letters E, V and I in the chart indicate reflectance area of
coal macerals exinite (liptinite), vitrinite and inertinite. Fusible inertinite has reflectance
less than the boundary reflectance. The boundary between fusible and non-fusible
inertinite is not well defined. Pearson [109], Barriocanal et al. [110] and Diessel [108]
correlated the amount of inert derived maceral components with coal rank. The
reflectance boundary moves to a greater value as the coal rank increases. Moreover,
Pearson [109] believes that the amount of vitrinite present may affect the fusibility of
inertinite. He assumed that the reflectance boundary of coals with the same rank varies
with vitrinite content; as the vitrinite content decreases the reflectance boundary moves
to the higher values. He also found that the fusibility of fusible inertinite could be
suppressed if the vitrinite content is very high.
41
Chapter 2 Literature Review
Figure 2.14 A typical reflectance distribution of macerals in coking coal [108]
Sakawa et al. [78] found a good agreement between the content of inertinite in the
parent coals and coke gasification rate; as the inertinite levels increased the reaction rate
increased.
2.5.1.3 Coal fluidity
Coal fluidity affects coke reactivity because it controls the size and shape of coke
anisotropy [31] and the nature of interfaces between reactive and inert macerals [110].
42
Chapter 2 Literature Review
Coal rank, maceral composition and coking rate determine coal plasticity [45]. Coal is
fluid over a limited temperature range during carbonisation. The initial softening
temperature and resolidification temperature describe the temperature range over which
the coal is fluid. The temperature of maximum fluidity and the range of temperature of
maximum fluidity increase with increasing rank [31]. The best coking coals have
optimum chemical reactivity and fluidity that ensure the growth of the anisotropic
domains. Low rank coals have solidification temperatures too low to allow growth of
the domain size. In very fluid systems, more than in normal coking coals the size of the
domains can be too large and the cokes produced do not have the necessary mechanical
and thermal resistance [111].
The mechanism of formation of coke microtexture is not similar for all coals. Fortin and
Rouzaud [32] described two mechanisms of formation of coke microtexture during
carbonisation that are a function of coal fluidity. One mechanism is for coals with high
fluidity and the other one for high rank coals of low fluidity. Medium rank bituminous
coals are characterized by higher plasticity than high rank bituminous coals. The high
plasticity of medium rank bituminous coals favours the reorientation of BSUs to form
MOD and the resultant coke mainly shows mosaic microtexture. High rank bituminous
coals show a pre-organized structure. Their limited plasticity allows only an
improvement of this structure developing a massive microtexture. Fortin and Rouzaud
[32] define massive microtexture as uniform anisotropy extended over the whole
particle.
Coal oxidation affects coal plasticity. Coal plasticity decreases as the oxidation time
increases [112]. Coal oxidation rate increases as the coal rank decreases. Oxidation of
low rank coals reduces the MODs in the coke because the amount of metaplast
decreases (see next section). The coke made from high rank coals contains very large
domains even if the coal plasticity is lowered by oxidation. These coals have initial
planar orientation and they are not affected by reduction of fluidity [38].
43
Chapter 2 Literature Review
2.5.2 Carbonization process
During carbonisation moisture and volatile matter are released, the mineral matter is
transformed and both coke microtexture and structure are developed. The coke
microtexture and structure is the result of coal behaviour during the plastic stage.
During carbonisation, chemical and physical processes occur, such as chemical
transformations and orientation of BSUs. Different models have been used to explain
coal plasticity and coke structure development during the carbonisation process.
Fitzgerald and van Krevelen [36] introduced the metaplast theory to explain coal
plasticity and chemical transformations during the carbonisation process. They consider
the metaplast to be responsible for the plastic behaviour of coal. The process is
presented as occurring in three steps:
I. Coking coal Metaplast
II. Metaplast Semi-coke + Primary volatiles
III. Semi-coke Coke + Secondary gas
This hypothesis shows that the metaplast is formed by a depolymerisation process.
Metaplast is defined as an unstable plastic phase formed during the first stage of coal
pyrolysis. The maximum plasticity is considered to be the point at which the
concentration of metaplast is at a maximum [34]. The plastic phase occurs at
temperatures between 300 and 500C [113].
The next stage consists of the cracking process; tar is evaporated and the non-aromatic
groups are split off [113]. The residual aromatic groups produce components less
polymerised than the coal and a large proportion of them are liquids at the pyrolysis
temperature. They form metaplast after their saturation with hydrogen, which is
generated by other aromatisation reactions [47]. The fluidity of the metaplast is
improved by increasing the content of hydrogen. Aromatisation and condensation are
the other reactions that take place during this stage. Aromatisation results from
44
Chapter 2 Literature Review
dehydrogenation of saturated rings [47]. A scheme of cracking and aromatization
reactions is presented in Figure 2.15.
Figure 2.15 Reactions of cracking and aromatisation of coal components [47].
Large molecules are also formed by condensation reactions. An example of a
condensation reaction is presented below (Reaction 2.14):
R-OH + RH R-R + H
2
O Reaction 2.14
where R and R are organic radicals. The metaplast solidifies to form anisotropic
ordered coke. In some cases coke formation is believed to proceed via liquid crystal
mesophase. Oxygen has an opposite role to that of the metaplast in the carbonisation
process. Oxygen acts as a cross-linking agent [114] as it induces condensation reactions
[34] lowering the fluidity of the coal.
45
Chapter 2 Literature Review
The density of the semi-coke increases during the third stage because methane and
hydrogen (especially at higher temperature) are released. At the end of this stage coke is
produced [113].
Rouzaud [37] described not only the chemistry of the process, but also the physical
process of formation of the coke microtexture during carbonisation in his two-
component structural model of coal (Figure 2.16). In this model the orientation of
polyaromatic basic structural units (BSUs) was observed using transmission electron
microscopy (dark field mode).
Figure 2.16 Transformation of coal during pyrolysis; a) raw coal, b) plastic stage
(~400-500C), c) semi-coke (~ 500C) and d) coke (~ 1000C) [37].
46
Chapter 2 Literature Review
He assumed that coal contains two components: a macromolecular network and a
molecular component. The macromolecular network is formed by BSUs in a random
distribution. These BSUs are bound to each other by either oxygen atoms (thick line) or
aliphatic bridges (zig-zag line) that act as cross-linkers and prevent the development of
large molecular orientation domains (MODs). The oxygenated groups (thin line) or
aromatic hydrogen (thin line) that is linked to BSUs do not affect their ordering. The
pores of the macromolecular network include the molecular component (dot), which is
composed of small hydrocarbon molecules that are more or less trapped by the
macromolecular network (Fig. 2.16a). Low rank coals contain large amounts of
oxygenated and aliphatic chemical groups, their content decreases with increasing coal
rank. High rank coals consist largely of molecular component and aromatic hydrogen.
At about 400C most of the bridges of the macromolecular structure are broken (Fig.
2.16b). Fragments of the macromolecular network and aliphatic bridges yield a new
macromolecular component, similar to the metaplast, where BSUs are free to reorient
and form a MOD. During coal pyrolysis a portion of the macromolecular component is
released as volatile matter and the remaining portion condenses. Cross-linking oxygen
and hydrogenated molecules play opposing roles. Hydrogenated molecules of the
macromolecular component reduce the viscosity of the fused coal and also retard cross-
linking reactions by donating hydrogen to free radicals. High hydrogen content
improves the plasticity and the BSU orientation [114].
At temperatures about 500C solidification occurs. A semi-coke is obtained after the
volatiles have been released (Fig. 2.16c).
From 500C to about 1000C significant changes in size and orientation of MODs do
not occur. At this stage only gases are released (Fig. 2.16d).
Deposition of hydrocarbon molecules from volatile matter obtained during the cracking
process produce a pyrolytic form of carbon [18,21] (Figure 2.17).
47
Chapter 2 Literature Review
Figure 2.17 Deposition of pyrolytic carbon (P) [21].
Coke microtexture and structure can be changed if the operating conditions of the coke
oven (heating rate, coking temperature and pressure) or other parameters such as bulk
density of the charge, position in the oven and heat treatment of coal and coke are
modified. The effect of these parameters on coke microtexture and structure will be
presented in the next sections.
2.5.2.1 Heating rate
Fluidity is low at a low heating rate and increases with increasing heating rate [115].
High heating rate increases the concentration of metaplast and the MOD size increases
[34]; therefore coke anisotropy increases [20].
Mitchell et al. [21] carbonised two bituminous coals, one of low rank and the other one
of high rank, at different heating rates. The low rank coal carbonised at higher heating
48
Chapter 2 Literature Review
rate produced a coke with lower content of isotropic carbon and greater proportion of
very fine and fine mosaic microtextures. The coke microtexture from the high rank coal
was also dependent on heating rate. The proportion of fine mosaic was greater and
lower for the flow-like anisotropic microtexture. This was explained by the shorter fluid
range and carbonization time.
Coke porosity was found to increase as the heating rate increases [52,116] because coal
swelling increases [36]. Nevertheless, the degree of increased porosity becomes smaller
at high heating rates [116].
2.5.2.2 Carbonization temperature
Coking temperature has a major influence on coke reactivity. It affects the degree of
ordering of the domains and porosity of coke. Increasing coking temperature produces
larger and more ordered BSUs and the carbon layer edges become less accessible to the
reactive gases [22]. The porosity of coke decreases as the temperature increases up to
about 800C then it becomes constant [116].
2.5.2.3 Bulk density
Increasing bulk density of the coal charge increases the apparent density of coke and
this implies a coke of lower porosity [7,17,26,52,70]. Two ways of increasing the bulk
density of the charge are stamping the coal [7] or preheating. The bulk density of a coal
charge also depends on moisture content and the grinding size of the charge. Graham et
al. [17] noticed that the density of the charge decreases until the moisture content is
10% and then rises with further increase in moisture content. Coal crushed more finely
amplified this effect above 10% moisture.
49
Chapter 2 Literature Review
2.5.2.4 Position in the coke oven
Coke properties vary with the position of coke in the oven from top to the bottom and
from wall to centre. Coke reactivity decreases from top to the bottom of the oven. This
can be explained by bulk density in the oven, which increases with increasing depth
[117]. Reactivity of coke next to the oven wall is lower than that in the middle of the
oven [45]. This phenomenon is amplified when the heating rate is lower [20]. Two
explanations have been proposed for this. One is the heating rate of coal near the oven
walls is faster than its centre producing more anisotropic coke [45]. The other one is the
coke from the centre of the oven has a higher porosity than that from the wall [46].
2.5.2.5 Pressure
The amount of MOD classes eight to ten (see chapter 2.2.1) increases when the external
pressure increases. Pressure improves the fluidity and increases the capacity of the
metaplast to increase MODs. The MOD formation rate increases more quickly than the
diffusion rate because the pressure improves the confinement. This permits the
formation of larger domains than those obtained at atmospheric pressure [33].
2.5.2.6 Heat treatment
Heat treatment can be applied to the coal charge and also to the produced coke. The heat
treatment affects both coke porosity and microtexture. The pore diameter observed in
preheated coal charges was smaller and the pore-wall thickness was less than in the case
of wet charges [17]; in this case the difference could be attributed to pre-drying of the
coal prior to entry to the coke oven.
On reheating coke at 1400C for two hours in inert atmospheres, it loses about 4-5%
weight due to the loss of volatile matter, sulfur and ash-coke reactions. Moreover, coke
structure is densified [26] as coke shrinking occurs. During heat treatment the mosaic
microtexture can become porous because of removal of sulfur and volatile material.
50
Chapter 2 Literature Review
Legin-Kolar et al.[118] and Zamalloa et al. [119] observed an intensive structural
arrangement and the crystallites height started to grow in the annealed cokes above
1300C. Kashiwaya and Ishii [41] annealed coke above 1000C and observe in increase
not only in the crystallite height (L
c
) but also in the crystallite length (L
a
).
Iwakiri et al. [116] observed that increasing the temperature of heat treatment (900
1300C) of cokes reduces coke reactivity.
2.6 Methods of measuring coke reactivity
The most widely used test for assessing quality of metallurgical cokes is one developed
by the Nippon Steel Corporation (NSC). A 200g sample of coke with particle size
between 19 to 21 mm is placed into a reaction vessel, which is heated by an electrical
furnace, and the coke is reacted with carbon dioxide at a flow rate of 5 L/min for two
hours at 11005C. After reaction, the Coke Reactivity Index (CRI) and Coke Strength
after Reaction (CSR) are measured. The Coke Reactivity Index is the percentage of
weight loss during the reactivity test (Equation 2.7):
CRI = 100
weight original
in weight loss
Equation 2.7
The coke sample from the reactivity test is used to determine the Coke Strength after
Reaction. The CSR is defined as the weight percentage of coke larger than 10 mm in
size after tumbling the reacted coke for 600 revolutions at 20 revolutions per minute
(Equation 2.8):
CSR = 100
reaction after weight
ling after tumb mm 10 fraction of weight
>
Equation 2.8
Many studies have shown a good relationship between CRI and CSR; as CRI increases
CSR decreases [70,90,105]. Although the NSC test does not reproduce the blast furnace
51
Chapter 2 Literature Review
conditions it gives information about coke quality and also a relatively good agreement
was observed between CSR and blast furnace permeability [120]. However the NSC test
cannot be used to determine the reaction kinetics during the test because it was not
designed to acquire data about the carbon loss during the test.
The most common techniques used to measure gasification reaction parameters use
either the thermogravimetric analyser (TGA) or the fixed-bed reactor (FBR). Each
apparatus has its shortcomings in determining some of the reaction parameters and these
must be considered in selection of the appropriate technique for an accurate
measurement of certain reaction parameters.
Thermogravimetric analyser (TGA)
Thermogravimetric analysers have been used in a series of studies [18] [24,60,76,77]
designed to measure reactivity under different specific conditions such as gas
composition, temperature, sample mass and particle size. Figure 2.8 shows a schematic
diagram of a TGA system used by Aderibigbe and Szekely [24] in their study. The
sample is placed in a basket suspended by an automatic recording balance. The basket is
located inside an electrical furnace and the reactant gas flows from the bottom to the top
of the furnace. Two thermocouples are located above and below the basket. The change
in mass of the sample during the reactivity test and the temperature are recorded.
The measurement of the reaction rate under kinetically controlled conditions using the
TGA could be affected to some extent by several factors:
- The flow of the reactant gas could affect to some degree the reading of the sample
mass because of the sensitivity of the balance;
- There could be some difference between the temperature of the sample and that
recorded by the thermocouple because the thermocouple cannot be placed inside the
sample and CO
2
gasification is an endothermic reaction, which can lower the
temperature in the middle of the sample;
52
Chapter 2 Literature Review
- A concentration gradient of the reactant and product gases can exist inside the
sample due to an inadequate gas flow, which is directly related to the mass of the
sample and particle size.
All the above mentioned factors have been previously identified as controlling the
reaction regime (see section 2.3.1). However, rigorous selection of reaction parameters
and conditions would minimise the effect of the above mentioned factors on the reaction
kinetics.
Figure 2.18 Schematic diagram of an experimental system using a
thermogravimetric analyser [24].
Fixed-bed reactor (FBR)
A schematic diagram of a fixed-bed reactor is shown in Figure 2.19 [55]. The reactor is
placed into an electric furnace and the sample sits on a fixed holder. The reactant gas
passes through the sample bed from top to the bottom and a thermocouple measures the
temperature in the middle of the sample bed. The reaction rate is measured using the gas
53
Chapter 2 Literature Review
flow-rate and the concentration of carbon monoxide in the exhaust gas. The design of
the fixed-bed reactor system has some advantages:
- The sample temperature measurement is accurate because the thermocouple is placed
in the middle of the sample bed;
- The gas flow is through the sample bed which allows quick removal of the gaseous
reaction product enabling conditions for regime I;
- The design of the system allows the measurement of the activation energy due to
possibility of quickly changing the reaction temperature.
Figure 2.19 Schematic diagram of a fixed-bed reactor system [55].
54
Chapter 2 Literature Review
2.7 Chapter overview
Gasification is one of the factors that affect coke degradation in the blast furnace, which
in turn affects furnace performance. The temperature and gas composition change as
coke descends in the blast furnace. The mechanism of coke gasification also changes
with increasing temperature. At low temperatures the reaction takes place throughout
the particle and is chemically controlled, but at higher temperatures the reaction is
limited to the surface. Gasification rate is influenced by combined factors such as coke
reactivity and blast furnace conditions. The combined effect of these factors in the blast
furnace makes difficult the assessment of their effect on gasification rate.
Increasing temperature increases the reaction rate in laboratory conditions. The
concentration of carbon dioxide in the blast furnace gas decreases significantly at high
temperature. Diminishing carbon dioxide levels in the gas reduces the reaction rate. The
inhibition effect of carbon monoxide on gasification rate can also have a contribution in
lowering the reaction rate. Alkalis and some iron compounds from the burden are
known to catalyse the gasification reaction, mostly at the accessible surface of the coke
lumps.
Coke properties such as carbon microtexture, microstructure and the composition of
inorganic matter control coke reactivity. Different types of carbon microtexture react
differently with carbon dioxide. Flow type anisotropic microtexture and coarse mosaic
have a relatively strong resistance to carbon dioxide attack. Medium mosaic was
consumed more rapidly and fine mosaic, isotropic microtexture and inertinite were the
most reactive components. Gasification is assumed to occur mainly at the edges of the
molecular orientation domain (MOD) and is less likely on the basal planes. As the
MOD size decreases the density of the free edges on the pore surface increases which
would explain the greater reactivity of the isotropic microtexture than that of the
anisotropic microtexture.
Carbon crystallite height in chars and cokes as measured by the XRD was not affected
by gasification in the early stages of the reaction (approximately up to 60% carbon
conversion) but decreased at later stages, whereas crystallite length decreased as
55
Chapter 2 Literature Review
gasification proceeded. However limited studies have investigated the effect of
gasification on carbon crystallite size in coke.
The access of carbon dioxide to the carbon active sites is through the pore network.
Particle surface area affects reaction rate when the reaction is chemically controlled
whereas pore size distribution is important at higher temperatures when the reaction is
limited by diffusion. During gasification with carbon dioxide the surface area of the
pores increases due to enlargement of the pores and then the pores coalesce at greater
conversion levels, which result in a decrease in surface area. Although it is generally
agreed that surface area increases as gasification proceeds in the early stages a good
relationship between surface area and the reaction rate was found only in some of the
studies.
The presence of mineral matter in coke was found to affect coke reactivity. Ash yield
and the elemental composition of the ash were considered in many studies as indicators
of coke reactivity. Several studies have found a good relationship between coke
reactivity and potassium, sodium, calcium and iron oxides from the ash analysis.
However oxides are not the only form of inorganic matter in coke, also other mineral
phases are present. There is currently limited information available in the literature
about the form of minerals present in the metallurgical cokes. Metallic iron, iron oxides
and pyrrhotite were the only catalysts present among the minerals identified in the
metallurgical coke by previous studies. The calcium, potassium and sodium minerals
identified so far in the coke do not catalyse gasification. Therefore using ash yield or
composition as indicator of coke reactivity should be reconsidered. The particle size and
dispersion of catalytic minerals are also of major importance in catalytic gasification.
The reactivity of coke increases as the catalyst particle size decreases and dispersion
increases. Quantitative analysis of the catalytic minerals identified in cokes and their
relationship with coke reactivity has not been previously investigated.
Although coke microtexture, porosity and catalytic minerals have been established as
major factors that influence coke reactivity by many studies, there is still uncertainty as
to the relative importance of these factors.
56
Chapter 2 Literature Review
57
Coal properties such as rank, maceral composition, fluidity and mineral matter, and
carbonization conditions are the factors that determine the development of coke
properties. Macerals from high volatile coals that fuse during the carbonisation process
form coke with small size anisotropic microtexture. With increasing coal rank the size
of anisotropic microtexture increases. Based on this observation coal rank and maceral
composition have been used to predict coke reactivity. However the prediction was not
valid for all cases. Other factors such coal fluidity, mineral matter and carbonization
conditions could be the explanation for the poor relationship found between coke
reactivity and both coal rank and maceral composition.
