Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Combustion Calculations
Caricato da
lutfi awn0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
159 visualizzazioni6 pagineengineering
Copyright
© © All Rights Reserved
Formati disponibili
XLS, PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoengineering
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato XLS, PDF, TXT o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
159 visualizzazioni6 pagineCombustion Calculations
Caricato da
lutfi awnengineering
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato XLS, PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 6
COMBUSTION CALCULATIONS
Solid & Liquid Fuels:
A
stoi
= 11.53*C + 34.34*(H
2
- (O
2
/8)) + 4.29*S
where
A
stoi
= Dry stoichiometric air required for combustion, lb/lb of fuel
C = weight fraction of Carbon in Solid/Liquid fuel
H
2
= weight fraction of Hydrogen in Solid/Liquid fuel
O
2
= weight fraction of Oxygen in Solid/Liquid fuel
S = weight fraction of sulfur in Solid/Liquid Fuel
Gaseous Fuels:
A
stoi
= 2.47*CO + 34.34*H
2
+ 17.265*CH
4
+ 13.3*C
2
H
2
+ 14.81*C
2
H
4
+ 16.12*C
2
H
6
+ 15.69*C
3
H
8
+ 15.44*C
4
H
10
- 4.32*O
2
where
A
stoi
= Dry stoichiometric air required for combustion, lb/lb of fuel
CO = weight fraction of Carbon Monoxide Gaseous fuel
H
2
= weight fraction of Hydrogen in Gaseous fuel
CH
4
= weight fraction of Methane in Gaseous Fuel
C
2
H
2
= weight fraction of acetylene in Gaseous Fuel
C
2
H
4
= weight fraction of ethylene in Gaseous Fuel
C
2
H
6
= weight fraction of ethane in Gaseous Fuel
C
3
H
8
= weight fraction of propane in Gaseous Fuel
O
2
= weight fraction of Oxygen in Gaseous fuel
HHV's (Higher Heating Value) for API fuel oils:
HHV = 17887 + 57.5*API - 102.2*S
where
API = Degree API of Fuel Oil (measure of specific gravity)
S = weight percent of sulfur in fuel oil
MM Btu method of combustion:
Examples for calculation of thoretical/stoichiometric Air:
Example 1:
A Fuel oil has the following characteristics:
Degree API 28 API
weight percent C = 87.5 %
weight percent H = 12.5 %
weight percent S = 0 %
weight percent O = 0 %
Calculate the amount of theoretical air required per million Btu fired for above fuel oil
This method for combustion is based on the concept that the weight of air required in the combustion of a unit
weight of any commercial fuel is more nearly proportional to the unit heat value than to the unit weight of the fuel.
Hence the quantity of air required is expressed in lb per million btu (lb/MMBtu) fired.
Page 1 of 6
COMBUSTION CALCULATIONS
Calculations:
HHV = 19497 Btu/lb
A
stoi
= 14.38 lb/lb fuel
1 MM Btu fired requires = 51.29 lb fuel
Hence air required per 1 MM Btu fuel = 737.6 lb
Example 2:
Natural gas is used as fired fuel for boiler. It has the following volumetric analysis:
CH
4
= 83.4 %
C
2
H
6
= 15.8 %
N
2
= 0.8 %
Calculate the amount of theoretical air required per million Btu fired for natural gas
Calculations:
Component volume %
Molecular wt
weight % wt fraction Combustion
Constants
or HHV,
Btu/lb
CH
4
83.4 16 72.89 0.7289 23876 yellow colored cell are inputs
C
2
H
6
15.8 30 25.89 0.2589 22320
N
2
0.8 28 1.22 0.0122 -
A
stoi
= 16.76 lb/lb fuel
HHV = 23181.0 Btu/lb
1 MM Btu fired requires = 43.14 lb fuel
Hence air required per 1 MM Btu fuel = 722.9 lb
Total Atmospheric Air required for Combustion in fired Heaters:
A
tot
= A
stoi
*(1000000/HHV of fuel)*(1+ H)*(1 + A
exc
)
where
A
tot
= total atmospheric air, lb/MM Btu
A
stoi
= Dry stoichiometric or theoretical air required, lb/lb fuel
HHV = Higher Heating Value, Btu/lb
H= absolute humidity of atmospheric air expressed as a fraction - lb of water vapor per 1 lb of dry air
A
exc
= excess air expressed as a fraction (10% excess air will be entered as 0.1, 15% = 0.15 etc.)
