Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Aldol Puzzle Lab Report
Caricato da
laurabruce27100%(1)Il 100% ha trovato utile questo documento (1 voto)
501 visualizzazioni11 pagineThis document summarizes an experiment to synthesize 2-methoxychalcone via an aldol condensation reaction between acetophenone and ortho-methoxybenzaldehyde. The reaction product was purified via recrystallization and characterized using melting point, IR spectroscopy, 1H NMR and 13C NMR. These analyses identified the product as 2-methoxychalcone. The yield was 52%, which was decent but not ideal, suggesting some product was lost during the recrystallization purification step. The document concludes the experiment successfully demonstrated an important carbon-carbon bond forming aldol condensation reaction.
Descrizione originale:
Organic Chemistry Laboratory
Copyright
© © All Rights Reserved
Formati disponibili
PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoThis document summarizes an experiment to synthesize 2-methoxychalcone via an aldol condensation reaction between acetophenone and ortho-methoxybenzaldehyde. The reaction product was purified via recrystallization and characterized using melting point, IR spectroscopy, 1H NMR and 13C NMR. These analyses identified the product as 2-methoxychalcone. The yield was 52%, which was decent but not ideal, suggesting some product was lost during the recrystallization purification step. The document concludes the experiment successfully demonstrated an important carbon-carbon bond forming aldol condensation reaction.
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
100%(1)Il 100% ha trovato utile questo documento (1 voto)
501 visualizzazioni11 pagineAldol Puzzle Lab Report
Caricato da
laurabruce27This document summarizes an experiment to synthesize 2-methoxychalcone via an aldol condensation reaction between acetophenone and ortho-methoxybenzaldehyde. The reaction product was purified via recrystallization and characterized using melting point, IR spectroscopy, 1H NMR and 13C NMR. These analyses identified the product as 2-methoxychalcone. The yield was 52%, which was decent but not ideal, suggesting some product was lost during the recrystallization purification step. The document concludes the experiment successfully demonstrated an important carbon-carbon bond forming aldol condensation reaction.
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 11
Synthetic #3: An Aldol Condensation Puzzle
Laura Bruce, CHEM 213
Synthetic FFR #3
TA: Kristin Beiswenger
December 11
th
, 2013
"#$%& '
I. Introduction
Much of the focus in pharmaceutical development lies in utilizing the full potential of previously
uncovered biologically active compounds. Fully understanding the role that certain molecules can play
in biochemical pathways is essential for the development of innovative treatment options in medicine.
1
For instance, a class of compounds known as chalconoids is currently under investigation in order to
exploit a wide range of biological applications.
1
Chalcones are trans-1,3-diaryl-2-propen-1-ones, containing an !,"-unsaturated ketone between
two aromatic rings.
1
Varying substituent groups on these rings allow for great diversity within the
chalconoid class, as they can serve as precursors, intermediates, or products in many biological
reactions.
1,2
Notably, chalcones are important in the natural synthesis of flavonoids and isoflavonoids,
which are plant metabolites. In humans, chalcones and their derivatives have been shown to exhibit
anticancer, antiprotozoal, antimalarial, antimicrobial, antioxidant, and antifungal properties with limited
toxicity.
2
Additionally, certain chalcones can serve as inhibitors for mammalian enzymes such as
monoamine oxidase (MAO) and cyclooxygenase (COX), which suggests that these ketones can be used
in pain management.
1,2
Accordingly, chalcone synthesis has become a topic of high interest in pharmaceutical
development. Chalcone formation can be achieved via the Claisen-Schmidt reaction, which is a
relatively simple and inexpensive example of a mixed aldol condensation.
2,3
Aldol condensation
reactions comprise of an aldol reaction (nucleophilic addition/alpha substitution) and subsequent
dehydration of the aldol product to yield an !,"-unsaturated carbonyl.
2
Carbonyl condensation reactions
are essential in organic syntheses, as they result in the formation of a carbon-carbon bond, allowing for
the construction of complex biomolecules and intermediates as seen in metabolic pathways.
3,4
Specifically, methoxychalcones can be synthesized from the base-catalyzed mixed aldol
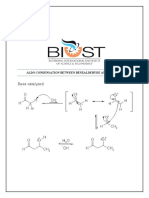
condensation of acetophenone and a methoxybenzaldehyde, as seen in Scheme 1.
3
"#$%& (
Scheme 1. Synthesis of a methoxychalcone from acetophenone and a methoxybenzaldehyde.