CHAPTER 3 Thesis Objectives
To make the blast furnace more sustainable the efficiency of the furnace operated under
current technologies must be improved and also new technologies must be considered.
In order to achieve this, a good understanding of coke degradation in the furnace is
required to be able to prepare cokes of suitable quality. Gasification has been identified
as an important factor that affects coke degradation in the furnace. Coke gasification is
controlled by coke properties and the blast furnace environment.
Many studies have investigated the effect of microtexture, surface area and to a lesser
extent mineral matter on coke reactivity. Although some agreement has been found
between coke properties and reactivity there are still inconsistencies in their
relationship. The aims of this project are to develop a fundamental understanding of the
effect of coke properties such as microtexture, surface area, carbon crystallite size (L
c
and L
a
), and mineral matter on coke reactivity and to determine their relative
importance in the gasification process. Because coke properties are strongly related to
the properties of the parent coal, the influence of coal properties such as coal rank,
maceral composition and mineral matter on coke properties will be also investigated.
Of all the coke properties the influence of mineral matter on coke reactivity is least
understood. Although previous studies have concentrated on ash yield and elemental ash
composition it has been recognized that the forms of the mineral matter in the coke play
an important role in controlling coke reactivity. However, there is currently limited
information about the form and the relative concentration of minerals present in
metallurgical cokes. In this project the mineral forms present in coke will be identified
and their relative proportions will be determined. The influence of the catalytic minerals
present in the cokes on coke reactivity will be also investigated. Because mineral matter
transformation during gasification has been poorly investigated previously another
58
Chapter 3 Thesis Objectives
59
objective will be to determine the effect of gasification on mineral matter, especially on
the catalytic mineral phases.
Coal rank, maceral composition and mineral matter determine coke properties.
Separating these factors and then determining their effect on the reaction rate will
improve the understanding of their effect on coke properties. A comprehensive analysis
regarding the effect of coal rank and maceral composition on coke properties and coke
reactivity will be carried out on carbonised maceral-enriched fractions prepared from
coals of different rank. To explain the mineral phases formed in cokes the mineralogy of
the parent coal will be identified.
Many blast furnace operators use parent coal properties such as rank, maceral
composition and to a lesser extent ash chemistry to predict coke reactivity. However,
the prediction of coke reactivity using these parameters has not been found valid at all
times. Therefore a better understanding of the importance of coal properties on coke
reactivity would improve the accuracy of the prediction of coke reactivity.
To summarise, the broad outlines of the project are as follows:
- Establish the effect of rank and maceral composition of the parent coal on coke
properties. Also, the influence of coke properties such as microtexture, surface area
and carbon crystallite size (L
c
and L
a
) on coke reactivity is investigated.
- Mineral matter characterisation (qualitative and quantitative) of the raw cokes and
their parent coals. The occurrence of catalytic mineral matter in the cokes is also
investigated.
- Establish the influence of the catalytic minerals present in cokes on coke reactivity.
- Investigate the effect of gasification on mineral matter in coke (qualitative and
quantitative). Also, the influence of gasification on catalytic mineral matter is
investigated.
CHAPTER 4 Experimental
4.1 Parent coals
Nine Australian bituminous coals from New South Wales and Queensland having a
wide range of rank, maceral composition and mineral matter were selected to examine
the effect of coal properties on the gasification reaction rate of cokes produced from
these coals. The coals were sent externally (Amdel laboratories, Newcastle, NSW) for
chemical analyses, namely proximate analysis and ash composition. The petrographic
analysis was carried out at Commonwealth Scientific Industrial Research Organisation
(CSIRO) Energy Technology laboratory. In Table 4.1 are listed the proximate
analysis, ash analysis and petrographic analysis.
60
Chapter 4 Experimental
Table 4.1 Proximate, petrographic and ash analyses of the parent coals used for
preparation of cokes in this work.
Coal A B C D E F G H I
Proximate Analysis (wt %, air-dried basis)
Moisture 1.9 2.4 2.5 2.4 2.7 1.4 1.1 1.5 1.5
Ash 6.2 5.6 7.7 7.10 9.1 7.0 9.8 9.7 9.6
Volatiles 34.0 28.9 26.2 22.3 22.8 21.3 20.2 20.2 17.6
Fixed Carbon 59.8 65.5 66.1 70.6 68.1 71.7 70.0 70.3 72.8
Coal Rank (%, mean maximum vitrinite reflectance in oil)
R
0
max. 0.95 1.00 1.05 1.18 1.19 1.27 1.29 1.40 1.61
Petrographic Analysis (vol %)
Vitrinite 80.0 59.0 49.6 55.2 51.2 57.7 31.6 67.7 75.0
Liptinite 3.7 3.4 2.6 0.0 0.0 0.0 0.0 0.0 0.0
Inertinite 13.3 36.2 43.8 42.0 45.4 39.7 65.4 27.5 20.3
Mineral Matter 3.0 1.4 4.0 2.8 3.3 2.6 3.0 4.8 4.7
Ash Analysis (wt %)
SiO2 50.80 61.40 53.60 47.30 53.80 56.90 48.30 61.60 58.10
Al2O3 37.9 28.3 28.4 36.3 33.1 18.3 37.9 28.1 27.4
Fe2O3 4.60 4.30 7.60 5.00 4.50 12.80 5.30 3.20 6.20
CaO 1.10 1.30 3.00 2.80 2.30 3.70 2.50 1.30 1.60
MgO 0.32 0.34 0.95 0.63 0.54 1.60 0.58 0.46 0.70
TiO2 1.90 1.50 1.40 1.90 1.50 1.10 1.40 1.40 1.60
Na2O 0.55 0.30 0.57 0.57 0.41 0.45 0.65 0.72 0.45
K2O 0.76 0.48 1.00 1.10 0.69 0.92 0.54 1.10 1.00
P2O5 0.74 0.79 1.70 1.80 1.50 1.30 1.90 0.84 0.80
Mn3O4 <0.02 <0.02 0.05 0.08 0.04 0.06 0.03 0.04 0.09
SO3 0.09 0.26 0.76 0.74 0.37 2.00 0.32 0.25 0.49
Cr2O3 <0.02 <0.02 <0.02 <0.02 <0.02 <0.02 <0.02 <0.02 <0.02
CuO <0.02 <0.02 <0.02 <0.02 <0.02 <0.02 <0.02 <0.02 <0.02
V2O5 0.09 0.05 0.05 0.05 0.04 0.04 0.02 0.05 0.07
ZnO 0.04 <0.02 <0.02 <0.02 <0.02 <0.02 <0.02 <0.02 <0.02
NiO <0.02 <0.02 <0.02 <0.02 0.03 <0.02 <0.02 <0.02 <0.02
BaO 0.10 0.03 0.15 0.22 0.10 0.09 0.22 0.09 0.04
SrO 0.08 0.04 0.08 0.29 0.09 0.05 0.13 0.05 0.05
Total 99.15 99.19 99.39 98.86 99.07 99.39 99.87 99.28 98.67
61
Chapter 4 Experimental
4.2 Preparation of maceral-rich fractions
Inertinite- and vitrinite-rich fractions were prepared from five of the nine coals, namely
B, C, D, F and G. Liptinite was present only in two of the selected coals (B and C) in
low concentration (3.4% and 2.6%), consequently it was not considered in this work.
The coals were crushed to less than 1 mm size and the fraction 0.45 1.00 mm was
used for preparation of the maceral-enriched fractions.
The method used to separate macerals in this study was sink-float, using organic liquids
of different densities. This method is based on the different densities of the macerals.
Vitrinite density is lower than that of inertinite [36]. Solutions of different densities
were prepared for maceral separation. The solution is a mixture of a light density
component and a heavy density component prepared to have a specific density. Hexane
( = 0.66 g cm
-3
) was the light density liquid and the heavy density component was
perchlorethylene ( = 1.62 g cm
-3
).
Figure 4.1a depicts the maceral separation device. It comprises two glass flasks,
connected by a ground glass joint. The top flask has a funnel shape and its volume is
two litres. The top flask was designed with a Teflon stopcock and an inlet at its top. The
bottom flask is a one litre Erlenmeyer flask. About three litres of solution was necessary
to fill the flasks. The coal was poured into the top flask through the inlet using a funnel
and then stirred well. After stirring the stopcock was turned on to allow to the coal
particles, with greater density than that of the liquid, to settle into the bottom flask. The
coal particles with lower density than that of the solution went to the top. The separation
process lasted about 16 hours. After the separation had been completed the stopcock
was turned off and the flasks were disconnected. Beneath the top flask, still supported
by a metallic ring fixed on the stand, were placed a funnel with filter paper supported by
a Berzelius beaker, Figure 4.1b. The filter paper used was no.1 Whatman qualitative
filter paper. Then the stopcock was turned on and the flask was emptied. The coal
particles from the bottom flask were collected just by pouring the content of the flask
into a similar device like that described in Figure 4.1b. The coal fractions still in the
filter papers were dried in an unheated vacuum oven under nitrogen flow.
62
Chapter 4 Experimental
(a)
(b)
Figure 4.1 Schematic of the maceral separation device.
63
Chapter 4 Experimental
Petrographic characterisation was performed on the maceral enriched fractions mounted
in resin ( see section 4.5.1).
In order to decide the density range of vitrinite- and inertinite-rich fractions a test was
performed for each coal. 100 g of each coal was separated using seven solution
densities, namely 1.25, 1.30, 1.35, 1.40, 1.45, 1.50 and 1.55 gcm
-3
. Figure 4.2 shows
the concentration of macerals and mineral matter of each separated fraction as a
function of density for coal F. This was a general trend for all five coals. Low density
fractions were rich in vitrinite and low in inertinite. The percentage of inertinite started
to increase significantly in fractions with density greater than 1.35 gcm
-3
. The content
of mineral matter increased with increasing density. The density of the fraction richest
in vitrinite or inertinite was then selected for the bulk separation of the coals. About 1
kg from each coal underwent the sink-float process. A maximum 150 g of coal was used
for each batch of sink-float separation.
0
20
40
60
80
100
<1.25 1.25-1.30 1.30-1.35 1.35-1.40 1.40-1.45 1.45-1.50 1.50-1.55 >1.55
Density fraction (g cm
-3
)
M
a
c
e
r
a
l
(
%
)Vitrinite
Inertinite
Mineral matter
Figure 4.2 The concentration of macerals and mineral matter in the separated
fractions of coal F.
64
Chapter 4 Experimental
Table 4.2 shows the petrographic analysis of the vitrinite-rich fractions, intermediary
fractions and inertinite-rich fractions after the separation was completed. The
concentration of vitrinite in the vitrinite-rich fractions varied from 83.9 to 96.2 %. The
inertinite concentration in the inertinite-rich fractions was lower than the vitrinite
concentration in vitrinite rich fractions; it ranged between 72.1 -77.0 %. Along with the
vitrinite- and inertinite-rich fractions an intermediary fraction was produced, which had
greater density than vitrinite-rich fractions and lower density than the inertinite-rich
fractions. The intermediary fractions were used to prepare synthetic low ash coals
(SLACs) with maceral composition similar to that of the original coal but with less ash
yield. The intermediary fractions of coals B and G were called synthetic low ash coals
because they had the maceral composition similar to the coal source. The intermediary
fractions from coals C, D and F were richer in inertinite than the corresponding original
coal. In order to prepare the synthetic low ash coals the intermediary fractions were
mixed with vitrinite-rich fractions from the same coal in certain ratios. The ash content
of the vitrinite and inertinite rich fractions, the synthetic low ash coals and the original
coals from the proximate analysis are shown in Table 4.3.
65
Chapter 4 Experimental
Table 4.2 Petrographic analysis of the coal fractions and the original coal.
Vitrinite
(vol. %)
Liptinite
(vol. %)
Inertinite
(vol. %)
Mineral
Matter
(vol. %)
Coal B
Vitrinite-rich fraction 94.8 1.8 3.2 0.2
Intermediary fraction 59.3 3.4 36 1.1
Inertinite-rich fraction 20.5 3.3 73.9 2.3
Original coal 59.2 3.2 35.8 1.7
Coal C
Vitrinite-rich fraction 85.2 1.5 12.3 1.0
Intermediary fraction 40.3 3 54.9 1.8
Inertinite-rich fraction 16.00 3.80 76.90 3.3
Original coal 51.2 2.7 42.8 3.3
Coal D
Vitrinite-rich fraction 89.3 0.3 10.3 0.1
Intermediary fraction 37.2 0.1 60.3 2.4
Inertinite-rich fraction 24.3 0.3 72.1 3.3
Original coal 52.8 0.3 43.6 3.3
Coal F
Vitrinite-rich fraction 96.2 0 3.4 0.4
Intermediary fraction 51.7 0 46.6 1.3
Inertinite-rich fraction 22.3 0 75.2 2.5
Original coal 57.3 0 40.0 2.7
Coal G
Vitrinite-rich fraction 83.9 0 15.4 0.7
Intermediary fraction 33.8 0 64.2 2
Inertinite-rich fraction 20.9 0 77.0 2.1
Original coal
32.9 0 63.7 3.4
66
Chapter 4 Experimental
Table 4.3 Ash concentration in the vitrinite- and inertinite-rich fractions, the
synthetic low ash coals and the original coals from the proximate
analysis.
Ash, db (wt. %) Proximate analysis
B C D F G
Vitrinite-rich fraction 0.79 2.11 1.92 1.18 2.54
Inertinite-rich fraction 9.68 9.66 11.08 10.06 9.73
Synthetic low ash coal (SLAC) 4.06 5.04 5.25 4.95 6.57
Original coal 5.60 7.70 7.10 7.00 9.80
4.3 Coke preparation
4.3.1 The large scale coke ovens
The coals as received were crushed in a jaw crusher and sieved at 6 mm to provide a
product with a nominal particle size of less than 6 mm. Prior to carbonization the
crushed samples were stored in drums in nitrogen to prevent oxidation.
The 9 kg coke oven
A schematic of the 9 kg coke oven is shown in Figure 4.3. The coking vessel was a
cylindrical retort with a nominal capacity of 9 kg (189 mm diameter and 800 mm high)
heated by a furnace fitted with silicon carbide elements. The temperature of the furnace
was controlled by a programmable controller. The coal was packed into the retort in
four increments of 2.45 kg each. Each of the increments was tamped down to a pre-
determined height of 100 mm in order to achieve the desired bulk density of the charge.
The bulk density of the dried coal charge was around 850 kg m
-3
. A thermocouple was
placed at the geometric centre of the charge. The retort was closed using an end plate
67
Chapter 4 Experimental
and gasket. The end plate was designed with a gas off-take tube to remove the evolved
gases and tars during the carbonization process.
Figure 4.3 A diagram of the 9 kg coke oven.
The retort was introduced into the furnace after the furnace wall temperature had
reached 1050C. The temperature of the furnace dropped to approximately 700C due to
the cooling effect of charging the retort. From this temperature the furnace started to
heat the retort with a heating rate of 3C per minute. After the temperature of the centre
of the charge had reached 900C, the retort was held in the furnace for a further 50
68
Chapter 4 Experimental
minutes to raise the temperature up to 1050C in the centre of the charge. The total time
in the furnace was about 3 hours. The furnace and charge temperatures were recorded
during the carbonization process by a computer. The retort was then removed from the
furnace and allowed to cool in a nitrogen atmosphere. The proximate and ash analyses
of the cokes are presented in Table 4.4.
Table 4.4 Proximate and ash analyses of the cokes prepared in the 9 kg oven.
Coke A B C D E F G H I
Proximate Analysis (wt %, air-dried basis)
Moisture 0.7 0.7 0.8 0.7 1.1 1.9 0.8 0.8 0.6
Ash 9.1 7.9 10.3 9.3 11.9 9.0 11.8 12.2 11.9
Volatiles 0.7 0.8 0.3 0.7 0.5 0.6 0.6 0.4 0.6
Fixed Carbon 90.2 91.3 89.4 90.0 87.6 90.4 87.6 87.4 87.5
Ash Analysis (wt %)
SiO2 51.50 62.00 54.50 48.10 54.30 58.00 48.00 61.30 56.90
Al2O3 38.30 29.00 29.30 36.80 33.80 19.10 37.50 28.30 27.00
Fe2O3 4.20 3.90 7.30 4.70 4.30 12.70 5.60 3.10 8.50
CaO 1.10 1.20 2.70 2.60 2.10 2.90 2.40 1.30 1.60
MgO 0.21 0.20 0.90 0.59 0.43 1.60 0.52 0.38 0.78
TiO2 1.80 1.50 1.40 1.80 1.40 0.99 1.40 1.40 1.40
Na2O 0.42 0.11 0.20 0.38 0.27 0.32 0.58 1.50 0.35
K2O 0.76 0.55 1.10 1.10 0.75 0.92 0.52 1.10 1.00
P2O5 0.72 0.74 1.60 1.80 1.40 1.20 1.80 0.84 0.71
Mn3O4 0.00 0.00 0.05 0.05 0.04 0.05 0.04 0.03 0.14
SO3 0.03 0.16 0.41 0.48 0.21 1.10 0.25 0.17 0.43
Cr2O3 0.00 0.02 0.00 0.04 0.00 0.17 0.00 0.02 0.00
CuO 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00
V2O5 0.06 0.03 0.03 0.05 0.05 0.03 0.02 0.05 0.06
ZnO 0.05 0.02 0.00 0.03 0.00 0.00 0.00 0.00 0.00
NiO 0.00 0.00 0.00 0.00 0.02 0.02 0.00 0.00 0.00
BaO 0.06 0.00 0.08 0.15 0.08 0.00 0.24 0.06 0.04
SrO 0.08 0.04 0.07 0.28 0.09 0.05 0.14 0.05 0.04
Total 99.29 99.47 99.64 98.95 99.24 99.15 99.01 99.60 98.95
69
Chapter 4 Experimental
The 400 kg coke oven
It is a 400 kg capacity, movable wall oven with a width of 0.450 m. The fixed and
moving walls are electrically heated. The nine test coals were each packed into one of
12 compartments (the remaining 3 compartments contained other test coals not
associated with this work). The 12 compartments were surrounded by a dummy layer of
moist coal placed at the bottom, ends and top of the 12 compartments. The mass charge
of each coal was 15.5 kg. The bulk density of the dried coal charge was 825 Kg m
-3
.
After the temperature in the centre of the charge had reached 900C, the charge was
held in the oven for another 4 hours and 15 minutes to reach 1039C in the centre. Then
the charge was pushed from the oven and quenched as monolith with water. The total
coking time was about 20 hours.
4.3.2 The 70 g coke oven
The maceral-enriched fractions prepared and the original coals were carbonised in a
small scale (70 g) oven. They were carbonized in two stages because the retort used in
the first stage was made of aluminium, which set the maximum temperature of
carbonisation to be 470C, since aluminium melts at 660 C. The semi-coke prepared in
the first stage was calcined to 1050C in a horizontal tube furnace.
Preparation of the semi-cokes
A diagram of the 70 g oven is presented in Figure 4.4a. The oven was a wire wound
vertical tube furnace with an internal diameter of 90 mm and a length of 300 mm. The
heating rate was controlled by a digital temperature controller. The oven was preheated
to 300C before the retort was introduced into the furnace. The furnace was continually
purged with nitrogen to avoid the oxidation of the sample. The retort was lined with
filter paper to prevent the plasticised coal adhering to it. Sub-samples of 70 g were
packed into the carbonization retort at a bulk density in the range 0.68 to 0.80 g cm
-3
.