(to be calculated from % Relative Humidity (RH) & Dry Bulb Temperature using psychrometric chart,
example: at 105F & 100% RH, H = 0.0507 from Humid Air chart)
Page 2 of 6
COMBUSTION CALCULATIONS
Common Combustion Reactions & the Heats of Combustion:
Heating Value (Btu/lb)
HHV LHV
H
2
+ 1/2O
2
= H
2
O 61100 51600
C + O
2
= CO
2
14093 14093
C + 1/2O
2
= CO 4440 4440
CO + 1/2O
2
= CO
2
4345 4345
S + O
2
= SO
2
10160 10160
CH
4
+ 2O
2
= CO
2
+ 2H
2
O 23885 21500
C
2
H
6
+ 3.5O
2
= 2CO
2
+ 3H
2
O 22263 20370
C
3
H
8
+ 5O
2
= 3CO
2
+ 4H
2
O 21646 19929
C
4
H
10
+ 6.5O
2
= 4CO
2
+ 5H
2
O 21293 19665
Note that where no water is formed during combustion reaction the HHV & LHV are the same.
Heating Value (LHV(net) & HHV(gross)) @ 60F, 14.696 psia (Source: Fig 23-2, GPSA Engg. Data Book)
Btu/ft
3
,
ideal gas,
14.696
psia
Btu/lb
m
Liquid
Btu/ft
3
,
ideal gas,
14.696
psia
Btu/lb
m
Liquid
Btu/gal
Liquid
Methane 909.4 - 1010 - -
Ethane 1618.7 20277 1769.6 22181 65869
Propane 2314.9 19757 2516.1 21489 90830
Isobutane 3000.4 19437 3251.9 21079 98917
n-Butane 3010.8 19494 3262.3 21136 102911
Isopentane 3699 19303 4000.9 20891 108805
n-Pentane 3706.9 19335 4008.9 20923 110091
Neopentane 3682.9 19235 3984.7 20822 103577
n-Hexane 4403.8 19232 4755.9 20783 115021
Hydrogen 273.8 - 324.2 - -
Carbon
Monoxide
320.5 - 320.5 - -
Prepared by: Ankur Srivastava
Chemical Engineer
e-mail: ankur2061@yahoo.co.in
Disclaimer : The information and methods included within this spreadsheet are presented for combustion air
calculations. It is intended to be used by technically skilled persons at their own discretion. I do not warrant the
suitability or accuracy of these methods.
Heating Value, 60F
Net Gross
Compound
Page 3 of 6
COMBUSTION CALCULATIONS
2.47*CO + 34.34*H
2
+ 17.265*CH
4
+ 13.3*C
2
H
2
+ 14.81*C
2
H
4
+ 16.12*C
2
H
6
+ 15.69*C
3
H
8
+ 15.44*C
4
H
10
- 4.32*O
2
Page 4 of 6
COMBUSTION CALCULATIONS
Page 5 of 6
COMBUSTION CALCULATIONS
Disclaimer : The information and methods included within this spreadsheet are presented for combustion air
calculations. It is intended to be used by technically skilled persons at their own discretion. I do not warrant the
suitability or accuracy of these methods.