The mechanism for the reversible conversion of 1 equivalent of acetophenone and 1 equivalent
of a methoxybenzaldehyde to 1 equivalent of a methoxychalcone is depicted in Scheme 2. As with all
mixed aldol condensations between a ketone and an aldehyde, the base-catalyzed Claisen-Schmidt
reaction includes the enolate ion of acetophenone reacting with the given aldehyde to form a "-
hydroxyketone (an aldol), followed by the dehydration of the aldol to afford an !,"-unsaturated
carbonyl.
3
In this experiment, the reversible reaction produces 2-, 3-, or 4-methoxychalcone.
3
Scheme 2. Electron-pushing mechanism for the synthesis of a methoxychalcone via the reversible
Claisen-Schmidt reaction.
"#$%& )
In the first step, a hydroxide ion from sodium hydroxide serves as a catalyst, deprotonating the
acetophenone at a reactive !-hydrogen from the methyl ketone. This converts acetophenone to an
anionic form, which tautomerizes to an enolate ion via resonance. Next, this enolate nucleophilically
attacks the electrophilic carbonyl carbon of the methoxybenzaldehyde, which also initiates an !-
substitution for the acetophenone enolate ion. The resulting anionic tetrahedral intermediate is next
protonated by hydroxyl hydrogen from ethanol, resulting in a neutral aldol product (a "-hydroxyketone).
In the final step, this aldol is easily dehydrated to yield the final enone product. Specifically, the
deprotonated ethanol removes an !-hydrogen from the aldol, forming the alkene and expelling a
hydroxide ion as a leaving group via an E1
C
B elimination reaction. Subsequently, the hydroxide base
catalyst is reformed and a methoxychalcone is produced.
2,3
The purpose of this experiment is to exemplify the synthesis of a biologically significant
molecule, a methoxychalcone, from acetophenone and an unknown methoxybenzaldehyde, employing
the Claisen-Schmidt mixed aldol condensation. Subsequently, the product can be characterized and
identified via melting point, infrared (IR) spectroscopy, 400 MHz
1
H and 100 MHz
13
C nuclear
magnetic resonance (NMR) data.
3
II. Experimental
2-Methoxychalcone. Acetophenone (1 mL, 8.573 mmol), ortho-methoxybenzaldehyde (1 mL, 8.278
mmol), and ethanol (95%, 3 mL) were combined and stirred briefly at 25 C. Sodium hydroxide
solution (50% by weight, 0.50 mL) was added to the mixture and shaken until a homogenous, cloudy
yellow liquid was evident. The reaction mixture was stirred at 5 minute intervals for 30 minutes at 25
C, yielding an orange, layered mixture. The reaction was monitored by TLC (1:1 ethyl
acetate/hexanes) until completion, and the crude product was crystallized at -4 C, isolated via vacuum
filtration, and washed with deionized water (2 x 3 mL), yielding large, peach-colored crystal aggregates.
Recrystallization (70:30 200-proof ethanol/water) afforded 2-methoxychalcone as light yellow, powdery
"#$%& *
crystals (1.026 g, 52%), mp 51 - 58 C; IR (ATR) #
max
2962, 1657, 1594, 1575, 1251 cm
-1
;
1
H NMR
(400 MHz, CDCl
3
) $ (ppm) 8.01 (d, 2H), 7.78 (d, 1H), 7.58 (t, 1H), 7.51 (d, 1H), 7.50 (t, 2H), 7.33 (t,
1H), 7.24 (d, 1H), 7.15 (t, 1H), 6.96 (dd, 1H), 3.85 (s, 3H);
13
C NMR (100 MHz, CDCl
3
) $ (ppm)
190.44, 159.84, 144.67 138.07, 136.15, 132.73, 129.87, 128.49, 122.26, 121.01, 116.21, 113.33, 99.88,
55.25.
III. Results, Discussion, and Conclusions
2-methoxychalcone was synthesized via the Claisen-Schmidt reaction from acetophenone and
ortho-methoxybenzaldehyde starting materials. While Scheme 3 depicts the three possible
methoxychalcone products, the synthesized product and unknown aldehyde starting material were
identified as such using multiple characterization techniques. This experiment is an important example
of a mixed aldol condensation, an important reaction that results in the formation of a carbon-carbon
bond, which lends applications in the organic synthesis of complex, biologically significant molecules
like chalcones.
Scheme 3. Possible methoxychalcone products, with the product of this synthesis boxed.
In the laboratory, the Claisen-Schmidt reaction between acetophenone and ortho-
methoxybenzaldehyde was initiated by a sodium hydroxide base catalyst and run at room temperature.