An aluminium plunger was used to compact the sample into the retort. After the sample
70
Chapter 4 Experimental
was packed-in a steel disc was placed between the sample and the plunger. The steel
disc supported a ceramic sheath, which was inserted through both the static mass and
the aluminium plunger. A micrometer barrel and a linear transducer were placed on top
of the ceramic sheath. The linear transducer transmitted the contraction/dilatation of the
charge to a computer to be recorded. The role of the micrometer barrel is to measure the
dilatation of the charge if it exceeded the measuring range of the linear transducer
during carbonization. During the carbonization process the plunger supported a mass
which applied a static load of 10 kPa. Two thermocouples measured the temperature in
the geometric centre of the charge and the temperature of the retort. The temperatures
were recorded by a computer. After the temperature in the centre of the charge reached
300C the coal sample was heated up to 470C at a specific rate and held at this
temperature for 2 hours. Then the semi-coke produced was cooled under nitrogen flow.
The vitrinite-rich fractions swelled more than the coals and inertinite-rich fractions.
High swelling leads to high porosity in the carbonization product. Therefore, to control
the porosity in the coke, the heating rate of the vitrinite-rich fractions had to be lower
than the heating rate of the coals and the inertinite-rich fractions. The heating rate from
300 to 470C was 1C per minute for the original coals and the inertinite-rich fractions.
The vitrinite-rich fractions of coals B, C, D and F were carbonized at 0.1C per minute.
The vitrinite-rich fraction of coal G was heated up at even lower heating rate, 0.05C per
minute.
Preparation of calcined cokes
An electrically heated, horizontal tube furnace was used to calcine the semi-coke
samples (Figure 4.4b). The furnace consisted of an alumina furnace tube with an
alumina sheath inside the furnace tube. The thermocouples measured the temperature of
the furnace and the semi-coke samples within the alumina sheath. These two
temperatures were recorded by a computer during the calcination process. The semi-
cokes samples were put into alumina boats and inserted into the centre of the furnace at
ambient temperature. The open end of the alumina sheath was sealed with a gas tight
end cap. The alumina sheath was purged with high purity nitrogen. The furnace was
71
Chapter 4 Experimental
heated to 500C at a heating rate of 1C per minute, then at 10C per minute from 500C
to 1050C. The purge gas was changed from high purity nitrogen to ultra high purity
argon after the temperature reached 500C. At the completion of the calcining stage the
samples were cooled down inside the furnace under ultra high purity argon. Below
500C the purge gas changed back to high purity nitrogen.
72
Chapter 4 Experimental
(a)
(b)
Figure 4.4 A schematic diagram of (a) the 70 g coke oven and (b) the calcination
furnace.
73
Chapter 4 Experimental
4.4 Coke reactivity test
The prepared cokes from both large and small ovens were then crushed to less than 1
mm and then the 0.6 1.0 mm fraction was used to carry out the reactivity test. Coke
specimens were dried at 105C overnight and then a 1.4 g specimen was taken for the
reactivity test.
4.4.1 Coke reactivity reactor system
A schematic diagram of the fixed-bed reactor system is provided in Figure 4.5. A
similar system was used by Harris and Smith [55] and Roberts [121] in their work. The
reactor system comprised a fixed bed of sample supported in a quartz reaction tube by a
sintered glass frit. The quartz tube was placed in an electrically heated furnace. The
temperature of the furnace was controlled by a Eurotherm temperature controller. The
reactant gas passed through the sample bed from top to the bottom at a flow rate of
approximately 0.750 Lmin
-1
. A series of Brook mass-flow controllers controlled the
flow rate of the gas. The design of the reactor allows for a thermocouple to measure the
temperature in the centre of the sample bed. Carbon monoxide was the gas product of
the reaction. The concentration of carbon monoxide in the exhaust gas was measured
continuously using a non-dispersive infrared analyser (Horiba Model PIR 2000), and
recorded by a computer system. The temperature of the sample was also recorded. The
fixed-bed reactor system also allows the activation energy to be measured for every
experiment. In order to do this the power of the furnace was switched off and the
reaction rate was measured as the temperature decreased. The weight loss of the sample
was insignificant during this period (less than 1%). The repeatability error of the
experiments was less than 10%. The reaction rate was calculated using the
concentration of CO measured by the infrared analyser (see Section 4.4.2).
Coke gasification with carbon dioxide was performed in 100% CO
2
anaerobic grade
(99.95%). Before entering into the reactor, the reactant gas (carbon dioxide) was passed
through an oxygen trap and a Drierite column to remove traces of oxygen and moisture,
respectively. The reaction temperature was selected on the basis of the reactivity of the
74
Chapter 4 Experimental
coke, so that the percentage of carbon monoxide in the outlet gas did not exceed 1%.
Low concentrations of carbon monoxide had negligible inhibition effect on the reaction
rate [55,65]. For these samples the temperature chosen ranged between 873C and
930C for the most reactive and the least reactive cokes, respectively. An example of
typical data obtained from the experiment, in which the reaction rate and activation
energy were determined, are presented in Figure 4.6.
Figure 4.5 A schematic diagram of the fixed-bed reactor system.
75
Chapter 4 Experimental
0
5
10
15
20
0 2 4 6 8 10 12 14 16 18
Carbon conversion (%)
A
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
Reactor cooling
(a)
-13.0
-12.5
-12.0
-11.5
-11.0
-10.5
0.00083 0.00085 0.00087 0.00089 0.00091
1/T (K
-1
)
l
n
(
R
a
t
e
)
(
g
g
-
1
s
-
1
)
(b)
Figure 4.6 Typical results from a FBR experiment; (a) Reaction rate versus
carbon conversion and the region were the furnace was cooling
down and (b) Arrhenius plot from the region were the furnace was
cooling down.
76
Chapter 4 Experimental
4.4.2 Reaction rate calculation
The apparent reaction rate (
a
) was calculated according to Equation 4.1, where w is the
mass of sample remaining at time t.
|
.
|
\
|
=
dt
dw
w
1
a
(g g
-1
s
-1
) Equation 4.1
This method for calculating the mass loss during carbon dioxide gasification was based
on the concentration of the carbon-containing gas product (carbon monoxide) and the
gas flow rate. Carbon monoxide production (n
CO
) at a given instant was determined
according to the Equation 4.2:
RT
F ] CO [ P
n
CO
= (mol s
-1
) Equation 4.2
where P (atm) is the pressure of the gas in the reactor, [CO] is the concentration of
carbon monoxide at this instant, F (L s
-1
) is the measured gas flow rate, R (J K
-1
mol
-1
)
is the universal gas constant and T (K) is the temperature of the gas. Using the carbon
monoxide production values from the IR analyser, the rate of carbon loss (r) at any
instant can be calculated using Equation 4.3:
2
12.01 n
r
CO
= (g s
-1
) Equation 4.3
The sample mass loss (m) to this time was given by Equation 4.4:
) t r ( m
t
0
A = A
_
(g) Equation 4.4
Equation 4.1 can be rewritten as a function of the weight loss of the sample and rate of
carbon loss. Therefore, the apparent rate at any instant can be calculated by Equation
4.5:
77
Chapter 4 Experimental
m m
r
0
a
A
= (g g
-1
s
-1
) Equation 4.5
where m
0
is the initial weight of the sample. The apparent reaction rate is expressed in
grams of carbon reacted per gram of carbon remaining per second. In the calculation the
sample mass was ash-free.
4.4.3 Activation energy measurement
The activation energies were measured to provide information about reaction conditions
and to normalise the reaction rates of the cokes during the experiment to a selected
temperature. The reaction rate could be expressed as an Arrhenius type equation:
|
.
|
\
|
=
RT
E
exp A
a
a
Equation 4.6
where E
a
(kJ mol
-1
) is the activation energy, A (g g
-1
s
-1
) is the pre-exponential factor, R
(J K
-1
mol
-1
) is the universal gas constant and T (K) is the coke bed temperature. Taking
logarithms on both sides, Equation 4.6 can be rewritten as:
T
1
R
E
A ln ln
A
a
= Equation 4.7
The apparent activation energy was calculated from the slope of the straight line
obtained by plotting ln
a
against 1/T (Figure 4.7b). The intercept of the line at 1/T = 0
(ln A) gives us the pre-exponential factor.
The reactivity of the cokes at a fixed temperature was calculated using Equation 4.8:
|
|
.
|
\
|
|
|
.
|
\
|
=
1
a
2
a
1 2
RT
E
exp
RT
E
exp
Equation 4.8
78
Chapter 4 Experimental
where
2
is the reaction rate at 900C,
1
is the measured reaction rate, T
2
is the selected
temperature (900C) and T
1
is the sample temperature during the experiment. Equation
4.8 can be rewritten as:
)
`
|
|
.
|
\
|
=
2 1
a
1 2
T
1
T
1
R
E
exp Equation 4.9
4.5 Coke samples characterisation
4.5.1 Optical microscopy
Sample preparation
The samples were prepared as polished particulate blocks. Approximately 0.5 - 1.0 g
representative sample was placed into a silicone rubber mould (25mm 25 mm 10
mm deep). The Epoxy Resin LC - 191 was mixed with Hardner HY 956 in a ratio 1:4
at 50C. A small amount of the mixture was poured into the mould to form a thick paste
consistency (about 3 mm deep) and then a 400 kPa pressure was applied for 16 hours.
The remaining volume of mould was filled with resin, labelled and again placed under
pressure to set. The block was polished in seven stages. In the first four stages was used
Struers Planopol 2/3 grinder and silicone carbide paper of different qualities P240,
P400, P800 and P1200. Struers Microcloth with 1 micron Linde C alumina, 0.05 micron
Linde B alumina and 0.04 micron Struers OPS colloidal silica on a Struers OP Chem
cloth were used for the last three stages.
79
Chapter 4 Experimental
Optical microscopy examination
A. Coal rank
Coal rank, expressed by its Maximum Reflectance (Rmax %), was measured with
reflected light microscope-photometer (Leitz-Orthoplan) in a green light (546nm) on
Vitrinite (maceral Telocollinite). In this technique the intensity of the light reflected
from the polished surface of coal under examination is compared to the intensity of the
light reflected from the standard of known reflectance. Since coals of higher rank are
distinctly anisotropic its maximum and minimum reflectance can be determined in
polarized light. To measure the Maximum Reflectance the stage of the microscope was
rotated 360 degrees and maximum value found was recorded.
B. Coal maceral analysis
Maceral analyses of coals and their fractions as well as coke texture analysis were
performed using a Zeiss Universal optical microscope. The maceral samples were
analysed in a reflected plane polarised light using a 40x oil objective (with total
magnification of 500x). The proportions of each maceral were determined by a point
counting procedure. The stage of the microscope allowed advancing the specimen by
equal steps. Each component falling under the cross fitted in the eye piece of the
microscope was determined and the counts in each category were registered by an
automatic Swift point counter. The sample content was expressed in % volume and
based on minimum 500-point counts. The analysis was performed according to
Australian Standard (AS2856) based on ICCP guidelines and ISO7404.
C. Coke textural analysis
For the analysis of cokes was used an accessory full wave plate to achieve optimum
optical effect to discriminate between its textural components (the plate is placed
between the specimen and analyser to impart the interference colours generated during
the rotation of analyser). Figure 4.7 shows photomicrographs of different types of
microtexture in coke.
80
Chapter 4 Experimental
F
(a)
C
F A
I
(b)
Figure 4.7 Different type of microtexture shown by (a) coke C and (b) coke F;
where FA is flow-like anisotropy, C is coarse mosaic, F is fine mosaic
and I is isotropic.
81
Chapter 4 Experimental
4.5.2 Surface area
Micropore surface area of the raw and reacted cokes was measured using nitrogen and
carbon dioxide adsorption techniques. Nitrogen was adsorbed on the surface of the coke
particle at 77 K and carbon dioxide was adsorbed at 273 K. The micropore range
includes slit-shaped pores with size less than 0.6-0.7 nm and larger pores
(supermicropores) with size between 0.6-0.7 nm and 1.5-1.6 nm [80]. Only carbon
dioxide can penetrate the slit-shape micropores. Nitrogen at 77K has lower kinetic
energy than carbon dioxide at 273K; therefore the diffusion of nitrogen molecules into
the narrow microporosity is limited [122]. Nitrogen measures the surface of the
supermicropores and the mesopores with size between 1.5-150 nm [123]. This means
that nitrogen and carbon dioxide supplement each other and can provide more complete
information about the microporous structure.
A Micromeritics ASAP 2400 surface area analyser was used for these measurements.
Carbon dioxide and nitrogen were 99.995% purity. Prior to analysis, samples were
vacuum degassed, at 300C, to an ultimate vacuum of less than 10 Pa. The BET
(Brunauer-Emmett-Teller) and Dubinin-Radushkevich equations were used to
determine the total surface area of the sample from nitrogen and carbon dioxide
isotherms.
4.5.3 Carbon structure
The raw and reacted coke samples were analysed using x-ray diffraction (XRD). The
samples before analysis were finely ground using a pestle mortar. The XRD analyses on
the cokes were run on a Philips PW1050 goniometer using CoK radiation at 45kV.
Step scans were undertaken from 3 90 2, with a step interval of 0.04 2 and 10
seconds count time per step.
Carbon crystallite size (L
c
and L
a
) was measured using the X-ray diffraction patterns.
The details of the carbon crystallite structure have been discussed in section 2.2.1. The
American Standard Test Method D 5187-91 was the procedure used to determine L
c
. A
82
Chapter 4 Experimental
similar procedure was used to determine L
a
. This technique allows measuring the
carbon crystallite height - L
c
(002 band; 2 ~ 30) and length - L
a
(100 band; 2 ~ 51).
The crystallite size was calculated using the Scherrer equation [40], which is:
Bcos
K
L
a/c
= Equation 4.10
where, K is a constant depending on the reflection plane (K = 0.89 for the 002 band, and
K = 1.84 for the 100 band [41]); is the wavelength of the incident X-rays (for cobalt
K radiation, = 1.7889); B is the width of the corresponding band at half maximum
intensity and is the peak position.
4.5.4 Qualitative and quantitative analysis of mineral phases
The mineral phases present in the raw and reacted cokes were identified using X-Ray
diffraction analysis. The carbon was removed from the mineral matter with minimal
alteration of the mineral species using radio-frequency oxygen plasma ashing at low
temperature (120C) LTA (low temperature ashing) [124]. The XRD analysis of the
cokes was carried out on a Philips PW1050 goniometer using CoK radiation at 45kV
and 30mA, with step scans from 3 90 2, a step interval of 0.04 2 and a 10sec count
time per step.
Mineral phases identification was performed using Bruker Eva search/match software
(Figure 4.8). SIROQUANT [125], a personal computer quantitative X-ray diffraction
analysis software package, was used to quantify the minerals in the ash. The software
was developed by CSIRO and uses the full-profile Rietveld method of refining the
shape of a calculated XRD pattern against the profile of a measured pattern. In order to
calculate a synthetic XRD pattern for a mineral, SIROQUANT use the data from the
hkl file that contains the XRD pattern of each mineral. The total calculated pattern is
the sum of the calculated patterns of the individual phases. SIROQUANT overlays the
measured XRD pattern with the calculated pattern to calculate a synthesized pattern.
SIROQUANT also calculates a difference pattern, which displays the difference in
intensity between the measured and calculated patterns at every point. The fit of a
83
Chapter 4 Experimental
calculated pattern to the measured (observed) data is a figure of merit, Chi-squared ().
A value below 3.0 indicates a well refined pattern. Classical Rietveld procedures can
only be applied to crystalline phases. A procedure has been developed in SIROQUANT
that allows the determination of poorly crystalline and amorphous phases such as some
clays (amorphous melt in cokes) to be quantified through the use of observed (hkl) files.
A typical plot from SIROQUANT is shown in Figure 4.9. The mineral phase
quantification error is typically less than 0.3% in the LTA samples, which translates to
less than 0.03% on a coke basis.
Coke F
00-037-0477 (*) - Troilite-2H - FeS
01-089-0552 (C) - Rutile, syn - Ti0.928O2
00-046-1045 (*) - Quartz, syn - SiO2
00-029-0725 (I) - Pyrrhotite-6T - Fe1-xS
00-008-0464 (I) - Oldhamite, syn - CaS
00-015-0776 (I) - Mullite, syn - Al6Si2O13
01-073-1959 (C) - Magnesium Aluminum Oxide - MgAl2O4
00-036-0427 (*) - Jarosite, hydronian syn - (K,H3O)Fe3(SO4)2(OH)6
00-006-0696 (*) - Iron, syn - Fe
01-070-1793 (C) - Iron Phosphate - FePO4
00-015-0876 (*) - Fluorapatite, syn - Ca5(PO4)3F
01-087-0700 (C) - Diopside, syn - CaMg.74Fe.26(Si2O6)
00-041-0224 (I) - Bassanite, syn - CaSO40.5H2O
00-021-1272 (*) - Anatase, syn - TiO2
00-035-0592 (*) - Akermanite, syn - Ca2MgSi2O7
File: lb3620.raw
I
n
t
e
n
s
i
t
y
(
c
o
u
n
t
s
)
0
200
400
600
800
1000
1200
1400
2 Theta
5 10 20 30 40 50 60 70 80 90
Figure 4.8 Identification of the mineral phases present in coke F using Bruker Eva
search/match software.
84
Chapter 4 Experimental
Figure 4.9 A typical SIROQUANT plot, which shows the observed and calculated
XRD patterns and the difference between them.
4.5.5 Visual analysis of mineral matter using FESEM
The distribution and association of the minerals in the raw and reacted cokes was
observed using scanning electron microscopy technique on polished blocks. The surface
of the specimens was made electrically conductive by the application of a thin coat of
carbon. The coating was few nanometres thick and did not interfere with the structure of
the specimen.
The field emission scanning electron microscope (FESEM) is essentially a traditional
scanning electron microscope, fitted with a field emission electron source, which
85
Chapter 4 Experimental
provides a very fine, but highly intense beam. This allows a higher resolution image
than with conventional SEMs. The model used in this work was a Hitachi S4500 fitted
with an Oxford Isis energy dispersive x-ray analyser (EDS). This enables a semi-
quantitative microchemical analysis of materials in the samples. The EDS can produce
microchemical analysis of a particular area on a sample, or can yield image maps, which
are based on a pre-defined set of elements. The depth of analysis is limited to the
interaction volume of the electron beam (typically ~ 2-3 m). Typical SEM image and
EDS analysis are presented in Figure 4.10.
Figure 4.10 A typical SEM image (top) and EDS analysis (bottom).
86
Chapter 4 Experimental
87
4.5.6 Analysis of mineral matter in coal using QEMSCAN
QEMSCAN
TM
[126] is an analytical technique which characterizes both coal and
associated mineral matter providing quantitative mineralogical, textural and chemical
data on a particle-by-particle basis. QEMSCAN creates digital images using both the
backscattered electrons (BSE) and energy dispersive X-ray signals (EDS) from a
scanning electron microscope (SEM). Each pixel of the digital images corresponds to a
mineral species or phase in a region under the electron beam. The identification of the
minerals is accomplished by comparing the collected X-ray spectrum with that of the
minerals, existent in a database, defined by their chemical composition.
CHAPTER 5 Kinetics of the Coke Carbon dioxide
reaction
Previous studies have identified a number of factors that influence coke reactivity such
as coke microtexture (defined by optical microscopy see section 2.2), carbon structure
(carbon crystallite size L
c
and L
a
see section 2.2), microporosity and mineral matter.
Also, the rank and maceral composition of the parent coal have been reported to
determine coke properties and consequently coke reactivity. Due to this relationship
coal rank [13,20,67,90] and, to a lesser degree, maceral composition [78] have been
frequently used to predict coke reactivity. The chemistry of coke ash has been also used
to predict coke reactivity [13,20,25,67,90].
In order to determine the influence of the above factors on the reactivity of the cokes
investigated in this project, the measurement of reaction kinetics and a thorough
characterisation of the cokes and parent coals were required. The reaction rate was
measured under chemically control conditions where the reaction is not limited by either
pore diffusion or external mass transfer (see section 2.3.1). Also, the reaction inhibition
by the reaction gas product (CO) was minimised (see section 4.4.1).
In this chapter the reaction kinetics of nine metallurgical cokes with carbon dioxide is
reported and the relationship between coke reactivity and both coke and parent coal
properties is discussed. Also, changes in the coke properties during gasification are
investigated.