Page 6 of 6
Potrebbero piacerti anche
- Steam and Gas Tables with Computer EquationsDa EverandSteam and Gas Tables with Computer EquationsValutazione: 3 su 5 stelle3/5 (2)
- COMBUSTION2222222222222222222Documento47 pagineCOMBUSTION2222222222222222222Habtamu Tkubet EbuyNessuna valutazione finora
- Combustion. Adiabatic Flame Temperature - Jeff MunicDocumento56 pagineCombustion. Adiabatic Flame Temperature - Jeff MunicChristopher LloydNessuna valutazione finora
- The Boudouard Reaction: C + CO2 2 CO: Thermodynamic Calculations Kj/mole T (C) T (K) LN (KR) KR KR KR Xco2 Xco GRDocumento6 pagineThe Boudouard Reaction: C + CO2 2 CO: Thermodynamic Calculations Kj/mole T (C) T (K) LN (KR) KR KR KR Xco2 Xco GRmksayshiNessuna valutazione finora
- Heavy Oil Upgrading by The Separation and Gasification of AsphaltenesDocumento11 pagineHeavy Oil Upgrading by The Separation and Gasification of Asphalteneslutfi awnNessuna valutazione finora
- QTM - Soap Battle CaseDocumento7 pagineQTM - Soap Battle CaseAshish Babaria100% (1)
- C3CYCLVDocumento13 pagineC3CYCLVUok RitchieNessuna valutazione finora
- Heat CalcDocumento13 pagineHeat Calciese027Nessuna valutazione finora
- Heatcalc: A Natural Gas Heat of Combustion CalculatorDocumento7 pagineHeatcalc: A Natural Gas Heat of Combustion CalculatorMuzzamilNessuna valutazione finora
- Heat Load Calculation-Rect-Tank-2019Documento8 pagineHeat Load Calculation-Rect-Tank-2019Hasmukh DaveNessuna valutazione finora
- Input Output: Combustion Calculations For Fuel GasDocumento33 pagineInput Output: Combustion Calculations For Fuel GasgsdaundhNessuna valutazione finora
- Combustion CalculationDocumento16 pagineCombustion Calculationmohamed Elsayed0% (1)
- Boiler Efficiency CalculationsDocumento8 pagineBoiler Efficiency CalculationsmanishxlriNessuna valutazione finora
- 0 Boiler Design SoftwearDocumento54 pagine0 Boiler Design SoftwearNITINNessuna valutazione finora
- Basic Design of A Heat ExchangerDocumento10 pagineBasic Design of A Heat ExchangerKvspavan KumarNessuna valutazione finora
- Mixing RuleDocumento2 pagineMixing Rulevictor javier nuñezNessuna valutazione finora
- Project No. 16279S Project Name Bapco - JVTST: Calculation of Emissivity of Process GasDocumento11 pagineProject No. 16279S Project Name Bapco - JVTST: Calculation of Emissivity of Process GasrajachemNessuna valutazione finora
- Combustion Table Industrial Energy ProcessDocumento4 pagineCombustion Table Industrial Energy Processsara_ghaemNessuna valutazione finora
- Distillation Column ReboilerDocumento13 pagineDistillation Column ReboilerLouie GresulaNessuna valutazione finora
- Boiler CalculatorDocumento4 pagineBoiler CalculatorMarc EdwardsNessuna valutazione finora
- Cooling Tower Calculations: I N P U TDocumento2 pagineCooling Tower Calculations: I N P U ThuangjlNessuna valutazione finora
- Standard Practice For Calculating Heat Value, Compressibility Factor and Relative Density of Gaseous FuelsDocumento10 pagineStandard Practice For Calculating Heat Value, Compressibility Factor and Relative Density of Gaseous FuelsChikkam Sathi Raju100% (1)
- Gas Properties, Flowrate and Conditions: Reciprocating Compressor Calculation SheetDocumento5 pagineGas Properties, Flowrate and Conditions: Reciprocating Compressor Calculation SheetNaqqash Sajid0% (2)
- A Guide To Assist in Evaluating Liquid Fuel FlamesDocumento16 pagineA Guide To Assist in Evaluating Liquid Fuel FlamestinuvalsapaulNessuna valutazione finora
- 14 ACC Air Side Performance PDFDocumento24 pagine14 ACC Air Side Performance PDFDSGNessuna valutazione finora
- Adiabatic Flame Temperature CalculationDocumento8 pagineAdiabatic Flame Temperature CalculationLuis VargasNessuna valutazione finora