TLC monitoring suggested that the reaction had reached completion after 30 minutes, as the reaction
lane was found to contain a spot likely indicative of the product (R
f
=0.59), which was close to that of
"#$%& +
the acetophenone standard (R
f
=0.54) due to similar polarities, but far from that of the unknown
aldehyde standard (R
f
=0.42). Since the unknown aldehyde was the limiting reagent in this reaction, this
TLC plate indicated that the reaction had reached completion.
In the workup, the crude product was cooled and crystallized to allow for easier purification.
Next, recrystallization was used as a purification technique to isolate the pure 2-methoxychalcone
product from the crude crystals, as the crude product was a suitable solid, containing relatively small
amounts of impurities (as indicated by TLC). 70:30 ethanol/water proved to be an effective solvent
system, although while heating the product in the solvent, a small amount of oiling out due to product
melting may have occurred, as indicated by oil aggregates. These aggregates were pipetted out of the
super-saturated solution before slow-cooling began, and did not seem to affect the resulting pure
crystals. Thus, the purification by recrystallization was effective, yet not perfect, and may have resulted
in some product loss.
The percent yield of pure 2-methoxychalcone from this synthesis was found to be 52%. This
value is decent, but not ideal, suggesting that some amount of product was lost. Likely, this product loss
is due to the purification by recrystallization. This can be inferred due to the crude percent yield, which
was found to be 86% before recrystallization, affording 1.688 g of crude crystals, compared to the
purified percent yield (52%), affording 1.026 g of purified 2-methoxychalcone. Though the goal of
recrystallization is to purify and remove all substances besides the product, it seems that the discarded
filtrate may have contained product in addition to impurities, as TLC did not indicate the presence of
aldehyde starting material. Additionally, it is much less likely that the product loss is due to the nature
of the Claisen-Schmidt reaction. Although this synthesis represented a reversible mixed aldol
condensation, the condensation product is heavily favored, especially when water is not present.
4
Since
all glassware was dried before use, and the TLC monitoring of the reaction mixture at 30 minutes
indicated the absence of limiting reagent (the ortho-methoxybenzaldehyde), it can be assumed that the
reaction itself was not significantly responsible for product loss in this synthesis.
3
"#$%& ,
Despite the below-ideal yield, the purified 2-methoxychalcone was characterized by melting
point, IR spectroscopy, 400 MHz
1
H NMR, and 100 MHz
13
C NMR, all of which led to the
identification of the product. The purified product had a melting point range of 51-58 C. The literature
values for the melting points of 2-, 3-, and 4-methoxychalcone are reported to be 53-57 C, 58-60 C,
and 73-76 C, respectively.
3
Therefore, melting point data was not sufficient to identify the product
alone, as the broad range reported could be indicative of all of the three possible products if impurities
were present. This is because contamination typically leads to melting point depression as the
crystalline lattice of the given solid is disrupted, requiring a lower temperature to melt the substance.
3
The purified crystals from this synthesis were then characterized by IR spectroscopy, which
provided strong evidence of a methoxychalcone, but not enough information to identify the specific
product or entirely rule out the presence of starting materials (Supplemental Information, Figure 1).
Beginning with the carbon-hydrogen stretch, the peak occurring at 2962 cm
-1
and those close to this
peak are indicative of the aromatic carbon-hydrogen bonds and the carbon-vinylic hydrogen bond of an
alkene functionality. Importantly, the peak at 1657 cm
-1
is likely indicative of a carbon-oxygen double
bond that would occur in a carbonyl functionality such as a conjugated ketone in 2-methoxychalcone. In
the carbon-hydrogen bending region of the IR spectrum, the peaks at 1594 cm
-1
and 1575 cm
-1
are likely
indicative of the carbon-carbon double bonds in alkene functionalities, such as those that would occur in
an aromatic ring or in other alkenes. Lastly, the peak at 1251 cm
-1
likely corresponds to the carbon-
oxygen single bond due to an aryl ether functionality, such as might occur due to the methoxy group in
2-methoxychalcone. The presence of these functional groups provides evidence that a methoxychalcone
could have been synthesized, due to the presence of peaks indicative of carbon-hydrogen
aromatic/alkene bonding, carbon-oxygen carbonyl bonding, carbon-carbon alkene bonding, and an
carbon-oxygen bonding due to an ether function.
3
However, all of these peaks would also be present in
the starting materials acetophenone and a methoxybenzaldehyde, so the IR spectroscopy data does not
absolutely suggest specific product formation.