88
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
5.1 Apparent reactivity experiments: Coke Carbon dioxide reactions
Figure 5.1 shows the apparent reaction rates with CO
2
(100%) of the nine cokes as a
function of carbon conversion. The apparent reaction rate was normalised to 900C
using the activation energy measured at the end of each experiment (see section 4.4.3).
The apparent reaction rate is reported as grams of reacted carbon per grams of
remaining carbon per second. A gradual increase of the apparent reaction rate with
increasing carbon conversion was observed at the initial stages of the reaction. The
reaction rate did not change significantly after 5 10 % carbon conversion for most
cokes (cokes A, D, E, H and I) whereas the reaction rate of the most reactive cokes,
namely coke F and coke C, still increased even after 15% carbon conversion.
The activation energy was derived from an Arrhenius plot of data collected during the
sample cooling at the end of the reactivity test. The Arrhenius plots are shown in Figure
5.2. The corresponding activation energies are presented in Table 5.1. All the activation
energies are in the range between 222 and 266 kJ mol
-1
.
0
10
20
30
40
50
60
0 5 10 15 20
Carbon conversion (%)
A
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
Coke F
Coke C
Coke D
Coke B
Coke G
Coke A
Coke E
Coke I
Coke H
Figure 5.1 The apparent reaction rate at 900C versus carbon conversion (100%
CO
2
).
89
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
-14
-13
-12
-11
-10
0.00080 0.00085 0.00090 0.00095 0.00100
1/T (K
-1
)
l
n
(
R
a
t
e
)
(
g
g
-
1
s
-
1
)
Coke F
Coke C
Coke D
Coke B
Coke G
Coke A
Coke E
Coke I
Coke H
Figure 5.2 Arrhenius plots obtained using data recorded during furnace cooling.
Table 5.1 The measured apparent activation energy of the reaction with carbon
dioxide of the cokes and their measured temperature ranges.
Coke Activation energy (E
a
)
(kJ mol
-1
)
Burn-off
(%)
Temperature range
(K)
A 247 16.3 1203 1128
B 266 16.8 1193 1119
C 245 17.5 1166 1079
D 252 18.1 1189 1128
E 256 15.3 1191 1118
F 222 17.5 1143 1043
G 258 18.4 1191 1116
H 239 17.6 1204 1137
I 229 17.3 1203 1128
90
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
Verification of regime I conditions
It was essential to establish that the reactivity measurements were made under
conditions of chemical rate control, free of any physical limitations due to gas pore
diffusion and mass transfer. Under chemical control conditions, the reaction rate should
not be affected by sample particle size and gas flow rate. Previous studies by Harris and
Smith [55,59] showed that the reaction rate of coke with carbon dioxide at 800C and
890C respectively was not affected by coke particle size between 0.2 2.0 mm and the
flow rate of the reactant gas (100% CO
2
) between 500 1000 mlmin
-1
at atmospheric
pressure. For this work the effect of the particle size and gas flow rate through the
reactor on the reaction rate was determined.
Table 5.2 lists the apparent reaction rates of cokes F and C at different gas flow rate and
particle size. Varying the size of the particles from 0.212 mm to 1.0 mm did not change
the reaction rate significantly. This means the partial pressure of the reactant gas could
be considered uniform through the coke particle. Also, changing the gas flow rate
through the sample bed did not change significantly the rate of reaction. It can be
concluded that the product gas (CO) was removed rapidly from the reaction site and did
not inhibit the reaction. Moreover, the relative density of all reacted samples showed a
linear relationship with burn-off (Figure 5.3), which means the particle size did not
change during reactivity test. This is a typical characteristic of chemically controlled
reactions (see section 2.3.1).
The activation energy values determined for the nine cokes were in the range 222266
kJ mol
-1
(Table 5.1). The magnitude of these numbers was consistent with the data
available in the literature for chemically controlled reaction rates: the activation
energies of the reaction of petroleum and metallurgical cokes with carbon dioxide
reported by Harris and Smith [59] and Pang et al. [58] were in the range 216-239 kJ
mol
-1
and 215-240 kJ mol
-1
, respectively. The reactions were carried out at 800C and
900C, respectively; the range of temperature for the activation energy measurement
was not specified for these experiments. Laurendeau [56] reported the activation energy
of the reaction of coke with carbon dioxide measured by Blake in a previous study. In
this case the activation energy was 239 kJ mol
-1
in the temperature range 850-900C.
91
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
Also, the activation energy measured by Kawakami et al. [57] for the reaction of coke
with carbon dioxide (regime I conditions) was 200 kJ mol
-1
, where the reaction
temperature was between 600 and 900C.
Table 5.2 The effect of particle size and flow rate on the reaction rate.
Coke C Coke F
Particle size
(mm)
Flow rate
(ml/min)
Rate*10
-6
(g g
-1
s
-1
)
Rate*10
-6
(g g
-1
s
-1
)
0.600 - 1.000 750 24.2 36.4
0.425 - 0.600 750 25.8 36.4
0.212 - 0.425 750 22.6 35.3
0.600 - 1.000 850 25.7 35.0
0.6
0.7
0.8
0.9
1.0
0 5 10 15 20 25 30 35
Conversion (%)
R
e
l
a
t
i
v
e
d
e
n
s
i
t
y
A
B
C
D
E
F
G
H
I
Theoretical line
Figure 5.3 Relative density as a function of burn-off; the theoretical line is the line
expected if Regime I conditions were followed.
92
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
5.2 Coke surface area and intrinsic reactivity
he surface areas of the raw and corresponding reacted cokes (approximately 15%
n increase in surface area, measured by both nitrogen and carbon dioxide, was
he greater surface area measured by nitrogen than carbon dioxide of the reacted cokes
mesopores (as measured by N
2
surface area) of the reacted cokes F and C.
T
carbon conversion) were measured using N
2
and CO
2
as adsorbate gases. Carbon
dioxide measures the surface area of the micropores (0.4 1.6 nm) and nitrogen
measures the surface area of larger micropores (supermicropores) with sizes between
0.6-0.7 nm and 1.5-1.6 nm and mesopores with sizes between 1.5-150 nm [123]. Figure
5.4 shows the N
2
and CO
2
surface areas of the raw and reacted cokes. Carbon dioxide
surface area was considerably greater than that measured using nitrogen, especially for
cokes F, C and H, and varied to a great degree from 2.3 to 64.5 m
2
g
-1
whereas nitrogen
adsorption in these cokes was very low, always less than 0.6 m
2
g
-1
. Greater surface
measured by CO
2
than N
2
indicates that the micropore structure of the raw cokes
consisted mainly of narrow (slit-shaped) microporosity that was inaccessible to nitrogen
at 77K.
A
observed after reaction indicating an increase in micro and mesoporosity (figure 5.4).
The increase in nitrogen surface area of the reacted cokes was dramatic and for most of
the cokes it was greater than the carbon dioxide surface area after reaction. However,
the most reactive cokes F and C still had greater carbon dioxide surface area than
nitrogen surface area. This suggests that in these cokes the narrow microporosity was
still significant. Narrow microporosity could be present in the rest of the cokes but it
could not be differentiated because nitrogen surface area was greater than carbon
dioxide surface area.
T
indicates that as gasification proceeds microporosity is enlarged and then followed by
coalescence, increasing both the supermicroporosity and mesoporosity. Also,
gasification did not only enlarge the existing micropores but also generated new
microporosity, which was probably developed at the early stages [43]. This continual
regeneration of new, accessible microporosity explains the significantly greater surface
area of micropores (as measured by CO
2
surface area) than both supermicropores and
93
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
0
20
40
60
80
100
N
2
s
u
r
f
a
c
e
a
r
e
a
(
m
2
g
-
1
)
Raw 0.24 0.32 0.56 0.44 0.31 0.39 0.49 0.56 0.48
Reacted 28.5 63.9 87.9 79.6 58.9 96.9 71.7 77.5 65.1
A B C D E F G H I
(a)
0
20
40
60
80
100
120
140
C
O
2
s
u
r
f
a
c
e
a
r
e
a
(
m
2
g
-
1
)
Raw 3.4 2.3 13.4 6.0 8.8 64.5 8.5 12.8 4.5
Reacted 21.7 54.5 96.6 64.2 45.5 131.9 56.4 31.3 23.9
A B C D E F G H I
(b)
Figure 5.4 Total surface area of raw and reacted cokes (approximately 15%
burnout) (a) measured using N
2
adsorption (b) measured using CO
2
adsorption.
94
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
C
mesoporosity
ores, measured by mercury porosimetry for different type of carbons, including
ences in the apparent reactivity
f the cokes. Figure 5.5a shows that the initial apparent reaction rate was poorly related
oorly related to the reaction rate (Figure 5.5b). These relationships suggest that
losed microporosity and mesoporosity could have a role in microporosity and
formation. Turkdogan et al. [43] concluded that the volume of the closed
p
metallurgical coke, is approximately 25-50%. Although mercury porosimetry does not
measure the volume of micropores, they may be present in the cokes. During
gasification, any closed microporosity and mesoporosity would open gradually and
contribute to the total microporosity and mesoporosity.
The initial and final apparent rates should increase with increasing surface area if
surface area was a major factor responsible for the differ
o
to nitrogen surface area. Also, the relationship between carbon dioxide surface area and
initial apparent reactivity was poor. It is concluded that the surface area of accessible
microporosity and mesoporosity plays little role in determining reaction rate at initial
stages for the cokes used in this study reactivity.
At later stages of the reaction (15% burnout) the relationship between reaction rate and
carbon dioxide surface area was much improved whereas nitrogen surface area was still
p
although surface area of micropores and mesopores increased significantly during
reaction and any closed porosity is believed to become more accessible, the reaction
occurred mostly on the surface of the micropores, and the surface of the mesopores
contributed less to total reactivity.
95
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
F
C
H
I
G
D
E
B
A
0
2
4
6
8
10
12
14
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7
N
2
initial surface area (m
2
g
-1
)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
B
G
I
E
D
A
H
C
F
0
2
4
6
8
10
12
14
0 20 40 60 8
CO
2
initial surface area (m
2
g
-1
)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
0
(a)
A
B
E
I
H
G
D
C
F
0
10
20
30
40
50
60
0 20 40 60 80 100 120
N
2
final surface area (m
2
s
-1
)
F
i
n
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
F
C
D
E
H
A
I
G
B
r
2
= 0.92
0
10
20
30
40
50
60
0 50 100 150
CO
2
final surface area (m
2
g
-1
)
F
i
n
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
(b)
igure 5.5 Dependence of apparent rates on total surface area measured using
both N
2
and CO
2
(a) initial apparent rate and (b) final apparent rate.
F
96
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
In order to remove the physical effect of surface area on the apparent rate, the intrinsic
te was calculated. The intrinsic rate was determined by dividing the apparent rate by
e surface area. Both carbon dioxide and nitrogen surface areas were used to determine
ra
th
the intrinsic rates at the initial and final (end of experiment) stages of the reaction. Table
5.3 shows the initial and final intrinsic rates and also the ratio (R) between the reaction
rate of the most reactive coke and the reaction rate of the least reactive coke. If the value
of R decreases after converting apparent to intrinsic rates, it suggests that surface area is
an important variable affecting the apparent rate. If R does not change, surface area is
less likely to be important. Normalising the initial apparent rates to both N
2
and CO
2
surface areas did not reduce significantly the range in reactivity of the cokes; the ratios
of the initial intrinsic rates calculated using both N
2
and CO
2
surface areas were 8.2 and
6, respectively. The final intrinsic rate determined using N
2
surface area still showed
significant difference between them as R was 6.3. The difference between the final
intrinsic rates, calculated using CO
2
surface area, was significantly reduced (R was
reduced from 6 to 2.4), which indicates a strong influence of surface area of the
micropores on the reaction rate. However, the difference in reactivity between cokes
could not be fully explained by the differences of the microporosity surface area (see
Chapter 9).
97
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
Table 5.3 The initial and final intrinsic rates of cokes with carbon dioxide
measured using N
2
and CO
2
surface areas.
Intrinsic rate using N surface area Intrinsic rate using CO surface area
Coke
2
(*10
-6
g m
-2
s
-1
)
2
(*10
-6
g m
-2
s
-1
)
al Final Initial Final Initi
A 5.19 0.30 0.37 0.44
B 6.26 0.17 0.96 0.22
C 12.09 0.30 0.56 0.31
D 8.35 0.17 0.68 0.24
E 9.63 0.12 0.39 0.18
F 26.90 0.44 0.18 0.36
G 7.05 0.14 0.46 0.21
H 3.27 0.07 0.16 0.19
I 4.60 0.09 0.56 0.29
Ratio 8.2 6.3 6.0 2.4
Ratio represents the ratio betw ion rate of the most reactive and
the reaction rate of the least reactive coke.
.3 Coal properties Coal rank and Maceral composition
revious studies [13,20,67,90] have shown that coke reactivity decreases with
e formation of
oke microtexture [26,29,67,105]; as the coal rank increases the size of the anisotropic
rsion) apparent rates of the nine cokes was poor
igure 5.6a), although the anisotropy size of the cokes increased as the rank of the
een the react coke
5
P
increasing rank of the parent coal. Coal rank has an important role in th
c
microtexture of the coke increases.
The relationship between rank of the parent coal and both the initial and final
(approximately 15 % carbon conve
(F
parent coal increased as shown in section 5.5. The influence of coal rank on coke
properties and consequently on coke reactivity is believed to be overshadowed by other
more significant factors for these cokes.
98
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
0
10
20
30
40
50
0
2
4
6
8
10
60
12
14
a
t
e
0.9 1.1 1.3 1.5 1.7
Coal rank (R
0
, %)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
(
*
1
0
-
6
g
g
-
1
s
-
1
)
0.9 1.1 1.3 1.5 1.7
Coal rank (R
0
, %)
F
i
n
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
(a)
0.0
0.2
0.4
0.6
0.8
1.0
1.2
0.9 1.1 1.3 1.5 1.7
Coal rank (R
0
, %)
I
n
i
t
i
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
0.0
0.1
0.2
0.3
0.4
0.5
0.9 1.1 1.3 1.5 1.7
Coal rank (R
0
, %)
F
i
n
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
(b)
0
5
10
15
20
25
30
0.9 1.1 1.3 1.5 1.7
Coal rank (R
0
, %)
I
n
i
t
i
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
0.0
0.1
0.2
0.3
0.4
0.5
0.9 1.1 1.3 1.5 1.7
Coal rank (R
0
, %)
F
i
n
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
(c)
Figure 5.6 Relationship between the rank of parent coal (expressed as mean
maximum vitrinite reflectance in oil) and (a) initial and final apparent
rates, (b) initial and final in sic rates calculated using CO surface trin
2
area and (c) initial and final intrinsic rates calculated using N
2
surface
area.
99
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
Although the
investigated
t in the parent coals was identified as a factor that influences the
action rate. Inertinite that does not fuse during carbonisation is the source of isotropic
ave found that both the rank and maceral composition of the parent
oals influence coke reactivity but their influence on coke reactivity was not evident for
influence of the rank of parent coal on the apparent reaction rate has been
in previous studies, the influence of the rank of parent coal on the intrinsic
reaction rate has not been previously reported. In this project it was investigated in order
to determine whether the poor relationship between the rank of the parent coal and
reaction rate was because of the differences in surface area. Figure 5.6b and 5.6c shows
the relationships between both initial and final intrinsic rates, measured using carbon
dioxide and nitrogen, and the coal rank. The intrinsic rates also showed a poor trend
with coal rank.
Inertinite conten
re
microtexture in the coke, which is the most reactive microtextural component [18,23].
Petrographic examinations of the reacted cokes showed that the non-fused inertinite was
the most reactive microtextural component (see section 5.4). Previous studies [78]
found a good relationship between the inertinite content of the parent coal and the
reaction rate; as the inertinite levels increased the reaction rate increased. However,
Figure 5.7 shows that no relationship was identified between both apparent and intrinsic
rates and inertinite content in the parent coal for either early or later stages of the
reaction. The influence of non-fused inertinite on the reaction rate is further discussed in
the next chapter.
Previous studies h
c
the series of cokes used in this study. Therefore, an in-depth study was required to
establish their importance on coke properties and coke reactivity. In the next chapter the
results of a study on maceral enriched fractions prepared from selected coals of different
rank is reported. This study was performed in order to establish the effect of rank and
maceral composition of the parent coals on coke properties and determine their relative
influence on coke reactivity.
100
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
0
2
4
6
8
10
12
14
10 20 30 40 50 60 70
Inertinite (%)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
0
10
20
30
40
50
60
10 20 30 40 50 60 70
Inertinite (%)
F
i
n
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
(a)
0.0
0.2
0.4
0.6
0.8
1.0
1.2
10 20 30 40 50 60 70
Inertinite (%)
I
n
i
t
i
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
0.0
0.1
0.2
0.3
0.4
0.5
10 20 30 40 50 60 70
Inertinite (%)
F
i
n
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
(b)
0
5
10
15
20
25
30
10 20 30 40 50 60 70
Inertinite (%)
I
n
i
t
i
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
0.0
0.1
0.2
0.3
0.4
0.5
10 20 30 40 50 60 70
Inertinite (%)
F
i
n
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
(c)
Figure 5.7 Relationship between inertinite content in the parent coal and (a) initia
and final apparent rates, (b) initial and final intrinsic rates calculated
using CO
2
surface area and (c) initial and final intrinsic rates calculated
using N
2
surface area.
l
101
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
5.4 Coke properties Coke microtexture
al analysis of the nine r
ealed that the size of anisotropic
coal increased. Figure 5.8 shows
hotomicrographs of three of the cokes (coke B, coke D and coke F) which were
onversion) showed that isotropic microtexture was selectively consumed during
asification. Figure 5.9 shows photomicrographs of coke F raw and reacted (15% and
ble 5.4 The content of isotropic microtexture in the raw and 75% burnout
cokes.
ke
I
Microtextur aw cokes rev
microtexture increased as the rank of the parent
p
prepared from coals of different rank (R
0
max is 1.00%, 1.18% and 1.27%, respectively).
Coke B was characterized by fine mosaic anisotropy. Medium mosaic anisotropy was
typical for coke D whereas coarse mosaic anisotropy was formed in coke F (the
microtexture classification used was according to that presented by Marsh [31] in his
study). This analysis confirms the influence of rank of the parent coal on size of the
anisotropic microtexture formed in coke observed in previous studies (see section
2.5.1).
Microtextural examination performed on the raw and reacted cokes (15% and 75%
carbon c
g
75% burnout). Isotropic microtexture in the 15% reacted coke showed preferential
dissolution. In the 75% burnout coke isotropic microtexture presence was very rare.
Petrographic analyses also indicate a very low content of isotropic microtexture in the
75% burnout cokes (Table 5.4). These findings are in accordance with those reported in
previous studies (see section 2.4.1).
Ta
Coke
A
Coke
B
Coke
C
Coke
D
Coke
E
Coke
F
Coke
G
Coke
H
Co
(Vol %, mmf)
Raw 13.9 27.2 36.2 29.1 29.3 25.6 32.6 21.2 17.7
75% burnout N/A 2.7 3.2 1.6 N/A 1.1 3.7 N/A 0.0
mmf mineral matter free
102
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
(a)
(b)
(c)
Figure 5.8 Microtexture of cokes made form coals of different rank (a) Coke B
(R
0
max1.00%), (b) Coke D (R
0
max1.18%) and (c) Coke F (R
0
max
1.27%); where F is Fine Mosaic, M is Medium Mosaic and C is Coarse
Mosaic.
F
M
C
103
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
(a)
I
I
I
I
I
I
(b)
(c)
Figure 5.9 Microtexture of coke F during gasification (a) raw coke, (b) 15%
burnout coke and (c) 75% burnout coke; where I indicates isotropic
microtexture.
104
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
5.5 Coke properties Carbon structure
Mean carbon crystallite size (L d L
a
) of the raw and reacted cokes were calculated
using the procedure presented in section 4.5.3. Crystallite height (L
c
) and width (L
a
) of
the raw cokes varied from 1.46 to 1.67 nm and 3.60 to 4.18 nm, respectively (Table
5.5).
able 5.5 Crystallite size (L
c
and L
a
) of the raw cokes.