- Firetube Heaters: FIG. 8-31 Convection HeaterDocumento7 pagineFiretube Heaters: FIG. 8-31 Convection Heatery149487Nessuna valutazione finora
- Heat DutyDocumento7 pagineHeat Dutyingegnere1234Nessuna valutazione finora
- Dearator Calculations Book1 Rev1Documento8 pagineDearator Calculations Book1 Rev1MechanicalVee18Nessuna valutazione finora
- 1.5 NPS RecuperatorDocumento7 pagine1.5 NPS RecuperatorAnonymous pVoSWn8yh0Nessuna valutazione finora
- Fuel Characteristic BiomassDocumento365 pagineFuel Characteristic BiomassCalvin JunNessuna valutazione finora
- Boiler Efficiency by Indirect Method Coal Fired BoilerDocumento4 pagineBoiler Efficiency by Indirect Method Coal Fired BoilerM Ziaul ArifNessuna valutazione finora
- D.A.Steam Load CalculatorDocumento2 pagineD.A.Steam Load CalculatorHarwi PatiNessuna valutazione finora
- Combustion. Volume Composition InputDocumento312 pagineCombustion. Volume Composition InputNITIN P SHAHNessuna valutazione finora
- Air Pollution Control Technologies: VOC IncineratorsDocumento51 pagineAir Pollution Control Technologies: VOC IncineratorsRay CNessuna valutazione finora
- Combustion H2 O2 SensitDocumento12 pagineCombustion H2 O2 SensitClarence AG YueNessuna valutazione finora
- Boiler CalculationDocumento4 pagineBoiler Calculationmohsin husen BargirNessuna valutazione finora
- Waste Heat Recovery: AHEF.120.MD. Energy Efficiency Audit Guide For CHP and HobDocumento30 pagineWaste Heat Recovery: AHEF.120.MD. Energy Efficiency Audit Guide For CHP and HobMoriyasuNguyen100% (1)
- Coal CombustionDocumento3 pagineCoal CombustionRahul ChandrawarNessuna valutazione finora
- Steam TablesDocumento16 pagineSteam TablesDanu MamlukatNessuna valutazione finora
- Che 2Documento25 pagineChe 2Jaynie Lee VillaranNessuna valutazione finora
- Heat Balance Computation and TFT DeterminationDocumento8 pagineHeat Balance Computation and TFT DeterminationJose Renz EspaltoNessuna valutazione finora
- Boiler CalcDocumento5 pagineBoiler CalcBhavana KewlaniNessuna valutazione finora
- Mae 5310: Combustion Fundamentals: Laminar Premixed Flames Example, Applications and CommentsDocumento22 pagineMae 5310: Combustion Fundamentals: Laminar Premixed Flames Example, Applications and CommentsAlex KeaneNessuna valutazione finora
- Modeling of Fire Tube BoilerDocumento16 pagineModeling of Fire Tube BoilerVignesh AlagesanNessuna valutazione finora
- Efficiency-Boiler & TGDocumento21 pagineEfficiency-Boiler & TGAnand SwamiNessuna valutazione finora
- Chemical KineticsDocumento48 pagineChemical Kineticsvikash kumarNessuna valutazione finora
- Ammonia Production PDFDocumento5 pagineAmmonia Production PDFJustine LagonoyNessuna valutazione finora
- Combustion ChemistryDocumento17 pagineCombustion Chemistryrajeev50588Nessuna valutazione finora
- Double FdexchangerDocumento8 pagineDouble FdexchangerBoško IvanovićNessuna valutazione finora
- Thermodynamic Computational Tools For Python: Christopher MartinDocumento55 pagineThermodynamic Computational Tools For Python: Christopher MartinBilal AbdullahNessuna valutazione finora
- Boiler Efficiency StudyDocumento3 pagineBoiler Efficiency Studyvinayg85Nessuna valutazione finora
- Furnace Heater DesignDocumento6 pagineFurnace Heater DesignINDRAJIT SAONessuna valutazione finora
- Desuperheater Boiler Feed Water RequirementDocumento2 pagineDesuperheater Boiler Feed Water Requirementmehul10941Nessuna valutazione finora
- Natural Gas Boiler CalculationsDocumento20 pagineNatural Gas Boiler Calculations@sss100% (1)
- Combustion CalcsDocumento8 pagineCombustion Calcs31331311313Nessuna valutazione finora
- FUELSDocumento15 pagineFUELSJohn Archie MendozaNessuna valutazione finora