"#$%& -
The 400 MHz
1
H NMR spectrum (Supplemental Information, Figures 2a-c) for the synthesized
crystals is very important for the identification of the purified product as 2-methoxychalcone, as
opposed to 3- or 4-methoxychalcone. This data also refutes the notion that starting material was present
in the purified product. Peaks corresponding to 10 unique hydrogens were integrated. Importantly,
many of the hydrogen peaks are shifted downfield, out of the expected range due to the significant
conjugation in the methoxychalcone molecules.
Beginning with the most downfield peaks, the doublet at 8.01 ppm with an integration value of 2
is suspected to designate the two hydrogens in the aromatic ring that are in the ortho position to the
carbon that is bonded to the ketone functionality. The doublet at 7.78 ppm with an integration value of 1
is likely due to the vinylic hydrogen around the enone alkene of the methoxychalcone that is closer to
the carbon-oxygen carbonyl double bond. This is shifted further downfield by the oxygen than the
peaks due to the other vinylic hydrogen, which likely appear at 7.51 ppm as a doublet with an
integration value of 1, and are clearly coupled with the former vinylic hydrogen peaks. The latter
vinylic hydrogen doublet peaks overlap with a triplet (7.50 ppm, integration value of 3), that likely
represent the two aromatic hydrogens that are in the meta position to the carbon that is bonded to the
ketone functionality.
The remaining peaks in the aromatic region (7.58 ppm (t, 1H), 7.33 ppm (t, 1H), 7.24 ppm (d,
1H), 7.15 ppm (t, 1H), 6.96 ppm (dd, 1H)) are difficult to identify due to complex splitting, and are not
significant for product identification. Lastly, the singlet occurring at 3.85 ppm with an integration value
of 3 is likely indicative of the methyl group from the methoxy functionality in the methoxychalcone
product. While all of the discussed peaks would be evident in NMR spectra for each of the possible
methoxychalcone products, 4-methoxychalcone can be ruled out based on this data because 10 unique
hydrogens were integrated. In 2- or 3-methoxychalcone, there are 10 unique proton types, yet in 4-
methoxychalcone, there are only 8 unique protons due to additional symmetry in the second aromatic
ring. Importantly, 3-methoxychalcone can also be ruled out based on splitting patterns. In 3-
"#$%& .
methoxychalcone, there would likely be a singlet with an integration value of 1 somewhere in the
1
H
NMR spectrum, due to the aromatic hydrogen that is in the ortho position to the carbon that is bonded to
the methoxy functionality and the carbon that is bonded to the enone functionality. Additionally, there
is no evidence of starting material in this spectrum, as there is no singlet in the 9.0-10.0 ppm range that
would be indicative of an aldehyde proton (from a methoxybenzaldehyde) nor a singlet in the 2.1-2.4
ppm range that would be indicative of a methyl ketone (from acetophenone). Thus, the
1
H NMR
strongly suggests that 2-methoxychalcone was synthesized without the presence of starting materials
acetophenone or ortho-methoxybenzaldehyde.
3
The 100 MHz
13
C NMR spectrum (Supplemental Information, Figure 3) for the synthesized
product also provides confirmation that 2-methoxychalcone was synthesized, though it cannot rule out
3-methoxychalcone as a product without the 400 MHz
1
H NMR data. There are 14 peaks in the
spectrum, while there are 16 carbons in each methoxychalcone. The number of peaks is consistent with
the structure of both 2-methoxychalcone and 3-methoxychalcone, which both have equivalent carbons
ortho and meta to the carbonyl group. However, the number of peaks is not consistent with the structure
of 4-methoxychalcone, eliminating it as an option because it has symmetry on both aromatic rings,
lending only 12 unique carbons. Beginning with the high chemical shift end of the spectrum, the peak at
190.44 ppm is likely indicative of the carbonyl carbon. The peak at 159.84 ppm is likely due to the
aromatic carbon that is bonded to the methoxy oxygen in a methoxychalcone, as this proximity to a
heteroatom would shift the peak downfield. The remaining 11 peaks in the aromatic region are not
easily identifiable, yet are not significant to product identification in this case. Lastly, the peak at 55.25
ppm likely corresponds to the methyl carbon in the methoxy group of methoxychalcones. Therefore,
13
C NMR is useful for collectively suggesting the presence of 2-methoxychalcone with other forms of
data.
3
In conclusion, this experiment was successful in achieving the purpose. The biologically
significant molecule 2-methoxychalcone was synthesized and identified from three product possibilities
"#$%& /0
via the Claisen-Schmidt reaction with acetophenone and ortho-methoxybenzaldehyde starting materials.