Coke
A
Coke
B
Coke
C
Coke
D
Coke
E
Coke
F
Coke
G
Coke
H
Coke
I
c
an
T
(n m)
L
c
1.53 1.51 1.51 1.56 1.52 1.46 1.51 1.59 1.67
L
a
4.02 3.73 3.82 3.87 3.69 4.18 3.82 3.77 3.60
Although the crystallite size (L
c
and L
a
) of the coals has been reported to increase as the
103,106], the crystallite size of the corresponding chars in the study
performed by Lu et al. [106] was not significantly influenced by the coal rank. The
influence of coal rank on the crystallite size of cokes has not been reported so far.
Therefore, the effect of rank of parent coal on crystallite size of the coke was
investigated. In this work the influence of rank of the parent coal on carbon crystallite
size (L
c
and L
a
) of the cokes was not significant, since a poor relationship was observed
between both L
c
and L
a
of the cokes with rank of the parent coals (Figure 5.10).
However, the maceral composition of the parent coals investigated here varied widely.
In order to separately investigate the influence of rank and maceral composition on coke
properties and reactivity, maceral-enriched fractions of coals of different rank were
repared (see next chapter).
coal rank increased [
p
105
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
Feng et al. [73] observed a decrease of the crystallite size (L
c
and L
a
) of a char, made
om a semi-anthracite, during gasification L
a
decreased from the beginning of fr
reaction whereas L
c
showed a significant decrease after approximately 60% carbon
conversion. In this project the effect of gasification on crystallite size was investigated
on cokes reacted with carbon dioxide to 15% and 75% burnout.
1.4
1.5
1.6
1.7
0.9 1.1
3.4
3.6
3.8
4
1.3
R
0
max (%)
L
m
)
1.5 1.7
c
(
n
.0
4.2
4.4
1 1.3 5
R
0
max (%)
)
igure 5.10 L and L of the raw cokes versus rank of parent coal (mean
hours had no significant effect on either L
c
or L
a
. Since the
okes were exposed to 1050C during carbonisation, this lack of change at 900C is not
unreasonable.
0.9 .1 1. 1.7
L
a
(
n
m
F
c a
maximum reflectance of vitrinite).
Coke gasification to high levels (75% carbon conversion) requires keeping the cokes for
at least 15 hours at 900C under a CO
2
flow. In order to establish the effect of
gasification on carbon crystallite size the effect of temperature alone on crystallite size
had to be determined. Three cokes (A, C and F) were subjected to a thermal treatment
(annealing) for at least 15 hours at 900C under nitrogen. The L
c
and L
a
values of the
raw and the corresponding thermal treated cokes are presented in Table 5.6. Exposure of
these cokes to 900C for 15
c
106
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
Table 5.6 The L
c
and L
a
of the raw cokes and cokes annealed at 900C for 15
hours.
Coke L
c
(nm) L
a
(nm)
Raw coke Annealed coke Raw coke Annealed coke
A 1.52 1.50 4.02 4.07
C 1.51 1.54 3.82 3.87
F 1.46 1.52 4.18 4.12
Figure 5.11a shows that crystallite height (L
c
) changed during gasification. L
c
showed a
slight increase at 15% carbon conversion but increased significantly at 75% burn-off,
and this pattern was observed for all of the cokes. Because temperature alone had no
effect on crystallite sizes, it is appropriate to conclude that the increase of crystallite
height is solely due to gasification.
Kashiwaya and Ishii [41] and Feng et al. [73] have found that at the early stages of
gasification (below approximately 30-40% conversion) gasification did not affect
ignificantly the L
c
; the data from this study are consistent with these observations. At
garding L
c
. Feng et al [73] noted a e of L
c
at greater carbon
onversion levels, during char gasification with carbon dioxide and also during char
ombustion. However, Lu et al. [127] observed an increase of L
c
during char
ombustion. The significant increase of L
c
at high carbon conversion levels observed in
t of L
c
because of the interference of the mineral matter peaks with the
arbon peak, which introduced errors into the fitting of the theoretical curve for L
a
peak.
s
high carbon conversion levels, no consistent trends were reported by previous studies
significant decreas re
c
c
c
this work could be due to preferential consumption of small crystallites or merging of
neighbouring crystallites [127]. The selective consumption of the small crystallites
would produce an increase of the average crystallite height (L
c
). The removal of the
disordered carbon between crystallites could result in a merger of the neighbouring
crystallites, which translates to a real growth in the crystallite height.
Crystallite length (L
a
) showed little significant variation during gasification across the
cokes investigated (Figure 5.11b). The measurement error of L
a
was relatively high
compared to tha
c
107
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
1.5
1.6
1.7
1.8
9
2.0
L
c
(
n
m
)
1.
Raw coke
15% burn-off
1.3
1.4
A B C D E F G H I
75% burn-off
(a)
3.0
3.2
3.4
3.6
3.8
4.0
4.2
4.4
A B C D E F G H I
L
a
(
n
m
)
Raw coke
15% burn-off
75% burn-off
(b)
Figure 5.11 Variation of (a) L
c
and (b) L
a
during gasification (the measurement
error bars are shown).
108
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
Because L
a
did not change significantly during gasification in this work, and previous
studies [41,73] have reported a decrease of L
a
during gasification, further investigations
were conducted in order to determine the importance of L
a
on the reaction rate (see next
chapter).
In order to determine the influence of crystallite height (L
c
) on the reaction rate, the
apparent and intrinsic rates at initial and final (approximately 15% burnout) stages were
plotted versus L
c
. The influence of L
a
on the reaction rate is not shown since no
significant change of L
a
was observed during gasification (Figure 5.11b).
Increasing ordering of carbon structure leads to a lower reactivity [22]. The initial
apparent rates and the initial intrinsic rates, measured by carbon dioxide, were not
related to crystallite height (L
c
) (Figure 5.12). The influence of other factors such as
catalytic mineral matter (see Chapter 8) rshadowed any influence of L
c
on the
action rate.
A trend is observed between the L
c
of the reacted cokes (approximately 15% burn-off)
and the final apparent rate; the most reactive cokes (F and C) had the lowest L
c
and the
least reactive cokes (I and H) had the greatest L
c.
The trend is not strong but it suggests
that L
c
may affect to some extent the final reaction rate (15% burnout). Also, the trend
between L
c
of the 15% burnout cokes and the final intrinsic rate (Figure 5.12b) is weak,
which indicates that L
c
did not influence significantly the final reaction rate. A strong
influence of L
c
on the reaction rate would show a strong relationship with the final
intrinsic rates (determined using CO
2
surface area), since micropore surface area of the
reacted cokes was found to have a strong influence on the reaction rate (see section 5.2).
In the next chapter, a study on carbonised maceral-enriched fractions was conducted in
order to establish the influence of coal properties on crystallite size of carbonised
maceral-enriched fractions, and the influen on their reactivity.
ove
re
ce of crystallite size
109
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
B
A
A
E
12
E
D
G
C
4
6
8
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
H
I
F
2
10
B
G
D
C
20
30
40
F
i
n
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
0
1.4 1.5 1.6 1.7
Initial Lc (nm)
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
H
I
F
10
50
0
1.4 1.5 1.6 1.7 1.8
Final Lc (nm)
(a)
A
G
B
D
F
E
I
H
C
0.0
0.5
1.0
1.5
2.0
2.5
3.0
1.4 1.5 1.6 1.7
Initial Lc (nm)
I
n
i
t
i
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
G
I
E
D
H
B
C
F
A
0.0
0.1
0.2
0.3
0.4
0.5
1.4 1.5 1.6 1.7 1.8
Final Lc (nm)
F
i
n
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
(b)
s vs.
L
c
and (b) initial and final intrinsic rates of the nine cokes, calculated
using CO
2
surface area, vs. L
c
.
Figure 5.12 (a) Initial and final (15% burn-off) apparent rates of the nine coke
110
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
5.6 Coke properties Ash composition
oke ash chemistry is believed to be strongly related to coke reactivity [20,25,70]. Total
iron oxide, calcium oxide, potassium oxide and sodium oxide from the ash analysis
were considered as reasonably good predictors of coke reactivity [89] [13,18,25,90].
Figure 5.13 shows the relationship between the initial and final apparent rates and
concentration of total iron, calcium, potassium and sodium in the nine cokes. A trend is
observed between total iron in coke and the initial apparent reaction rate. The
concentration of calcium, potassium and sodium in cokes showed a poor relationship
with initial and final apparent rates.
Iron, calcium, potassium and sodium occur in different mineral phases in cokes. For
instance, iron in the mineral matter in coke urs not only as oxide but also as metallic
iron [50] and as a component of other mineral phases such as pyrrhotite (Fe
1-x
S) [23]
and calcium ferrite (CaFe
2
O
4
) [50]. Some of the minerals identified in the cokes that
contain iron, calcium, potassium and sodium are not catalysts of gasification (see
section 2.4.3) and also those that are catalysts of gasification could have different
activity as catalysts. Therefore, it is more appropriate to identify the catalytic mineral
matter in the coke and then determine the relationship between the catalytic mineral
phases in cokes and reaction rate. The identification of the catalytic mineral phases in
the coke and their relationship with the gasification reaction rate are presented in
Chapters 7 and 8.
C
occ
111
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
B
H
A
E
G
D
I
C
r
2
= 0.50
0
2
4
6
8
0.0 0.5 1.0 1.5
Fe2O3 (%)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
(
*
1
0
-
6
g
g
-
1
s
F
10
t
r
a
t
-
1
)
12
e
A
E
B
H
G
D
I
C
0
10
20
30
0.0 0.5 1.0 1.5
Fe
2
O
3
(%)
F
i
n
a
l
a
p
p
a
r
e
n
t
(
*
1
0
-
6
g
g
-
1
s
F
40
50
r
a
t
e
-
1
)
H
I
A
B
E
D
G
0
2
4
0.0 0.1 0.2 0.3 0.4
CaO (%)
I
n
i
t
i
a
l
a
(
*
1
0
-
6
H
C
6
p
p
a
g
g
-
1
F
8
10
12
r
e
n
t
r
a
t
e
s
-
1
)
I
A
E
B
G
D
0
10
20
0.0 0.1 0.2 0.3 0.4
CaO (%)
F
i
n
a
l
a
p
(
*
1
0
-
6
C
F
30
40
p
a
r
e
n
t
r
a
t
g
g
-
1
s
-
1
)
50
e
A
G
B
E I
H
D
C
F
0
10
20
30
40
50
F
i
n
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
A
B
G
E
I
D
H
C
F
0
2
4
6
8
10
12
0.0
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
0.1 0.2
K2O (%)
0.0 0.1 0.2
K2O (%)
E
B
I
D
A
G
H
C
F
0
2
4
6
8
10
12
0.0 0.1 0.2
Na2O (%)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
A
E
I
B
D
G
H
C
F
0
10
20
30
40
50
0.0 0.1 0.2
Na2O (%)
F
i
n
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
Figure 5.13 Initial and final apparent rates of the nine cokes against total iron,
calcium, potassium and sodium concentration in the cokes.
112
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
5.7 Summary
In this chapter the effect of coke properties (coke microtexture, surface area, carbon
structure (L
c
and L
a
) and ash composition) and the parent coal properties (rank, maceral
composition) on reactivity of nine metallurgical cokes was investigated.
The apparent reaction rates of the nine cokes varied to a great extent from
approximately an order of magnitude between the highest and the lowest reactive cok
used in this study.
During gasification the micropore and mesopore surface area, measured by CO
2
and N
2
,
increased dramatically. The CO
2
surface area of the raw cokes and the 15% burnout
cokes ranged 2.364.5 m
-1
g and 21.7131.9 m
-1
g, respectively. The N
2
surface area of
the raw cokes was always less than 0.6 m
-1
g. The N
2
surface area of the 15% burnout
cokes varied from 28.5 to 96.9 m
-1
g. The increase of both N
2
and CO
2
surface area of
the reacted cokes is consistent with the previous data reported by other studies.
The initial apparent rate was poorly related to both N
2
and CO
2
surface area, which
indicates that the surface area of the micropores and mesopores did not significantly
affect the reaction rate. A poor relationship was also observed between N
2
surface area
of the 15% burnout cokes and apparent rate. However, the relationship between CO
2
surface area and the apparent rate of the reacted cokes was strong. Micropore surface
area (CO
2
surface area) appeared to affect significantly the reaction rate at about 15%
carbon conversion. Also, by calculating the intrinsic rates of the 15% burnout cokes
using micropore surface area (CO
2
surface area) of the 15% burnout cokes, the variation
in the reaction rates of the cokes diminished significantly.
Although rank and to a less degree, maceral composition of the parent coals have been
reported as good indicators of coke reactivity, the apparent and intrinsic reaction rates
were poorly related to coal rank and maceral composition in this suite of cokes.
he size of the anisotropic microtexture increased as the rank of the parent coal
e
T
increased. Non-fused inertinite derived component was more reactive than the
113
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
anisotropic texture as optical microscopy analysis showed. These observations are in
greement with those made in previous studies.
k of the parent
oal. Previous studies also indicate no strong influence of coal rank on crystallite height
vious studies also reported no significant change in L
c
during
asification at early stages, but it has been reported a decrease of L
c
at high carbon
k trend
as observed between crystallite height (L
c
) and intrinsic rate. Previous studies reported
nd carbon structure (L
c
and
a
)) and coke reactivity will be reported.
ate, but
alcium, sodium and potassium were poorly related to coke reactivity. The chemistry of
a
Carbon crystallite height (L
c
) and length (L
a
) of the raw cokes were in the range 1.46-
1.67 nm and 3.60-4.18 nm, respectively. L
c
and L
a
were not related to ran
c
(L
c
). During gasification, crystallite height (L
c
) slightly increased at the early stages of
reaction (15% burnout) but a significant increase was observed at high conversion levels
(75% burnout). Pre
g
conversion levels. Crystallite length (L
a
) did not change significantly during
gasification. But, a decrease of L
a
during gasification was observed in previous studies.
Crystallite height (L
c
) of the raw cokes was poorly related to initial apparent and
intrinsic rates. At 15% burnout a trend was observed between crystallite height (L
c
) and
apparent rate; the apparent reaction rate decreased as L
c
increased. Also, a wea
w
a decrease in reactivity as the ordering of carbon structure increased. However, in this
study L
c
did not show a strong effect on the reaction rate.
Coal macerals and rank of the parent coal appeared to have no effect on coke reactivity.
Also, the influence of carbon structure (L
c
and L
a
) on the reaction rate could not be
clearly established. In order to determine their relative importance on the reaction rate a
study was carried out on carbonised maceral-enriched fractions, which enabled partial
separation of some of the factors. In the next chapter the influence of coal macerals on
coke properties (in terms of microtexture, microporosity a
L
The total iron, calcium, potassium and sodium in coke were used as indicators of coke
reactivity in previous studies. A trend was observed between iron and reaction r
c
the ash yield is not considered a reliable indicator for coke reactivity, as iron, calcium,
potassium and sodium can be found in cokes in minerals that do not catalyse the
114
Chapter 5 Kinetics of the Coke Carbon dioxide reaction
115
8).
gasification reaction. Therefore mineral characterisation and the influence of the
catalysts require investigation (see Chapter 7 and
CHAPTER 6 Influence of coal macerals on coke properties
In this chapter the effect of maceral composition (see section 2.5.1.2) of the starting coal
on coke properties, including its reactivity, will be investigated. Five coals were
selected for further investigation of coal rank and maceral composition effect on coke
reactivity. The selection was based on the differences in rank and maceral composition
of the coals. The maceral-enriched fractions (inertinite and vitrinite) were prepared from
sub-samples of coals B, C, D, F and G. The maceral composition of the vitrinite-rich
fractions and inertinite-rich fractions is shown in Table 4.2. The mineral content in the
inertinite-rich fractions was almost similar to that in the original coals. But the vitrinite-
rich fractions had very low mineral matter content.
The maceral-enriched fractions obtained were carbonised in a 70 g oven. Also, sub-
samples of the original coals were carbonised in the same 70 g oven. The procedures for
maceral separation and carbonization were described in Chapter 4. Reactivity
measurements (using 100% CO
2
)
were performed on all carbonised maceral-enriched
fractions and the cokes made from the original coals.
In this chapter, the inertinite that did not fuse during carbonisation process is referred as
either non-fused inertinite or inert maceral derived component (IMDC). Macerals that
fuse during carbonisation and form anisotropic microtexture are called reactive maceral
derived component (RMDC).
116
Chapter 6 Influence of coal macerals on coke properties
6.1 Effect of carbonisation conditions on coke reactivity and coke
properties
ue to the small quantity of maceralenriched fractions a small oven was required for
eir carbonisation. In order to observe the effect of preparation conditions on both
roperties and reactivity of the coke, sub-samples of coals were also carbonised in the
on rate of the 70 g oven cokes (B,
, D and G) became greater than that of those made in the 9 kg oven. In order to explain
The amount of each maceralenriched fraction prepared was several hundred grams.
D
th
p
small oven. Then the reactivity test was performed on these cokes. The reactivity of the
cokes prepared in the 70 g oven was compared to that of their corresponding cokes from
the 9 kg oven (Figure 6.1).
The cokes from 70 g oven were more reactive than those carbonised in the 9 kg oven,
with one exception, coke F, which showed similar reactivity for samples from both
ovens. At the initial stages small differences were observed between the reaction rates
of the cokes carbonised in the 70 g oven and that of their corresponding cokes from 9 kg
oven. But, as carbon conversion increased the reacti
C
the differences in reactivity of the cokes produced in different carbonisation conditions
the raw cokes were characterised by micropore surface area, microtexture and carbon
structure (L
c
and L
a
).
117
Chapter 6 Influence of coal macerals on coke properties
Coke B
25
Coke C
0
5
10
15
0 2 4 6 8 10 12 14 16 18
Carbon conversion (%)
A
p
p
a
r
e
n
t
r
a
(
*
1
0
-
6
g
g
-
1
s
20
t
e
-
1
)
70 g oven 9 kg oven
0
10
20
30
0 5 10 15 20
Carbon conversion (%)
A
p
p
a
r
e
n
t
r
a
(
*
1
0
-
6
g
g
-
1
s
40
50
t
e
-
1
)
70 g oven 9 kg oven
Coke D
0
5
10
15
20
25
0 5 10 15 20
Carbon conversion (%)
A
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
70 g oven 9 kg oven
Coke F
0
10
20
30
40
50
60
0 2 4 6 8 10 12 14 16 18
Carbon conversion (%)
A
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
70 g oven 9 kg oven
Coke G
0
5
10
15
20
0 4 8 12 16 2
Carbon conve rsi on (%)
A
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
0
70 g oven 9 kg oven
C versus carbon conversion (100%
CO
2
) of the cokes B, C, D, F and G prepared in the 70 g and 9 kg
ovens.
Figure 6.1 The apparent reaction rate at 900
118
Chapter 6 Influence of coal macerals on coke properties
6.1.1 Surface area and intrinsic reactivity
Table 6.1 shows the surface area, measured by CO
2
, of the raw cokes B, C, F, and G
prepared in the 70 g and 9 kg ovens. The influence of carbonisation conditions on the
micropore surface areas of cokes B and F were significant, but the micropore surface
areas of cokes C and G were only slightly affected by the carbonisation conditions.
Although carbonisation conditions influenced to some degree the micropore surface
area, the impact of carbonisation conditions on micropore surface area cannot be easily
predicted because the lack of systematic variation of micropore surface area with
carbonisation.
In order to determine the influence of micropore surface area on the reaction rate the
intrinsic reaction rates were determined (Figure 6.2). The initial intrinsic rates of the
cokes B, C and G prepared in the 70 g oven and their corresponding cokes made from 9
kg oven were different. Therefore, the differences in the microporosity surface area did
not account for the observed differences in reaction rate between the cokes B, C, and G
from 70 g oven and their corresponding cokes from 9 kg oven. Moreover, the initial
apparent rate of coke F prepared in both 70 g and 9 kg ovens was similar although coke
F from 70 g oven had a significantly smaller surface area than coke F made in the 9 kg
oven. It can be concluded that the apparent reaction rate was affected by factors other
than the surface area of micropores.