- h-101 TwiceDocumento20 pagineh-101 TwiceAdela ShofiaNessuna valutazione finora
- Thermal Design - 2 Effect Forward-Feed Evaporator Nama Aristya Kurniawan NIM 114 152 5005 Design BasisDocumento4 pagineThermal Design - 2 Effect Forward-Feed Evaporator Nama Aristya Kurniawan NIM 114 152 5005 Design BasisAristya KurniawanNessuna valutazione finora
- Design 2 ReboilerDocumento5 pagineDesign 2 ReboilerAbdulrazzaqAL-MalikyNessuna valutazione finora
- High Pressure Phase Behaviour of Multicomponent Fluid MixturesDa EverandHigh Pressure Phase Behaviour of Multicomponent Fluid MixturesNessuna valutazione finora
- Practical Chemical Thermodynamics for GeoscientistsDa EverandPractical Chemical Thermodynamics for GeoscientistsNessuna valutazione finora
- Air Compressor Curve ToolDocumento14 pagineAir Compressor Curve Toollutfi awnNessuna valutazione finora
- Bitumen Upgrading Shell Canada LimitedDocumento301 pagineBitumen Upgrading Shell Canada Limitedlutfi awnNessuna valutazione finora
- Liquid Pressure DropDocumento8 pagineLiquid Pressure Droplutfi awnNessuna valutazione finora
- Liquid and Gas Velocity Gas Compressibility and Renolds Number.Documento30 pagineLiquid and Gas Velocity Gas Compressibility and Renolds Number.lutfi awnNessuna valutazione finora
- E10 Blending Study Final ReportDocumento107 pagineE10 Blending Study Final Reportlutfi awnNessuna valutazione finora
- 04 Jet Mixing Design ApplicationsDocumento6 pagine04 Jet Mixing Design Applicationslutfi awnNessuna valutazione finora
- Air Compressor Tools APODocumento7 pagineAir Compressor Tools APOlutfi awnNessuna valutazione finora
- Hydraulic Calculations MKIIDocumento8 pagineHydraulic Calculations MKIIlutfi awnNessuna valutazione finora
- Lubricating Oils: Test Methods Test MethodsDocumento32 pagineLubricating Oils: Test Methods Test MethodsCorina StanculescuNessuna valutazione finora
- Viscosity-Classifications Astm d2422Documento8 pagineViscosity-Classifications Astm d2422Francisco TipanNessuna valutazione finora
- Plumbing H 2 Osu Pair Gap CalculatorDocumento9 paginePlumbing H 2 Osu Pair Gap Calculatorlutfi awnNessuna valutazione finora
- Astm D 86Documento8 pagineAstm D 86lutfi awnNessuna valutazione finora
- Mixer Tank Jet Pumps Gea - tcm11 34889Documento4 pagineMixer Tank Jet Pumps Gea - tcm11 34889Venodaren VelusamyNessuna valutazione finora
- Fonds Bombes - Surface MouilleeDocumento3 pagineFonds Bombes - Surface MouilleesegunoyesNessuna valutazione finora
- Petroleum Average Conversion FactorsDocumento2 paginePetroleum Average Conversion Factorslutfi awnNessuna valutazione finora
- 2011 Chaudhuri U R Fundamentals of Petroleum and PetrochDocumento33 pagine2011 Chaudhuri U R Fundamentals of Petroleum and Petrochlutfi awnNessuna valutazione finora
- Water Demand Calculation 1Documento2 pagineWater Demand Calculation 1lutfi awnNessuna valutazione finora
- Calculation ExampelDocumento21 pagineCalculation Exampellutfi awnNessuna valutazione finora
- H2S RiskDocumento6 pagineH2S Risklutfi awnNessuna valutazione finora
- Valve Sizing Worksheet InstructionsDocumento11 pagineValve Sizing Worksheet Instructionslutfi awnNessuna valutazione finora
- PumpDocumento31 paginePumpkajale_shrikant2325Nessuna valutazione finora
- PCVDocumento2 paginePCVlutfi awnNessuna valutazione finora
- Conversion of Milligrams Per Cubic Meter To PPMDocumento2 pagineConversion of Milligrams Per Cubic Meter To PPMlutfi awnNessuna valutazione finora
- Molecular Sieve Dehydration OptimizationDocumento24 pagineMolecular Sieve Dehydration Optimizationlutfi awn100% (1)
- Gas TurbDocumento10 pagineGas Turblutfi awnNessuna valutazione finora