3
The melting point, IR, 400 MHz
1
H NMR, and 100 MHz
13
C NMR data collectively provide strong
evidence that 2-methoxychalcone was the synthesized product, without evidence of impurities and a
decent percent yield of 52%.
Evidently, there were several sources of error in this laboratory that may have led to the less than
ideal percent yield, although the results were respectable. Since product loss due to the nature of the
Claisen-Schmidt reaction itself is not likely, and the reaction went to completion according to TLC, it
seems that product was indeed lost during the purification step (recrystallization). This may have
occurred due to the slight oiling out that was evident, indicating that some of the product in solution may
have melted if the solvent temperature was too high. Further, slow cooling may not have been
conducted slowly enough or at a cool enough temperature. To solve this issue, a more precise
recrystallization technique could have been employed, with extended cooling time or reduced
temperature (refrigeration). Additionally, a second crop of crystals could have been recrystallized using
the primary filtrate from the first crop, although this crop would be more likely to include impurities.
Moreover, as opposed to using recrystallization as a purification method, column chromatography could
have been used, and may have avoided the complications associated with recrystallization.
3
These
improvements would likely augment the purified percent yield of 2-methoxychalcone in the experiment.
Despite these drawbacks, this laboratory was successful in both exemplifying the increasingly
pertinent Claisen-Schmidt synthesis of a chalcone, and in synthesizing an unknown product for
identification. Formation of pure 2-methoxychalcone with an acceptable percent yield was confirmed
by various characterization techniques. As the broad applications of pharmaceutically active chalcones
are further appreciated and explored, syntheses such as this one will become increasingly relevant. In
future, it is likely that researchers will strive to produce less toxic, more efficient chalconoids.
1,2
"#$%& //
IV. References
(1) Batovska, D. I.; Todorova, I. T. J. Current Clinical Pharmacology. 2013, 5, 1-29.
(2) Rahman, M.A. Chem. Sci. J. 2011, 29, 1-16.
(3) Wink, D.; Angelo, N. G.; Henchey, L. K.; Waxman, A. J.; Canary, J. W.; Arora, P. S. J. Chem.
Educ. 2007, 84, 1816-1818.
(4) Hathaway, B.A. J. Chem. Educ. 1987, 64, 367-368.
Potrebbero piacerti anche
- Transition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesDa EverandTransition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesNessuna valutazione finora
- Sustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeDa EverandSustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeNessuna valutazione finora
- FFR 4 PDFDocumento7 pagineFFR 4 PDFCamille Loscalzo100% (1)
- Pre LabghvyicDocumento6 paginePre LabghvyicGobe JamNessuna valutazione finora
- Chem 305 Lab OneDocumento6 pagineChem 305 Lab OneGobe JamNessuna valutazione finora
- Effect of Poly (Acrylic Acid) End-Group Functionality On Inhibition of Calcium Oxalate Crystal GrowthDocumento7 pagineEffect of Poly (Acrylic Acid) End-Group Functionality On Inhibition of Calcium Oxalate Crystal GrowthPencils SharpenerNessuna valutazione finora
- Chalcone: A Versatile Molecule: IntroductionDocumento12 pagineChalcone: A Versatile Molecule: IntroductionKavita TyagiNessuna valutazione finora
- Synthesis of 2-Acetylcyclohexanone Using Pyrrolidine-EnamineDocumento3 pagineSynthesis of 2-Acetylcyclohexanone Using Pyrrolidine-Enaminerobet12Nessuna valutazione finora
- DehydrationDocumento19 pagineDehydrationapi-338215029Nessuna valutazione finora
- Synthesis of Cyclohexanol To Cyclohexene - Lab ReportDocumento5 pagineSynthesis of Cyclohexanol To Cyclohexene - Lab ReportparisdelapenaNessuna valutazione finora