Table 6.1 Surface area of the raw cokes B, C, F and G carbonised in the 70 g and 9
kg ovens.
Coke Surface area (m
2
g
-1
)
70 g oven 9 kg oven
B 10.4 2.3
C 11.0 13.4
F 14.1 64.5
G 11.1 8.5
119
Chapter 6 Influence of coal macerals on coke properties
0.0
0.4
0.8
1.2
n
i
t
i
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
B C F G
I
70 g oven
9 kg oven
Figure 6.2 The initial intrinsic reaction rates of cokes B, C, F and G prepared in
the 70 g and 9 kg ovens.
6.1.2 Coke microtexture
Heating rate has a major effect on microtextural composition and the size of the
nisotropic texture of the product coke; both the amount of fused inertinite [21] and size
f anisotropy [20,34] in the product coke decrease as the heating rate decreases.
etrographic analysis showed that the amount of fused inertinite in the cokes from the 9
.3); inertin oals B and C did not fuse at all in the 70 g oven and a small
proportion of the inertinite in coals D and F fused in the 70 g oven.
a
o
P
kg oven was greater than that in the corresponding cokes made in the 70 g oven (Figure
ite in the c 6
120
Chapter 6 Influence of coal macerals on coke properties
0
10
20
30
40
50
60
70
B C D F G
I
n
e
r
t
i
n
i
t
e
(
v
o
l
,
%
)
Coal
70 g oven
9 kg oven
Figure 6.3 Percentage of non-fused inertinite in the cokes B, C, D, F and G
prepared in the 70 g and 9 kg ovens compared to percentage of
inertinite in the coal.
he anisotropic microtexture in the cokes from 70 g oven was smaller than that in the
re 6.4). The anisotropic microtexture of both cokes B and C
ade in the 70 g oven was mainly fine mosaic whereas the anisotropic microtexture
stic stage than that
xisting in the 9 kg oven. The influence of heating rate on coke microtexture, observed
ork, is in agreement with that reported by previous studies [20,21].
T
cokes from 9 kg oven (Figu
m
which characterise cokes B and C carbonised in the 9 kg oven was mostly medium
mosaic. Medium mosaic was the typical anisotropic microtexture observed in cokes F
and G carbonised in the 70 g oven whereas coarse mosaic and flow-like anisotropic
microtextures characterised cokes F and G prepared in the 9 kg oven.
The greater content of non-fused inertinite and smaller anisotropy formed in the 70 g
oven cokes is explained by the lower heating rate during the pla
e
in this w
121
C
h
a
p
t
e
r
6
I
n
f
l
u
e
n
c
e
o
f
c
o
a
l
m
a
c
e
r
a
l
s
o
n
c
o
k
e
p
r
o
p
e
r
t
i
e
s
1
2
2
(
a
)
(
c
)
(
d
)
F
i
g
u
r
e
6
.
4
M
i
c
r
o
t
e
x
t
u
r
e
o
f
(
a
)
c
e
p
a
r
e
d
i
n
t
h
e
7
0
g
9
k
g
(
b
o
t
t
o
m
)
o
v
e
n
s
;
w
h
e
r
e
F
i
s
f
i
n
e
m
o
s
a
i
c
,
M
i
s
m
e
d
i
u
m
m
o
s
a
i
c
,
C
i
s
c
o
a
n
d
F
A
F
A
F
A
M
M
F
M
(
b
)
o
k
e
F
a
n
d
(
d
)
c
o
k
e
G
p
r
i
s
f
l
o
w
-
l
i
k
e
a
n
i
s
o
t
r
o
p
y
.
M
F
(
t
o
p
)
C
)
c
o
k
e
B
,
(
b
)
c
o
k
e
C
,
(
c
a
r
s
e
m
o
s
a
i
c
a
n
d
Chapter 6 Influence of coal macerals on coke properties
Also, microtexture formation was not significantly affected during re-solidification after
the plastic stage, although different heating rates were used to carbonise the sem kes.
During the plastic stage of the carbonisation process in the 70 g oven the heating rate
was 1 C min
-1
whereas the heating rate after 470C was much higher (10 in
-1
),
even greater than that of the 9 kg oven (3 C min
-1
). Therefore microtexture
formation was mainly controlled by the heating rate during the plastic stage than that
after the plastic stage, which confirms the observations made in previous studies [37].
Marsh [31] and Mitchell and Benedict [21] showed that the non-fused inertinite is the
most reactive m xtural component with carbon dioxide, followed by very fine, fine,
medium and coarse mosaic microtexture. The greater reactivity of cokes B, C and G
prepared in the 70 g oven than that of their corresponding cokes made in the 9 kg oven
could be influenced to some degree by their greater content of no sed
derived component and finer anisotropic microtexture. However, both cokes F
carbonised in the 70 g and 9 kg oven had similar reactivity althoug e fo had
greater inertinite content and the smaller size of anisotropy. The influence of non-fused
inertinite component on coke reactivity cannot be clearly established from these data
and further characterisation is required (see section 6.2).
6.1.3 Carbon structure
Carbon crystallite height (L
c
) and length (L
a
) of the raw cokes were d mined for the
cokes B, C, F and G prepared in both 70 g and 9 kg ovens (Figure 6.5). Carbonisation
conditions had a greater effect on L
c
than on L
a
. Cokes B, C and G produced in the 70 g
oven had smaller L
c
than their corresponding cokes made in the 9 kg oven. Only coke F
made in the 70 g oven had slightly greater L
c
than coke F prepar e 9
Smaller L
c
of cokes produced in the 70 g oven could be explained by the lower heating
rate of carbonization process, which reduces coal fluidity [115] during the plastic stage
hindering carbon crystallite development [34].
i-co
C m
inertinite
rmer
kg oven.
the
n-fu
h th
eter
in th
icrote
ed
123
Chapter 6 Influence of coal macerals on coke properties
1.5
1.6
(
n
m
)
L
c
1.3
1.4
70 g oven 1.36 1.37 1.54 1.40
9 kg oven 1.51 1.52 1.46 1.51
B C F G
4.6
3.8
4.2
4.4
L
a
)
4.0
(
n
m
3.2
3.4
3.6
70 g oven 3.85 3.96 3.76 3.53
9 kg oven 3.73 3.82 4.18 3.82
B C F G
Figure 6.5 Crystallite size (L
c
and L
a
) of the raw cokes B, C, F and G carbonised in
the 70 g and 9 kg ovens (the vertical lines represent the measurement
error bars).
124
Chapter 6 Influence of coal macerals on coke properties
Crystallite length (L
a
) of cokes B and C from the 70 g oven was slightly greater than
eir corresponding cokes from the 9 kg oven whereas cokes F and G made in the 70 g
oven had slightly smaller L
a
than their corresponding cokes from the 9 kg oven.
Considering the measurement error of L
a
, the carbonisation conditions could not be
concluded to affect significantly the crystallite length (L
a
) of the cokes prepared from
the same coal.
Crystallite height (L
c
) may have some influence on reactivity of cokes B, C and G
prepared in the 70 g oven as their L
c
compared to that of their corresponding cokes
carbonised in the 9 kg oven is lower. However, the influence of crystallite height on
coke reactivity cannot be established due to the small number of sample. The influence
of crystallite size on coke reactivity will be further discussed in section 6.2.
It can be concluded that the effect of low heating rate results in a decrease of the amount
f inertinite fused and smaller size of anisotropic microtexture. Also, the carbon
rystallite height (L
c
) was smaller.
6.2 Effect of macerals on coke reactivity
A study by Huang et al. [101] on reactivity of carbonised vitrinite-rich and inertinite-
rich fractions showed that the carbonised vitrinite-rich fractions had lower reactivity
than their corresponding carbonised inertinite-rich fractions, which was explained by
the increased ordering of carbon structure (L
c
) of the carbonised vitrinite-rich fractions.
They observed no influence of both micropore and mesopore surface area on reactivity
of the carbonised maceral-enriched fractions. However, Czechowski and Kidawa [102]
in some cases the reactivity of the carbonised vitrinite-
their corresponding carbonised inertinite-rich
e coals. They assumed that the reactivity of the carbonised vitrinite-rich
content of Na
th
o
c
and Huang et al. [101] found that
rich fractions was greater than that of
fraction for som
fractions was increased due to the greater content of catalyst, which was based on the
, K, Ca and Fe in the ash chemistry.
125
Chapter 6 Influence of coal macerals on coke properties
Sakawa et al. [78] concluded from the investigation of several coals of different rank
that the reactivity of carbonised fractions of consecutive densities, increased with
increasing both inertinite content and alkali index (B) of the fraction (the inertinite
content and the alkali index increased as the density fraction increased). However, they
could not differentiate between the influence of inertinite content and alkali index on
coke reactivity.
he data from the reactivity test showed that the carbonised inertinite-rich fractions
e from coal G at the early stages, but it increased
fter approximately 4% carbon conversion. Carbonised vitrinite-rich fractions were less
active than the cokes made from the original coals.
bonised vitrinite-rich fractions was
maller than that of their corresponding carbonised inertinite-rich fractions, because the
In this section the reactivity of the cokes prepared from the original coals in the 70 g
oven and their corresponding carbonized maceral enriched-fractions will be determined,
and also the influence of surface area and carbon structure (crystallite size) on reactivity
will be investigated.
T
were more reactive than the coke from the original coals prepared in the same oven
(Figure 6.6). Only the carbonised inertinite-rich fraction of coal G showed similar
reactivity with that of the coke mad
a
re
The size of the anisotropic microtexture of the car
s
vitrinite-rich fractions were carbonised at lower heating rate than inertinite-rich
fractions (to avoid swelling). The effect of heating rate on the size of anisotropic
microtexture was more significant for the maceral-enriched fractions from medium rank
coals than for those from low rank coals. The anisotropic microtexture of the vitrinite-
rich fraction B was predominantly very fine mosaic whereas the carbonised inertinite-
rich fraction B had mainly fine mosaic (Figure 6.7a). Vitrinite-rich fraction G was
characterized by fine mosaic microtexture whereas inertinite-rich fraction G was
characterized by medium mosaic microtexture (Figure 6.7b).
126
Chapter 6 Influence of coal macerals on coke properties
B
0
10
20
30
40
50
A
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
C
10
20
30
40
50
60
A
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
0
0 5 10 15
Carbon conversion (%)
0 2 4 6 8 10 12 14 16 18
Carbon conversion (%)
Inertinite Original Vitrinite
Inertinite Original Vitrinite
D F
50
0
10
20
30
0 2 4 6 8 10 12 14 16
Carbon conversion (%)
A
p
p
a
r
e
n
t
r
a
t
(
*
1
0
-
6
g
g
-
1
s
-
1
40
e
)
0
10
20
30
40
50
0 2 4 6 8 10 12 14 16
Carbon conversion (%)
A
p
p
a
r
e
n
t
r
a
(
*
1
0
-
6
g
g
-
1
s
60
70
t
e
-
1
)
Iner Inertinite Original Vitrinite
tinite Original Vitrinite
G
0
5
10
15
20
0 2 4 6 8 10 12 14 16 18
Carbon conversion (%)
A
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
Inertinite Original Vitrinite
Figure 6.6 The apparent reaction rate of the cokes produced from the original
coals (B, C, D, F and G) and their corresponding maceral-enriched
fractions versus carbon conversion.
127
Chapter 6 Influence of coal macerals on coke properties
(a)
(b)
Figure 6.7 Anisotropic microtexture of (a) carbonised inertinite-rich fraction B
(left) and carbonised vitrinite-rich fraction B (right) and (b)
carbonised inertinite-rich fraction G (left) and carbonised vitrinite-
rich fraction G (right); where VF is very fine mosaic (it has a uniform
grey appearance), F is fine mosaic (it has a grey mottled appearance)
and M is medium mosaic.
F
F
V F
M
F
V F
128
Chapter 6 Influence of coal macerals on coke properties
The initial apparent rates of the coke made from the original coal and its corresponding
carbonised vitrinite and inertinite-rich fractions were strongly influenced by the content
of non-fused inertinite; this pattern was observed for all cokes (Figure 6.8a-e).
However, no trend was observed between initial apparent rates and non-fused inertinite
content when data for all the cokes and maceral-enriched fractions were considered
(Figure 6.8f). This suggests that actually it is not the content of non-fused inertinite that
affects reaction rate but its intrinsic properties, which varies between cokes. A
further investigation was conducted on the carbonised inertinite-rich fractions properties
and their influence on reactivity and the results are presented in section 6.3.1.
not show any obvious influence on the
action rate. The reactivity of anisotropic microtexture has been reported [21,31] to
crease as its size decreases. Therefore, the presence of finer anisotropic microtexture
the carbonised vitrinite-rich fraction compared to that of the corresponding coke and
arbonised inertinite-rich fraction, should result in an increased reactivity of the
carbonised vitrinite-rich fraction, which would affect the relationship between reaction
rate and non-fused inertinite content observed for the cokes and maceral-enriched
fractions prepared from the same coal source (Figure 6.8a-e). The in nce of the size
of the anisotropic microtexture on the reaction rate will be discussed further in section
6.3.2.
the
The size of the anisotropic microtexture did
re
in
in
c
flue
129
Chapter 6 Influence of coal macerals on coke properties
B
r
2
= 0.99
0
2
4
6
8
10
0 20 40 60 80
Non-fused inertinite (vol, %)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
C
r
2
= 0.99
0
3
6
9
12
15
0 20 40 60 80
Non-fused inertinite (vol, %)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
(a) (b)
D
r
2
= 0.99
0
3
6
9
12
0 20 40 60 80
Non-fused inertinite (vol, %)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
F
r
2
= 0.98
0
6
12
18
24
0 20 40 60
Non-fused inertinite (vol, %)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
(c) (d)
G
r
2
= 0.92
0
1
2
3
4
0 20 40 6
Non-fused inertinite (vol, %)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
0
All
0
6
12
18
24
0 20 40 60 80
Non-fused inertinite (vol, %)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
(e) (f)
Figure 6.8 The initial apparent reaction rate of the cokes produced from the
original coals (B, C, D, F and G) and their corresponding maceral
enriched fractions versus their content of non-fused inertinite.
130
Chapter 6 Influence of coal macerals on coke properties
6.2.1 Surface area and intrinsic reactivity
The surface area of the carbonised original coals (B, C, F and G) and their
corresponding maceral-enriched fractions are shown in Table 6.2. The carbonised
vitrinite-rich fractions showed lower micropore surface area than their corresponding
carbonised inertinite-rich fractions. Figure 6.9 shows that generally, surface area
increased with the amount of non-fused e derived component. Huang et al. [101
surface area of micropores than that of the carbonised vitrinite-rich fractions. It can be
concluded that most of the microporosity in coke occurs in the non-fused inertinite
derived component and little is associated with the anisotropic microtexture. This
suggests that the increased microporosity of the non-fused inertinite can be one of the
factors that make the non-fused inertinite derived component more reactive than the
anisotropic microtexture; as greater surface is exposed to gasification.
Table 6.2 Surface area of the raw cokes made from the original coals (B, C, F and
G) and their corresponding carbonised maceral-enriched fractions.
Coke Surface area (m
2
g
-1
)
inertinit ]
also observed that the carbonised inertinite-rich fractions were characterised by greater
Inertinite-rich fraction Original Vitrinite-rich fraction
B 19.0 10.4 4.1
C 17.0 11.0 8.0
F 30.7 14.1 6.5
G 11.8 11.1 7.3
Since surface area of the micropores was shown to be a potential factor that influences
rate, the intrinsic rates of the cokes and their corresponding carbonised
inertinite-rich fractions were determined (Figure 6.9). Micropore
the reaction
surface
ces between reactivity of the carbonised
vitrinite and
area cannot explain entirely the differen
131
Chapter 6 Influence of coal macerals on coke properties
vitrinite-rich factions and their corresponding carbonised inertinite-rich factions, as the
itial intrinsic rates of the former were smaller than that of the latter. However, most of
in
the differences between reactivity of the cokes and their corresponding carbonised
inertinite rich-fractions appeared to be largely due to differences in micropores surface
area, as smaller differences were observed between their intrinsic rates than apparent
rates.
132
Chapter 6 Influence of coal macerals on coke properties
B
0.2
0.4
0.6
n
i
t
i
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
B
0
5
10
15
20
S
u
r
f
a
c
e
a
r
e
a
(
m
2
g
-
1
)
0 20 40 60 80
Non-fused inertinite (vol,%)
0.0
V O I
I
C
0
5
10
15
20
0 20 40 60 8
Non-fused inertinite (vol,%)
S
u
r
f
a
c
e
a
r
e
a
(
m
2
g
-
1
)
0
C
0.0
0.2
0.4
0.6
0.8
1.0
V O I
I
n
i
t
i
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
F
0
10
20
30
40
0 20 40 6
Non-fused inertinite (vol,%)
S
u
r
f
a
c
e
a
r
e
a
(
m
2
g
-
1
)
0
F
0.0
0.2
0.4
0.6
0.8
V O I
I
n
i
t
i
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
G
0
5
10
15
0 20 40
Non-fused inertinite (vol,%)
S
u
r
f
a
c
e
a
r
e
a
(
m
2
g
-
1
)
60
G
0.0
0.1
0.2
0.3
0.4
V O I
I
n
i
t
i
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
Figure 6.9 Surface area of the raw cokes made from the original coals (B, C, F
and G) and their corresponding carbonised maceral-enriched fractions
versus the concentration of non-fused inertinite (left-side charts). The
right-side charts shows the initial intrinsic rates of the cokes made from
the original coals (B, C, F and G) and their corresponding carbonised
maceral-enriched fractions, where O is the coke made from the original
coal, and V and I are the carbonised vitrinite- and inertinite-rich
fractions.
133
Chapter 6 Influence of coal macerals on coke properties
6.2.2 Carbon structure
Crystallite height (L
c
) and length (L
a
) were determined for the carbonised maceral-
enriched fractions and the cokes prepared from the original coals. The carbonised
inertinite-rich fractions, which contain high levels of non-fused inertinite, had lower L
c
than their corresponding carbonised vitrinite-rich fractions, which were characterised by
low levels of non-fused inertinite (Figur . It can be concluded that the non-fuse
inertinite has lower L
c
than the reactive derived maceral component (the macerals that
fuses during carbonisation).
Crystallite height (L
c
) increased with decreasing content of non-fused inertinite in the
cokes made from coals of higher rank (F and G) and their corresponding carbonised
maceral enriched fractions. However, the cokes from lower rank coals (B and C) had
greater L
c
than their corresponding carbonised inertinite-rich fractions but similar L
c
with their carbonised vitrinite-rich fractions. This suggests that the non-fusible inertinite
in the parent coals affects the L
c
of cokes made from higher rank coals more than that of
the cokes produced from lower rank coals. As the amount of non-fused inertinite
increases the L
c
decreases because it produces a decrease of the average crystallite
height (L
c
). Also, the non-fusible inertinite in the coals may affect the development of
the carbon crystallites during carbonisation, which can result in a real decrease of L
c
,
more likely for the higher rank coals.
Crystallite height (L
c
) of the vitrinite-rich fractions could be affected to some extent by
the heating rate during carbonisation as vitrinite-rich fractions were carbonised at lower
heating rate (0.1C min
-1
for B, C and F and 0.05C min
-1
for G) than both original coals
and inertinite-rich fractions (1C min
-1
) to avoid swelling during carbonisation. It has
been shown in section 6.1.3 that carbonisation conditions affected L
c
; the crystallite
eight of three of four cokes prepared at higher heating (9 kg oven) was greater than
Therefore t
fractions m
e 6. 0) d 1
h
their corresponding cokes prepared at lower heating rate (70 g oven) (Figure 6.4).
he degree of ordering of carbon structure (L
c
) of the carbonised vitrinite-rich
ay be lesser because of the lower heating rate.