- Gas ReservesDocumento8 pagineGas Reserveslutfi awnNessuna valutazione finora
- ZM KV Idag eDocumento6 pagineZM KV Idag elutfi awnNessuna valutazione finora
- GOR Vs GVFDocumento8 pagineGOR Vs GVFlutfi awnNessuna valutazione finora
- Centrifugalcompressorpower Si UnitsDocumento4 pagineCentrifugalcompressorpower Si UnitsJoshi DhvanitNessuna valutazione finora
- Color Codes and Irregular MarkingDocumento354 pagineColor Codes and Irregular MarkingOscarGonzalezNessuna valutazione finora
- Streamline SWR (S) - Rev - 00-04-2019 PDFDocumento2 pagineStreamline SWR (S) - Rev - 00-04-2019 PDFarjun 11Nessuna valutazione finora
- Phonetics ReportDocumento53 paginePhonetics ReportR-jhay Mepusa AceNessuna valutazione finora
- Aplikasi Sistem Penuaian Air Hujan (Spah) Di Kawasan PerumahanDocumento18 pagineAplikasi Sistem Penuaian Air Hujan (Spah) Di Kawasan PerumahanFarid Che DeramanNessuna valutazione finora
- Food Poisoning: VocabularyDocumento9 pagineFood Poisoning: VocabularyHANG WEI MENG MoeNessuna valutazione finora
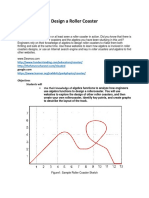
- Design A Roller Coaster ProjectDocumento4 pagineDesign A Roller Coaster Projectapi-3564628400% (1)
- MX 400Documento231 pagineMX 400Percy JimenezNessuna valutazione finora
- Mercedes (DTC) 976990001963 20220615144147Documento3 pagineMercedes (DTC) 976990001963 20220615144147YB MOTOR Nissan - Datsun SpecialistNessuna valutazione finora
- Ge Druck PTX 7535Documento2 pagineGe Druck PTX 7535ICSSNessuna valutazione finora
- Output Process Input: Conceptual FrameworkDocumento4 pagineOutput Process Input: Conceptual FrameworkCHRISTINE DIZON SALVADORNessuna valutazione finora
- Aerodrome Advisory Circular: AD AC 04 of 2017Documento6 pagineAerodrome Advisory Circular: AD AC 04 of 2017confirm@Nessuna valutazione finora
- Mobile Communication Networks: Exercices 4Documento2 pagineMobile Communication Networks: Exercices 4Shirley RodriguesNessuna valutazione finora
- EY Enhanced Oil RecoveryDocumento24 pagineEY Enhanced Oil RecoveryDario Pederiva100% (1)
- Group Collaborative Activity TaskonomyDocumento2 pagineGroup Collaborative Activity TaskonomyTweeky SaureNessuna valutazione finora
- K&J Magnetics - Demagnetization CurvesDocumento4 pagineK&J Magnetics - Demagnetization CurvessubbannachrsNessuna valutazione finora
- Chemistry Mid Term Exam 2014Documento8 pagineChemistry Mid Term Exam 2014Adham TamerNessuna valutazione finora
- Introduction To Reproduction PDFDocumento8 pagineIntroduction To Reproduction PDFLmssvNessuna valutazione finora
- Fluid Solids Operations: High HighDocumento20 pagineFluid Solids Operations: High HighPriscilaPrzNessuna valutazione finora
- 100 Yer PM PPM 0605Documento40 pagine100 Yer PM PPM 0605biplabpal2009Nessuna valutazione finora
- Ays 082914 3331 PDFDocumento18 pagineAys 082914 3331 PDFFabian R. GoldmanNessuna valutazione finora
- Manual de Taller sk350 PDFDocumento31 pagineManual de Taller sk350 PDFLeo Perez100% (1)
- Nano ScienceDocumento2 pagineNano ScienceNipun SabharwalNessuna valutazione finora
- Diels-Alder Reaction: MechanismDocumento5 pagineDiels-Alder Reaction: MechanismJavier RamirezNessuna valutazione finora
- 173 EvidenceDocumento6 pagine173 EvidenceSantiago RubianoNessuna valutazione finora
- Timer Relay ERV-09Documento1 paginaTimer Relay ERV-09wal idNessuna valutazione finora
- ScheduleDocumento1 paginaScheduleparag7676Nessuna valutazione finora
- Signals and Systems: Dr. Shurjeel WyneDocumento3 pagineSignals and Systems: Dr. Shurjeel Wynemarryam nawazNessuna valutazione finora
- Epilepsy Lecture NoteDocumento15 pagineEpilepsy Lecture Notetamuno7100% (2)
- RA9275Documento49 pagineRA9275znarf_ryanNessuna valutazione finora