- CHEM F110 - Lab Manual - Nov 5-2020Documento45 pagineCHEM F110 - Lab Manual - Nov 5-2020STUTI MATHUR100% (2)
- Aldol Condensation Reaction PDFDocumento6 pagineAldol Condensation Reaction PDFaizatNessuna valutazione finora
- Laboratory ReportDocumento11 pagineLaboratory ReportElsayed Refaat Aly MareyNessuna valutazione finora
- OrgochemsampleworkDocumento10 pagineOrgochemsampleworkMakcaNessuna valutazione finora
- Experiment #1Documento7 pagineExperiment #1Lakani Tindiwi YangalaNessuna valutazione finora
- Jurnal EugenolDocumento9 pagineJurnal EugenolMillenia AgustinaNessuna valutazione finora
- Appendix Attempts Towards Synthesis of IbuprofenDocumento12 pagineAppendix Attempts Towards Synthesis of IbuprofenJayNessuna valutazione finora
- Katch UmbelliferoneffrDocumento9 pagineKatch Umbelliferoneffrapi-456902531Nessuna valutazione finora
- CyclohexeneDocumento11 pagineCyclohexeneanon-407590100% (10)
- (R) - 3-Hydroxybutanoic Acid Methyl Ester Butanoic Acid, 3-Hydroxy-, (R) - Butanoic Acid, 3-Hydroxy-, Methyl Ester, (R)Documento6 pagine(R) - 3-Hydroxybutanoic Acid Methyl Ester Butanoic Acid, 3-Hydroxy-, (R) - Butanoic Acid, 3-Hydroxy-, Methyl Ester, (R)ArifSheriffNessuna valutazione finora
- Synthesis Report 41 Part 1Documento8 pagineSynthesis Report 41 Part 1tubagussinggihNessuna valutazione finora
- Synthesis of Acetophenone DerivativesDocumento6 pagineSynthesis of Acetophenone DerivativesAwad SaidNessuna valutazione finora
- Inorganic Chemistry Volume 50 Issue 20 2011Documento12 pagineInorganic Chemistry Volume 50 Issue 20 2011Lee ToulouseNessuna valutazione finora
- Expt6 Synthesis of An Alkyl Halide DraftDocumento6 pagineExpt6 Synthesis of An Alkyl Halide DraftAnna Sophia EbuenNessuna valutazione finora
- Wacker Oxidation: A. General Description of The ReactionDocumento6 pagineWacker Oxidation: A. General Description of The Reactionnabila OktavianiNessuna valutazione finora
- Chm557 Laboratory Report: Experiment 4 The Aldol Condensation Reaction: Preparation of DibenzalacetoneDocumento17 pagineChm557 Laboratory Report: Experiment 4 The Aldol Condensation Reaction: Preparation of DibenzalacetonesyafNessuna valutazione finora
- Alquilacion de Arenos Con AlcoholesDocumento5 pagineAlquilacion de Arenos Con AlcoholesJosé Guadalupe García EstradaNessuna valutazione finora
- Articulo 10.-Boric Acid As A Green Catalyst For The Conversion of Aldehydes and KetonesDocumento8 pagineArticulo 10.-Boric Acid As A Green Catalyst For The Conversion of Aldehydes and KetonesPaul Delgado MendozaNessuna valutazione finora
- Preparation of Cyclohexene From CyclohexanolDocumento7 paginePreparation of Cyclohexene From CyclohexanolDumile Nombasa100% (5)
- Experimen 5 Organic ChemistryDocumento8 pagineExperimen 5 Organic ChemistryAbd RaHmanNessuna valutazione finora
- Analysis of Elimination Reaction of CyclohexanolDocumento4 pagineAnalysis of Elimination Reaction of CyclohexanolPratiwi Surya RahayuNessuna valutazione finora
- Working With Hazardous Chemicals: A Publication of Reliable Methods For The Preparation of Organic CompoundsDocumento6 pagineWorking With Hazardous Chemicals: A Publication of Reliable Methods For The Preparation of Organic Compoundsrajesh kothariNessuna valutazione finora
- Aldol Condensation 1Documento8 pagineAldol Condensation 1Bilal100% (2)
- (2S) - ( ) - 3-Exo - (DIMETHYLAMINO) ISOBORNEOL ( (2S) - ( ) - DAIB)Documento6 pagine(2S) - ( ) - 3-Exo - (DIMETHYLAMINO) ISOBORNEOL ( (2S) - ( ) - DAIB)Alex CumbaNessuna valutazione finora
- Catalysis Communications: Suresh D. Salim, Krishnacharya G. AkamanchiDocumento4 pagineCatalysis Communications: Suresh D. Salim, Krishnacharya G. AkamanchinileshsalunkheNessuna valutazione finora