134
Chapter 6 Influence of coal macerals on coke properties
B B
1.5
1.1
1.2
1.3
1.4
0 20 40 60 80
Non-fused inertinite (vol,%)
L
c
(
n
m
)
r
2
= 0.91
0
2
4
6
8
1.0 1.2 1.4 1.6
Lc (nm)
I
n
i
t
i
a
l
a
p
p
a
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
10
r
e
n
t
-
1
)
C
C
1.5
1.1
1.2
0 20 40 60 80
Non-fused inertinite (vol,%)
L
c
1.3
1.4
(
n
m
)
0
5
1.1 1.2 1.3 1.4
Lc (nm)
I
n
i
t
i
a
l
a
r
a
(
*
1
0
-
6
10
15
p
p
a
r
e
n
t
t
e
g
g
-
1
s
-
1
)
F
1.2
1.4
1.6
1.8
0 20 40 60
Non-fused inertinite (vol,%)
L
c
(
n
m
)
F
r
2
= 0.99
0
5
10
15
20
25
1.2 1.4 1.6 1.8
Lc (nm)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
G G
r
2
= 0.95
1.2
1.4
1.6
1.8
0 20 40 6
Non-fused inertinite (vol,%)
L
c
(
n
m
)
0
0
1
2
3
4
1.2 1.4 1.6 1.8
Lc (nm)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
Figure 6.10 Crystallite height (L
c
) of the raw cokes made from the original coals
(B, C, F and G) and their corresponding carbonised maceral-enriched
fractions versus the concentration of non-fused inertinite (left-side
charts). The initial apparent rates versus L
c
(right-side charts).
135
Chapter 6 Influence of coal macerals on coke properties
The L
c
of the cokes B, F and G and their corresponding vitrinite and inertinite-rich
fractions showed a good relationship with initial apparent rate (Figure 6.10). The
reactivity of coke C was lower than that of its corresponding carbonised vitrinite-rich
fraction even though they have similar L
c
. Although the relationship between the initial
apparent rate and L
c
of the carbonised maceral-enriched fractions and coke made from
the same coal was good in some cases, no trend was observed between the initial
apparent rate and L
c
when all the carbon nriched fractions and cokes were
considered (Figure 6.12). It can be concluded that the influence of L
c
on the reaction
rate was overshadowed by other factors.
Crystallite length (L
a
) of the coke and its corresponding carbonised vitrinite- and
inertinite-rich fractions showed no consistent trend with the content of non-fused
inertinite (Figure 6.11). Feng et al. [73] observed that the carbon structure became more
ordered in the vicinity of clays and iron s. Therefore, the crystallite length may
be influenced by the mineral matter present in the coke.
It has been reported that the gasification reaction occurs mainly at the edges of the
crystallites rather than on the basal planes [34,53]. Therefore the density of the free
edges increases as the length (L
a
) decreases implying an increase of carbon reactivity.
In this study, the L
a
was poorly related to the initial apparent rate (Figure 6.11). No
consistent trend was observed between the initial apparent rate and L
a
of the carbonised
maceral-enriched fractions and coke from the same coal either. The poor relationship
could be explained by the reduced accessibility of the reactant gas to the edges of the
crystallites at the initial stages due to closed porosity [43] and/or presence of catalysts,
which can create pits in the carbon basal planes [92].
In conclusion, the reaction rate at the in ges was not significantly influenced by
either L
c
or L
a
.
ised maceral-e
par le tic
itial sta
136
Chapter 6 Influence of coal macerals on coke properties
B
3.2
3.4
3.6
3.8
4.0
4.2
0 20 40 60 80
Non-fused inertinite (vol,%)
L
a
(
n
m
)
B
0
2
4
6
8
10
3.2 3.4 3.6 3.8 4.0
La (nm)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
C C
4.2
3.2
3.4
3.6
0 20 40 60 80
Non-fused inertinite (vol,%)
L
a
(
n
3.8
4.0
m
)
0
5
3.2 3.4 3.6 3.8 4.0 4.2
La (nm)
I
n
i
t
i
a
l
a
r
a
(
*
1
0
-
6
10
15
p
p
a
r
e
n
t
t
e
g
g
-
1
s
-
1
)
F
3.2
F
0
4.4
3.4
3.6
3.8
4.0
4.2
0 20 40 60
Non-fused inertinite (vol,%)
L
a
(
n
m
)
5
10
15
20
3.2 3.4 3.6 3.8 4.0 4.2
La (nm)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
25 t
G
3.6
3.8
4.0
4.2
(
n
m
)
G
3
4
l
a
p
p
a
r
e
n
t
r
a
t
e
-
6
g
g
-
1
s
-
1
)
3.0
0 20 40 60
Non-
3.2
3.4
fused inertinite (vol,%)
L
a
0
3.2 3.4 3.6 3.8 4.0
I
1
2
La (nm)
n
i
t
i
a
(
*
1
0
Figure 6.11 Crystallite length (L
a
) of the raw cokes made from the original coals
(B, C, F and G) and their corresponding carbonised maceral-
enriched fractions versus the concentration of non-fused inertinite
(left-side charts). The initial apparent rates versus L
a
(right-side
charts).
137
Chapter 6 Influence of coal macerals on coke properties
0
5
10
15
20
25
1.0 1.2 1.4 1.6 1.8
L
c
(nm)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
Figure 6.12 Initial apparent rate of th okes made from the original coals (B
C, F and G) and their carbonised maceral-enriched fractions versus
L
c
.
6.3 Factors influencing rea of carbonised inertinite-rich
fractions and carbonised vitrinite-rich fractions
In the previous section (6.2) the influence of maceral composition on reactivity was
investigated. In order to understand the greater reactivity of carbonised inertinite-rich
fractions compared to that of the carbonised vitrinite-rich fractions, the influence of
their properties on the reaction rate are investigated.
6.3.1 Carbonised inertinite-rich fractions
Figure 6.13 sh
the carbonised
e raw c ,
ctivity
ows that the carbonised inertinite-rich fractions had different reactivity;
inertinite-rich fraction of coal F was the most reactive followed by that
138
Chapter 6 Influence of coal macerals on coke properties
of coal C. The reaction rate of carbonised inertinite-rich fractions increased gradually
during the reactivity test.
0
10
A
20
30
40
50
60
70
0 5 10 15
Carbon conversion (%)
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
F
C
B
D
G
igure 6.13 The apparent reaction rate of the carbonised inertinite-rich fractions
nised inertinite-rich fractions of
oals B, C and D were similar, about 70%, whereas the percentage of non-fused
ertinite in the carbonised inertinite-rich fractions of both coals F and G was smaller,
e anisotropic microtexture varied between
arbonised inertinite-rich fractions. The anisotropic carbon in carbonised inertinite-rich
carbonised inertinite-rich fractions B and C (Figure 6.14).
F
against carbon conversion.
The reactivity of the carbonised inertinite-rich fractions varied to a great degree (Figure
6.13). The content of non-fused inertinite in the carbo
c
in
approximately 56% (Table 6.3). The size of th
c
fractions F and G was characterized mainly by medium mosaic whereas smaller size of
anisotropic microtexture, such as very fine and fine mosaic, was characteristic of the
139
Chapter 6 Influence of coal macerals on coke properties
Table 6.3 The percentage of non-fused inertinite in the carbonised inertinite-rich
fractions.
B C D F G
Non-fused inertinite derived
aceral component (vol. %)
68.0 71.0 69.3 56.4 56.0
m
Because the content of non-fused inertinite varied between the carbonised inertinite-rich
macerals, the influence of the content of non-fused inertinite on the initial apparent rate
was determined. A poor relationship is observed between the content of non-fused
inertinite and initial apparent rate (Figure 6.15a), which shows that the content of non-
fused inertinite has no dominating influence on the reaction rate.
The influence of the anisotropic microtexture in the carbonised inertinite-rich fractions
on the reaction rate was considered as the percentage of anisotropic microtexture was
significant (28-44 %). Coke reactivity has been reported to decrease as the size of the
anisotropic microtexture increased [21,31]. The size of anisotropic microtexture
d from the relationship between initial
al. A poor relationship is observed (Figure
microtexture of different sizes had no
n the reaction rate. The influence of the anisotropic microtexture on the
increases as the coal rank increases [26,105]. Therefore the influence of the anisotropic
texture on the reaction rate can be observe
apparent rate and rank of the parent co
6.15b), which suggests that the anisotropic
influence o
reaction rate is further discussed in section 6.3.2.
140
Chapter 6 Influence of coal macerals on coke properties
(a) (b)
(c) (d)
M
M
Figure 6.14 Microtexture of the carbonised inertinite-rich fractions of (a) coal B,
(b) coal C, (c) coal F and (d) coal G; where VF is very fine mosaic (it
has a uniform grey appearance), F is fine mosaic (it has a grey mottled
appearance) and M is medium mosaic.
F
V F
V F
141
Chapter 6 Influence of coal macerals on coke properties
C
D
B
G
F
0
5
10
15
20
25
50 55 60 65 70 75
Non-fused inertinite (vol, %)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
B
C
D
F
G
0
5
10
15
20
25
0.9 1.0 1.1 1.2 1.3 1.4
R
0
(%)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
(a) (b)
Figure 6.15 Initial apparent rate of the carbonised inertinite-rich fractions versus
Surface area and intrinsic rates
The carbonised inertinite-rich fractions were characterized by the greatest micropore
surface area (CO
2
surface area) compared to that of their corresponding carbonised
vitrinite-rich fractions and cokes (section 6.2.1). However, the surface area was poorly
related to the content of non-fused inertinite derived component (Figure 6.16). The
tinite-rich
were reduced when they were normalised to surface area, but there are still
icant differences between the intrinsic reaction rates (Figure 6.17). Micropore
lain completely the difference between the reaction rates of the
both non-fused inertinite and rank of the parent coal.
difference between the apparent reaction rates between the carbonised iner
fractions
signif
surface area does not exp
carbonised inertinite-rich fractions.
142
Chapter 6 Influence of coal macerals on coke properties
C
B
F
G
0
5
10
15
20
25
30
35
50 55 60 65 70 75
Non-fused inertinite (vol,%)
S
u
r
f
a
c
e
a
r
e
a
(
m
2
g
-
1
)
Figure 6.16 Surface area measured by carbon dioxide against non-fused inertinite.
0
4
8
12
16
20
B C F G
0.0
0.2
0.4
0.6
0.8
1.0
B C F G
I
n
i
t
i
a
l
i
n
t
r
i
n
s
i
c
r
a
t
e
(
*
1
0
-
6
g
m
-
2
s
-
1
)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
Figure 6.17 Initial apparent and intrinsic rates of the carbonised inertinite-rich
fractions.
143
Chapter 6 Influence of coal macerals on coke properties
Carbon structure (carbon crystallite size)
Figure 6.18 shows that the crystallite height (L
c
) of the carbonised inertinite-rich
actions of coals F and G were similar, but greater than the crystallite height (L
c
) of the
carbonised inertinite-rich fractions of coals B and C. The greater L
c
could be due to the
presence of reactive maceral derived com isotropic microtexture). In the
previous section (6.2), it was shown that the carbonised vitrinite-rich fractions, which
are characterised by over 80% of reactive maceral derived component, had greater L
c
than the carbonised inertinite-rich fractions (Figure 6.10). Crystallite length (L
a
) was
similar for carbonised inertinite-rich fractions for coals B, C and G. Only the carbonised
inertinite-rich fraction of coal F had greater L
a
.
No trend was observed between initial apparent rate and either L
c
or L
a
(Figure 6.19).
Although the carbonised inertinite-rich fractions F and G had similar crystallite height
they had different reactivity. Also, the carbonised inertinite-rich fractions B and C had
different reactivity but similar L
c
. A poor relationship was also observed between L
a
and
reaction rate. Crystallite length (L
a
) was similar for the carbonised inertinite-rich
fractions B, C and G but their reactivity was also different. It can be concluded that
neither L
c
nor L
a
influenced significantly the initial apparent rate.
fr
ponent (an
1.1
1.2
1.3
1.4
1.5
B C F G
L
c
(
n
m
)
3.0
3.2
3.4
3.6
3.8
4.0
4.2
4.4
B C F G
L
a
(
n
m
)
Figure 6.18 Crystallite size (L
c
and L
a
) of the carbonised inertinite-rich fractions
B, C, F and G (the error bars are shown).
144
Chapter 6 Influence of coal macerals on coke properties
G
B
C
F
B
C
G
F
0
1.1 1.2 1.3 1.4 1.5
L
c
(
5
10
15
20
25
nm)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
0
3.2 3.4 3.6 3.8 4.0 4.2
5
10
15
20
25
L
a
(nm)
I
l
a
p
p
a
r
e
n
t
r
a
t
e
*
1
0
-
6
g
g
-
1
s
-
1
)
igure 6.19 Initial apparent rate versus crystallite size (L
c
and L
a
) of the
fractions.
bonised inertinite-rich fractions. The
arbonised inertinite-rich fractions contained, in most cases, a greater content of mineral
B, D, F and G were
uite similar (Figure 6.20). Their reaction rate initially increased with increasing carbon
onversion but after about 4-6% conversion the reaction rate did not change
n
i
t
i
a
(
F
carbonised inertinite-rich
In summary, the micropore surface area of the carbonised inertinite-rich fractions
influenced the reaction rate to some extent but it did not explain entirely the differences
between their reaction rates since differences between the intrinsic reaction rates were
still observed. Also, the carbon crystallite size (L
c
and L
a
) did not influence significantly
the reaction rate of the carbonised inertinite-rich fractions since a poor relationship was
observed between the apparent rate and both L
c
and L
a
. Therefore, other factors had a
stronger influence on the reactivity of the car
c
matter than the cokes made from the original coals. The influence of the catalytic
mineral matter on the reaction rate will be presented in Chapter 8.
6.3.2 Carbonised vitrinite-rich fractions
The reactivity of the carbonised vitrinite-rich fractions of coals
q
c
145
Chapter 6 Influence of coal macerals on coke properties
significantly. The carbonised vitrinite-rich fraction of coal C was the only one that
showed greater reactivity and the reaction rate increased gradually until the reactivity
test was completed.
0
2
4
6
8
10
12
g
g
-
1
s
14
16
18
0 2 4 6 8 10 12 14 16
Carbon conversion (%)
A
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
-
1
)
C
F
B
D
G
Figure 6.20 The apparent reaction rate of the carbonised vitrinite-rich fractions
against carbon conversion.
he size of anisotropic microtexture was smaller in the carbonised vitrinite-rich
actions made from high volatile coals (coal B and coal C) than that of the carbonised
itrinite-rich fractions made from medium volatile coals (coal F and coal G) (Figure
.21). The anisotropic microtexture of the carbonised vitrinite-rich fractions B and C
T
fr
v
6
was characterised mainly by very fine mosaic and low levels of fine mosaic. Mainly
fine mosaic and low levels of medium mosaic formed the anisotropic microtexture of
the carbonised vitrinite-rich fractions F and G. The content of anisotropic microtexture
in the carbonised vitrinite-rich fractions was very high, greater than 85% (Table 6.4).
146
Chapter 6 Influence of coal macerals on coke properties
147
(a) (b)
(c) (d)
Figure 6.21 Microtexture of the carbonised vitrinite-rich fractions of (a) coal B,
(b) coal C, (c) coal F and (d) coal G; where VF is very fine mosaic (it
has a uniform grey appearance) and F is fine mosaic (it has a grey
mottled appearance).
V F
F
F
V F
F
F
Chapter 6 Influence of coal macerals on coke properties
Table 6.4 The percentage of non-fused inertinite in the carbonised vitrinite-rich
fractions.
B C D F G
Inertinite (vol. %) 3.2 12.3 10.3 3.4 15.4
As discussed in the previous section, it is generally accepted that the reactivity increases
with decreasing the size of the anisotropic microtexture. However, the relationship
between initial apparent rate and the rank of parent coal was poor (Figure 6.22). This
indicates that the size of the anisotropic microtexture in the range very fine mosaic -
medium mosaic did not affect significantly the initial apparent rate.
In the previous section, it has been shown that the content of inertinite on the reaction
rate was more dominant than that of the anisotropic microtexture. The relationship
between initial apparent rate and the content of non-fused inertinite in the carbonised
vitrinite-rich fractions was poor, which suggests that non-fused inertinite derived
component did not affect the reaction rate (Figure 6.22).
G
F
D
C
B
0
1
2
3
4
0.9 1.0 1.1 1.2 1.3 1.4
R
0
(%)
I
n
i
t
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
C
D
F
B G
0
1
2
3
n
t
r
s
-
1
)
4
0 3 6 9 12 15 18
Non-fused inertinite (vol, %)
I
n
i
t
i
a
l
a
p
p
a
r
e
(
*
1
0
-
6
g
g
-
1
Figure 6.22 Initial apparent rate of the carbonised vitrinite-rich fractions versus
both rank of the parent coal and non-fused inertinite.
a
t
e
148
Chapter 6 Influence of coal macerals on coke properties
Surface area and intrinsic rates
The surface area of micropores (CO
2
surface area) did not vary significantly between
the carbonised vitrinite-rich fractions (Table 6.5). Moreover, the differences in apparent
reaction rates between the carbonised vitrinite-rich fractions were only slightly
diminished when the intrinsic rates are used for comparison (Figure 6.23). Therefore,
the micropore surface area of the carbonised vitrinite-rich fractions did not explain the
B C F G
differences between their reaction rates.
Table 6.5 Surface area, measured by CO
2
, of the carbonised vitrinite-rich
fractions.
Surface area (m
2
g
-1
) 4.1 8.0 6.5 7.3
4
0
1
B C F G
I
n
i
t
2
3
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
0.0
B C F G
I
n
i
t(
*
Figure 6.23 Initial apparent and intrinsic rates of the carbonised
0.2
0.4
0.6
i
a
l
i
n
t
r
i
n
s
i
c
r
a
1
0
-
6
g
m
-
2
s
-
1
)
vitrinite-rich
fractions.
t
e
149
Chapter 6 Influence of coal macerals on coke properties
Carbon structure (carbon crystallite size)
The effect of the rank of the parent coal on crystallite height (L
c
) of the carbonised
vitrinite-rich fractions was significant (Figure 6.24). The carbonised vitrinite-rich
actions F and G, prepared from medium volatile bituminous coals, had considerably
reater L
c
than the carbonised vitrinite-rich fractions B and C, made from high volatile
ituminous coals.
Although the rank of the parent coal showed its influence on the crystallite height (L
c
)
of the carbonised vitrinite-rich fractions, it did not appear to affect the L
c
of the cokes
prepared in the 9 kg oven (see section 5.5). The presence of inertinite in greater
proportion in the original coals than in the vitrinite-rich fractions may affect the
microtexture formation. Mitchell et al. [21] and Graham et al. [17] showed that the
distribution and size of macerals in coals controls the development of coke
microtexture. Gray and Champagne [52] concluded that coarse inertinite particles limit
the size of anisotropic microtexture because the space between the inertinite particles is
arrow and the molecular orientation domains cannot grow. Also, in section 6.2 it was
inite-rich f er rank (F and
poor relationship observed between the rank of the
arent coal and the L
c
of the cokes was due to inertinite content that lowered the L
c
of
ffect significantly L
a
of the coke.
he influence of both crystallite height (L
c
) and length (L
a
) on the reaction rate was
oor (Figure 6.25). Although the carbonised vitrinite-rich fractions F and G had greater
c
than the carbonised vitrinite-rich fraction B the differences between their reaction
fr
g
b
n
shown that the cokes made from the original coals had lower L
c
than that the carbonised
ractions, particularly for the cokes made from coals of high vitr
G). It can be concluded that the
p
the cokes made from higher rank coal.
Crystallite length (L
a
) of the carbonised vitrinite-rich fractions did not vary significantly
across the carbonised vitrinite-rich fractions from coals B, C and F, and it was slightly
greater for the carbonised vitrinite-rich fraction from coal G (Figure 6.24). It appears
that the rank of the parent coal does not a
T
p
L
rates were small.