- Cyclohexanol DehydrationDocumento4 pagineCyclohexanol DehydrationVersiformNessuna valutazione finora
- Oxidation of Cyclohexane and Ethylbenzene by Hydrogen Peroxide Over Co-Substituted Heteropolytungstate CatalystDocumento6 pagineOxidation of Cyclohexane and Ethylbenzene by Hydrogen Peroxide Over Co-Substituted Heteropolytungstate Catalystrungrawin ngamkhumNessuna valutazione finora
- Preparation of A-Perfluoroalkyl Ketones From Ab-UnDocumento5 paginePreparation of A-Perfluoroalkyl Ketones From Ab-UnnkazemiNessuna valutazione finora
- New Montmorillonite Silylpropylethylenediamine Palladium (II) Complex in Oxidation of Terminal OlefinsDocumento6 pagineNew Montmorillonite Silylpropylethylenediamine Palladium (II) Complex in Oxidation of Terminal OlefinsChamula K MasNessuna valutazione finora
- A Simple, Green and One-Pot Four-Component Synthesis of 1,4-Dihydropyridines and Their AromatizationDocumento6 pagineA Simple, Green and One-Pot Four-Component Synthesis of 1,4-Dihydropyridines and Their AromatizationEdgar HernándezNessuna valutazione finora
- Methyl SalicylateDocumento10 pagineMethyl Salicylatekab56067% (3)
- A Practical Synthesis of (-) - Kainic AcidDocumento11 pagineA Practical Synthesis of (-) - Kainic AcidNikhil SarpateNessuna valutazione finora
- Studies On ClaisenDocumento14 pagineStudies On ClaisenU4x DanteNessuna valutazione finora
- 2 Functional Group Conversion - 2004 - Advanced Free Radical Reactions For Organic SynthesisDocumento18 pagine2 Functional Group Conversion - 2004 - Advanced Free Radical Reactions For Organic SynthesisShuai LiuNessuna valutazione finora
- Formal Report For Synthesis of An Alkyl HalideDocumento5 pagineFormal Report For Synthesis of An Alkyl HalideLovelyn Marie Morada Nievales80% (5)
- Preparation of Butyl Acetate PDFDocumento6 paginePreparation of Butyl Acetate PDFjoiya100133% (3)
- Synthesis and Characterization of DibenzalacetoneDocumento7 pagineSynthesis and Characterization of DibenzalacetoneTan Yong Jie100% (7)
- Esterification Process To Synthesize Isopropyl Chloroacetate Catalyzed by Lanthanum Dodecyl SulfateDocumento6 pagineEsterification Process To Synthesize Isopropyl Chloroacetate Catalyzed by Lanthanum Dodecyl SulfateVinay JainNessuna valutazione finora
- Radical Coupling ReactionDocumento7 pagineRadical Coupling ReactionGobe JamNessuna valutazione finora
- Acetaldehyde 16.05.2020Documento3 pagineAcetaldehyde 16.05.2020sathiya sathiyaNessuna valutazione finora
- Aldol Condensation LabDocumento5 pagineAldol Condensation Labnmc515288% (8)
- Acetaldehyde Report - Final PDFDocumento20 pagineAcetaldehyde Report - Final PDFDinesh guhanNessuna valutazione finora
- Α-Allenic Esters From Α-Phosphoranylidene Esters And Acid ChloridesDocumento5 pagineΑ-Allenic Esters From Α-Phosphoranylidene Esters And Acid ChloridesJarrett RobinsonNessuna valutazione finora
- Exp 3-Reduction of Cyclohexanone With Sodium BorohydrideDocumento11 pagineExp 3-Reduction of Cyclohexanone With Sodium Borohydrideakuserai100% (3)
- Octyl AcetateDocumento6 pagineOctyl AcetateKristine BautistaNessuna valutazione finora
- Synthesis of Tert-Butyl Chloride Through Hydrochlorination of Tert-Butyl Alcohol and Purification Using DistillationDocumento9 pagineSynthesis of Tert-Butyl Chloride Through Hydrochlorination of Tert-Butyl Alcohol and Purification Using DistillationAnonymous GO6JVW9Wud100% (2)
- Chem 31.1 FR StuffDocumento4 pagineChem 31.1 FR StuffKazaTsuki100% (1)
- Etherification ReportDocumento7 pagineEtherification ReportEwout KesselsNessuna valutazione finora
- Benzylidene AcetalDocumento9 pagineBenzylidene AcetalsadiaNessuna valutazione finora
- Advanced Pharmaceutical analysisDa EverandAdvanced Pharmaceutical analysisValutazione: 4.5 su 5 stelle4.5/5 (2)
- 123.312 Advanced Organic Chemistry: Retrosynthesis: TutorialDocumento10 pagine123.312 Advanced Organic Chemistry: Retrosynthesis: TutorialĐàoTrungHiếuNessuna valutazione finora