150
Chapter 6 Influence of coal macerals on coke properties
The greater reactivity f the carbonised vit te-rich f ion C c ot be ex ined by
L
c
, as the h fraction B with s r L
c
had lower reaction rate.
lso, crystallite length (L
a
) did not explain the increased reactivity of the carbonised
itrinite-rich fraction C because it was comparable to that of the carbonised vitrinite-
ch fractions B and F, which had lower reactivity. The increased reactivity of the
arbonised vitrinite-rich fraction C was due to its greater content in catalytic mineral
atter. This is further discussed in Chapter 8.
o rini ract ann pla
carbonised vitrinite-ric imila
A
v
ri
c
m
1.2
1.4
1.6
1.8
B C F G
L
c
(
n
m
)
3.0
3.2
3.4
3.6
3.8
4.0
B C F G
L
a
(
n
m
)
Figure 6.24 Crystallite siz
e (L
c
and L
a
) of the carbonised vitrinite-rich fractions.
C
F
1
2
3
i
a
l
a
p
p
a
r
e
n
t
r
a
t
e
(
*
1
0
-
6
g
g
-
1
s
-
1
)
B G
4
I
n
i
t
C
F
2
3
p
p
a
r
e
n
t
r
a
t
e
-
6
g
g
-
1
s
-
1
)
G B
1
4
I
n
i
t
i
a
l
a
(
*
1
0
0
1.3 1.4 1.5 1.6 1.7 1.8
L
c
(nm)
0
3.2 3.4 3.6 3.8 4.0
L
a
(nm)
Figure 6.25 Initial apparent rate versus crystallite size (L
c
and L
a
) of the
carbonised vitrinite-rich fractions.
151
Chapter 6 Influence of coal macerals on coke properties
6.4 Summary
The properties of the cokes made in the 70 g oven were affected to some degree by the
lower heating rate used during carbonisation compared to that used for carbonisation of
the coals in the 9 kg oven. The extent to which inertinite fused decreased, which result
in greater content of non-fused inertinite in the cokes prepared in the 70 g oven, and the
size of the anisotropic texture was smaller. Previous studies also reported a decrease of
both percentage of inertinite that fuses and size of the anisotropic microtexture as
heating rate decreased. Also, the carbon crystallite height (L
c
) was lower in the cokes
ade in the 70 g oven than in the 9 kg oven.
ing vitrinite-
ch fractions. The size of anisotropic microtexture of the vitrinite-rich fractions was
arbonised inertinite-rich fractions were characterised by much greater
icropore surface area than the vitrinite-rich fractions.
influence the reaction rate.
he reaction rate of the carbonised inertinite-rich fractions showed significant variation.
The differences in the reactivity of the carbonised inertinite-rich fractions was reduced
when the apparent rate was normalised by the micropores surface area. However,
crystallite height (L
c
) showed no significant influence on the reaction rate. Therefore,
m
It was shown that the carbonised inertinite-rich fractions were far more reactive than the
carbonised vitrinite-rich fractions. Consistent differences were observed between the
properties of the carbonised inertinite-rich fractions and their correspond
ri
lower than that for the inertinite-rich fractions because of the lower heating rate used for
their preparation. The carbonised vitrinite-rich fractions had greater L
c
than the
carbonised inertinite-rich fractions, which is consistent with data reported in previous
studies. Also, the c
m
The micropore surface area of the carbonised inertinite-rich fractions and their
corresponding carbonised vitrinite-rich fractions was found to affect to some extent the
reaction rate. Crystallite height (L
c
) appeared to have some influence on the reaction
rate of the carbonised maceral-rich fractions prepared from the same coal, but when the
data for all the samples were considered together, a poor relationship was observed with
the reaction rate, which indicates that L
c
did not
T
152
Chapter 6 Influence of coal macerals on coke properties
153
other factors had stronger influence on the reactivity of the carbonised inertinite-rich
fractions.
The carbonised vitrinite-rich fractions showed small variation between their reaction
rates for most of them (B, D, F and G). The variation of size of anisotropic microtexture
from very fine to medium mosaic did not affect the reaction rate of the carbonised
vitrinite-rich fractions B, D, F and G. The crystallite height (L
c
) of the carbonised
vitrinite-rich fractions B, F and G, which was in the range 1.391.72 nm, did not
fluence significantly their reaction rates. The carbonised vitrinite-rich fraction C had
e
igh reactivity of the carbonised vitrinite-rich fraction C was due to its greater content
f catalytic mineral phases (see Chapter 8).
The poor relationship observed between L
c
of the cokes and the rank of their parent coal
(see Chapter 5) is believed to be due to the presence of non-fusible inertinite in the
coals. The non-fusible inertinite in the medium volatile coals decreased significantly
the L
c
of the cokes, compared to that of their corresponding carbonised vitrinite-rich
fractions, but had almost no influence on L
c
of the cokes made from high volatile coals.
This suggests that the influence of the coal rank on crystallite height (L
c
) of the cokes is
diminished by the presence of non-fusible inertinite in the parent coals.
study, the infl er on coke reactivity was also
Characterisation of mineral matter present in the cokes used in this study is
er 7 and the influence of the catalytic mineral matters on the reaction
te is presented in Chapter 8. Also, the transformations that occur in the mineral matter
in
significantly greater reactivity than the other carbonised vitrinite-rich fractions. Th
h
o
In order to explain the difference in reactivity across the cokes investigated in this
uence of the catalytic mineral matt
investigated.
presented in Chapt
ra
during gasification and also the importance of catalysts during gasification are presented
in Chapter 9.
Potrebbero piacerti anche
- Coal For Metallurgical Coke Production PDFDocumento24 pagineCoal For Metallurgical Coke Production PDFS SITAPATI100% (1)
- Design of Lifting Beams PDFDocumento10 pagineDesign of Lifting Beams PDFAnonymous x9STa9Nessuna valutazione finora
- 2011 Exam GeotechnicalDocumento9 pagine2011 Exam GeotechnicalAhmed AwadallaNessuna valutazione finora
- Utilization of Coking Coal in Metallurgical ProcessDocumento14 pagineUtilization of Coking Coal in Metallurgical ProcessVinay MathadNessuna valutazione finora
- How Is Crude Coal Tar DerivedDocumento8 pagineHow Is Crude Coal Tar DerivedRudy MadianNessuna valutazione finora
- Learning Center. SailDocumento5 pagineLearning Center. SailpdiconpNessuna valutazione finora
- Session-1-Introduction and Coke MakingDocumento51 pagineSession-1-Introduction and Coke MakingHeroNessuna valutazione finora
- Notes For COALTRANS Presentation2 - ManualDocumento15 pagineNotes For COALTRANS Presentation2 - ManualAgus BudiluhurNessuna valutazione finora
- CupolaDocumento5 pagineCupolaRajan Goyal100% (2)
- 4 CokeDocumento6 pagine4 CokePoshin ShrivastavNessuna valutazione finora
- Factors Affecting Iron QualityDocumento31 pagineFactors Affecting Iron QualitySandhya MukherjeeNessuna valutazione finora
- Blast Furnace IronmakingDocumento83 pagineBlast Furnace IronmakingKumar Varun100% (1)
- Blast Furnace Shaft StructureDocumento25 pagineBlast Furnace Shaft StructureKrishnaNessuna valutazione finora
- Lesson 1 Iron and Steel ManufacturingDocumento28 pagineLesson 1 Iron and Steel ManufacturingMercie KishNessuna valutazione finora
- Blast Furnace Iron Making, IIT, KGP, Oct 26, 2010Documento85 pagineBlast Furnace Iron Making, IIT, KGP, Oct 26, 2010Vikas Solanki100% (1)
- Applications Steel: Plexicoke in The VmezuelanDocumento8 pagineApplications Steel: Plexicoke in The VmezuelanJose Marval RodriguezNessuna valutazione finora
- CSR Vs CRI Graph Coalformetallurgicalcokeproduction PDFDocumento24 pagineCSR Vs CRI Graph Coalformetallurgicalcokeproduction PDFJANET GTNessuna valutazione finora
- Gas Holders Risk AssessmentDocumento48 pagineGas Holders Risk AssessmentRonak MotaNessuna valutazione finora
- S K HazraDocumento6 pagineS K HazraRicky MenonNessuna valutazione finora
- Coke Quality Predictions and Future RequirementsDocumento24 pagineCoke Quality Predictions and Future RequirementsSamanway DasNessuna valutazione finora
- Smelting Corex ProcessDocumento19 pagineSmelting Corex ProcessKrishna Teja JayanthiNessuna valutazione finora
- COREX Process in IronmakingDocumento16 pagineCOREX Process in IronmakingSamsul BahriNessuna valutazione finora
- Producing Metals Through Blast Furnace MetallurgyDocumento32 pagineProducing Metals Through Blast Furnace MetallurgyShailesh SharmaNessuna valutazione finora
- Coal Industry PDFDocumento10 pagineCoal Industry PDFVineet SharmaNessuna valutazione finora
- Mini bf3Documento5 pagineMini bf3Maheswar SethiNessuna valutazione finora
- Iron Metallurgy by Nwaogbe JohnDocumento26 pagineIron Metallurgy by Nwaogbe JohnDavid UdohNessuna valutazione finora
- Pig Iron - Blast Furnace RouteDocumento3 paginePig Iron - Blast Furnace RouteRaden Pambudi PratamaNessuna valutazione finora
- Coal Carbonization and Coke Oven Plant: Course: Chemical Technology (Organic) Module IIDocumento10 pagineCoal Carbonization and Coke Oven Plant: Course: Chemical Technology (Organic) Module IITom Jose KooduthottiyilNessuna valutazione finora
- Presentation On Preparation of Coke in Coke Oven Batteries: Presented By: Anil Kumar KhataiDocumento18 paginePresentation On Preparation of Coke in Coke Oven Batteries: Presented By: Anil Kumar KhataiKhatai Anil KumarNessuna valutazione finora
- Effects of preheating on coking coalDocumento14 pagineEffects of preheating on coking coalperekwamaNessuna valutazione finora
- Blast Furnace Iron Making IIT KGP Oct 26 2010Documento85 pagineBlast Furnace Iron Making IIT KGP Oct 26 2010Samanway DasNessuna valutazione finora
- Ironmaking and Steelmaking Theory and PracticeDocumento9 pagineIronmaking and Steelmaking Theory and PracticeRasul BzNessuna valutazione finora
- New Technology PDFDocumento27 pagineNew Technology PDFbasavarajNessuna valutazione finora
- Comparison of Cupola Furnace and BLDocumento9 pagineComparison of Cupola Furnace and BLst5154706Nessuna valutazione finora
- BF ThermoDocumento5 pagineBF ThermoLucky AliNessuna valutazione finora
- 1 Introduction of The Blast Furnace ProcessDocumento9 pagine1 Introduction of The Blast Furnace Processhemant patilNessuna valutazione finora
- Manufacture of Metallurgical Coke and Recovery of Coal ChemicalsDocumento166 pagineManufacture of Metallurgical Coke and Recovery of Coal ChemicalsMarco Milos Trentu100% (1)
- Blast Furance OperationDocumento9 pagineBlast Furance Operationjaved alamNessuna valutazione finora
- Coke Making TechnologyDocumento166 pagineCoke Making TechnologyManab DuttaNessuna valutazione finora
- Forging Processes MEE 3024 2014Documento25 pagineForging Processes MEE 3024 2014Ali M. ElghawailNessuna valutazione finora
- High-Temperature and Low-Oxygen Combustion Technology For Carbon Rotary KilnsDocumento5 pagineHigh-Temperature and Low-Oxygen Combustion Technology For Carbon Rotary KilnsGauranka MoranNessuna valutazione finora
- Proceso Bof en InglesDocumento25 pagineProceso Bof en InglesMossstazzzaNessuna valutazione finora
- Coke Quality and Thermal Reserve Zone PDFDocumento6 pagineCoke Quality and Thermal Reserve Zone PDFhalder_kalyan9216Nessuna valutazione finora
- Iron Steel ETSADocumento6 pagineIron Steel ETSAVarun RajNessuna valutazione finora
- Tecnored Process - High Potential in Using Different Kinds of Solid FuelsDocumento5 pagineTecnored Process - High Potential in Using Different Kinds of Solid FuelsRogerio CannoniNessuna valutazione finora
- CipolaDocumento2 pagineCipolanimish1991Nessuna valutazione finora
- Unit 3 NotesDocumento12 pagineUnit 3 NotesarunkumarnoolaNessuna valutazione finora
- International Journal of Thermal Sciences: Sen Li, Tongmo Xu, Qulan Zhou, Houzhang Tan, Shien HuiDocumento9 pagineInternational Journal of Thermal Sciences: Sen Li, Tongmo Xu, Qulan Zhou, Houzhang Tan, Shien HuiMatias MancillaNessuna valutazione finora
- Nox Emissions of An Opposed Wall-Fired Pulverized Coal Utility BoilerDocumento7 pagineNox Emissions of An Opposed Wall-Fired Pulverized Coal Utility BoilerAnonymous knICaxNessuna valutazione finora
- Vebsar: Specific Gravity PorousDocumento2 pagineVebsar: Specific Gravity Porousrahul srivastavaNessuna valutazione finora
- Iron Making MM-15020 5 Sem B Tech Department of Metallurgy and Materials Engineering V.S.S.U.T, BurlaDocumento83 pagineIron Making MM-15020 5 Sem B Tech Department of Metallurgy and Materials Engineering V.S.S.U.T, BurlaAshishNessuna valutazione finora
- Decreasing Blast Furnace Process Costs at Iscor Long ProductsDocumento6 pagineDecreasing Blast Furnace Process Costs at Iscor Long ProductsPaul VermeulenNessuna valutazione finora
- Reduction Behavior of Carbon Composite Iron Ore Hot Briquette in Shaft Furnace and Scope On Blast Furnace Performance Reinforcement PDFDocumento9 pagineReduction Behavior of Carbon Composite Iron Ore Hot Briquette in Shaft Furnace and Scope On Blast Furnace Performance Reinforcement PDFAgustine SetiawanNessuna valutazione finora
- KilnDocumento20 pagineKilnzabira50% (2)
- 7.carbonization and Combustion CalculationsDocumento27 pagine7.carbonization and Combustion CalculationsMuhammad Irfan Malik100% (1)
- Furnaces Classification and FeaturesDocumento75 pagineFurnaces Classification and FeaturesNiladri BiswasNessuna valutazione finora
- Calcination and Reduction of Laterite Nickel OresDocumento10 pagineCalcination and Reduction of Laterite Nickel OresrikocahyopNessuna valutazione finora
- 1.2 Layout of Modern Coal Power Plant, Super Critical Boilers, FBC Boilers, Steam and Heating RatesDocumento32 pagine1.2 Layout of Modern Coal Power Plant, Super Critical Boilers, FBC Boilers, Steam and Heating Rateskarthikeyan SNessuna valutazione finora
- High Efficiency Pulverized Coal-Fired Power Generation Technology (Ultra Super Critical Steam Condition)Documento2 pagineHigh Efficiency Pulverized Coal-Fired Power Generation Technology (Ultra Super Critical Steam Condition)Hns Kvn Chavez100% (2)
- The Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelDa EverandThe Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelNessuna valutazione finora
- The Working of Steel Annealing, Heat Treating and Hardening of Carbon and Alloy SteelDa EverandThe Working of Steel Annealing, Heat Treating and Hardening of Carbon and Alloy SteelValutazione: 5 su 5 stelle5/5 (4)
- Extractive Metallurgy 3: Processing Operations and RoutesDa EverandExtractive Metallurgy 3: Processing Operations and RoutesNessuna valutazione finora
- Customer Price List: Certified Code Compliant Steel Stud Manufacturer Manufacturer of Supreme Framing SystemsDocumento5 pagineCustomer Price List: Certified Code Compliant Steel Stud Manufacturer Manufacturer of Supreme Framing SystemsRamonNessuna valutazione finora
- Material Testing Lab Tensile Test ReportDocumento1 paginaMaterial Testing Lab Tensile Test Reportwajid0652Nessuna valutazione finora
- PulpDocumento382 paginePulpBeerBie100% (1)
- DOWSIL™ 795 Structural Glazing Sealant Technical Data SheetDocumento5 pagineDOWSIL™ 795 Structural Glazing Sealant Technical Data SheetTrung Nguyễn NgọcNessuna valutazione finora
- Readymix ProfileDocumento33 pagineReadymix ProfileAyman HammodehNessuna valutazione finora
- Forged Engineering DataDocumento4 pagineForged Engineering DataretrogradesNessuna valutazione finora
- Troubleshooting GuideDocumento25 pagineTroubleshooting GuideIjabiNessuna valutazione finora
- Dow Science Paper-A Commercially Viable Solution Process To Control Long-Chain Branching in PolyethyleneDocumento1 paginaDow Science Paper-A Commercially Viable Solution Process To Control Long-Chain Branching in Polyethylenefengshaohua_gmailNessuna valutazione finora
- Internship SeminarDocumento28 pagineInternship Seminarkavya kruthiNessuna valutazione finora
- Beseva Group Product Listing for Bleaching, Dyeing, Finishing and Printing ChemicalsDocumento9 pagineBeseva Group Product Listing for Bleaching, Dyeing, Finishing and Printing ChemicalsNoumanKhanNessuna valutazione finora
- Electric Water Heaters GuideDocumento36 pagineElectric Water Heaters GuidearshadNessuna valutazione finora
- Economic analysis of wastewater treatment processesDocumento12 pagineEconomic analysis of wastewater treatment processesEmanuel VillegasNessuna valutazione finora
- Lesson 1 Electron Configuration and Octet RuleDocumento23 pagineLesson 1 Electron Configuration and Octet RuleAngel CapinpinNessuna valutazione finora
- Anochrome Coating Data Sheet 15-10-14Documento3 pagineAnochrome Coating Data Sheet 15-10-14ellisforheroesNessuna valutazione finora
- SSPC News Bulletin - July 2020Documento26 pagineSSPC News Bulletin - July 2020JlkKumarNessuna valutazione finora
- Graphite Degeneration in The Superficial Layer ofDocumento11 pagineGraphite Degeneration in The Superficial Layer ofBehdad ShariatiNessuna valutazione finora
- T Printing 1Documento19 pagineT Printing 1Amrinder DhimanNessuna valutazione finora
- A Practical Guide For HOHDocumento164 pagineA Practical Guide For HOHDan AngheleaNessuna valutazione finora
- Heavy MetalsDocumento4 pagineHeavy MetalsGeorona KANessuna valutazione finora
- Annex II - AMC 20-29Documento36 pagineAnnex II - AMC 20-29Victor Manuel Peña EsparteroNessuna valutazione finora
- Tribological Analysis of Thin Films by Pin-On-Disc Evaluation of Friction PDFDocumento10 pagineTribological Analysis of Thin Films by Pin-On-Disc Evaluation of Friction PDFDavid Rafael RamírezNessuna valutazione finora
- A Predictive Approach To Fitness-For-Service Assessment of Pitting CorrosionDocumento9 pagineA Predictive Approach To Fitness-For-Service Assessment of Pitting CorrosionMaría Vaquero TxapartegiNessuna valutazione finora
- Hydrostatic Testing of Yankee Dryers Often Does More HarmDocumento4 pagineHydrostatic Testing of Yankee Dryers Often Does More HarmmgmqroNessuna valutazione finora
- Understanding The Quenchant Report PDFDocumento8 pagineUnderstanding The Quenchant Report PDFSandeep SarafNessuna valutazione finora
- Viscosity of c5h802 - 19Documento1 paginaViscosity of c5h802 - 19CharlesNessuna valutazione finora
- Cement and Steel Rates For The Month of August 2013Documento1 paginaCement and Steel Rates For The Month of August 2013Guru PrasadNessuna valutazione finora
- Technical Submission of PudloDocumento82 pagineTechnical Submission of PudlotcthomasNessuna valutazione finora
- Cotton ListDocumento22 pagineCotton ListL.N.CHEMICAL INDUSTRYNessuna valutazione finora