- Lab Activity 5Documento5 pagineLab Activity 5Jasmin CeciliaNessuna valutazione finora
- Structural IsomerismDocumento17 pagineStructural IsomerismMotivational BabaNessuna valutazione finora
- Bacterial Phenylalanine and Phenylacetate Catabolic Pathway RevealedDocumento6 pagineBacterial Phenylalanine and Phenylacetate Catabolic Pathway RevealedKarla KetiNessuna valutazione finora
- Aldol ConclusionDocumento1 paginaAldol Conclusionapi-235187189100% (1)
- Solutions - AIATS Medical-2019 (XII Studying&Passed) - Mock Test-4 - (Code-A & B) - (28-04-2019) PDFDocumento36 pagineSolutions - AIATS Medical-2019 (XII Studying&Passed) - Mock Test-4 - (Code-A & B) - (28-04-2019) PDFHarshNessuna valutazione finora
- Carbonyl Compounds 230Documento60 pagineCarbonyl Compounds 230mohtasim hasanNessuna valutazione finora
- Acs Jchemed 5b00170Documento5 pagineAcs Jchemed 5b00170Aitor PastorNessuna valutazione finora
- CHEM 2425. Chapter 22. Carbonyl Alpha-Substitution Reactions (Homework) WDocumento17 pagineCHEM 2425. Chapter 22. Carbonyl Alpha-Substitution Reactions (Homework) WPhương NguyễnNessuna valutazione finora
- (Lecture 3) Carbonyls and AminesDocumento34 pagine(Lecture 3) Carbonyls and AminesKasraSrNessuna valutazione finora
- Green Adeptness in The Synthesis and Stabilization of Copper Nanoparticles: Catalytic, Antibacterial, Cytotoxicity, and Antioxidant ActivitiesDocumento15 pagineGreen Adeptness in The Synthesis and Stabilization of Copper Nanoparticles: Catalytic, Antibacterial, Cytotoxicity, and Antioxidant ActivitiesWindah AnisyahNessuna valutazione finora
- Aldehydes and Ketones FinalDocumento67 pagineAldehydes and Ketones FinalAnil Kumar VermaNessuna valutazione finora
- Keto-Enol Tautomerism - WikipediaDocumento4 pagineKeto-Enol Tautomerism - WikipediafaustoNessuna valutazione finora
- Reaction Mechanism: C C H H H O Hgso H SO CHO H CDocumento6 pagineReaction Mechanism: C C H H H O Hgso H SO CHO H CFATHIMA THANHA T NNessuna valutazione finora
- Organic SynthesisDocumento35 pagineOrganic SynthesisAshok PareekNessuna valutazione finora
- CarboanionDocumento23 pagineCarboanionrajendraNessuna valutazione finora
- Part-1 Stereochemistry of Organic CompoundsDocumento28 paginePart-1 Stereochemistry of Organic CompoundsIct Pfa ClubNessuna valutazione finora
- Chapter 9 Lecture PDFDocumento128 pagineChapter 9 Lecture PDFjoseph changNessuna valutazione finora
- Carbon-Carbon Bond FormationDocumento18 pagineCarbon-Carbon Bond FormationFaTin AziEyatiNessuna valutazione finora
- Vinylic Carbon, Ambient Nucleophilic and Substrate ReactionDocumento11 pagineVinylic Carbon, Ambient Nucleophilic and Substrate Reactiongmanooj_484213209Nessuna valutazione finora
- Enol Dan EnolatDocumento40 pagineEnol Dan EnolatRiyan KateeNessuna valutazione finora
- Organic ChemDocumento116 pagineOrganic ChemNidhi Sisodia100% (1)
- Organic Chemistry ACS Sample QuestionsDocumento20 pagineOrganic Chemistry ACS Sample QuestionsNajmusawwa Aulia RahmahNessuna valutazione finora
- Reaction Mechanism IDocumento15 pagineReaction Mechanism IFilmodeNessuna valutazione finora
- Stereo ChemistryDocumento45 pagineStereo Chemistrysanjanaganguly2005Nessuna valutazione finora
- Heterocyclic ChemistryDocumento57 pagineHeterocyclic ChemistryGuillaume WNessuna valutazione finora
- Selective Hydrogenation of Phenol: Chemnanomat February 2018Documento21 pagineSelective Hydrogenation of Phenol: Chemnanomat February 2018Deo TolloNessuna valutazione finora
- MCAT Organic ChemistryDocumento7 pagineMCAT Organic ChemistryjoNessuna valutazione finora
- Lab 1 Dehydrogenation HydrogenationDocumento3 pagineLab 1 Dehydrogenation HydrogenationJason Chen100% (1)
- DPP 01Documento3 pagineDPP 01Rajeev GangwarNessuna valutazione finora