Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Flameless Combustion and Its Applications
Caricato da
Damy ManesiTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Flameless Combustion and Its Applications
Caricato da
Damy ManesiCopyright:
Formati disponibili
FLAMELESS COMBUSTION AND ITS APPLICATIONS
J oachim G. Wnning
President
WS Inc.
719 Sugar Lane, Elyria, OH 44035
U.S.A.
ABSTRACT
Flameless Combustion was first developed to suppress thermal NOx formation in burners
for heating industrial furnaces using preheated combustion air. While this technique is applied in
large numbers now, there are a number of other applications emerging. This presentation will
give an introduction into flameless combustion and then show industrial applications and
applications which are at a research stage.
heat treating and heating furnaces in the steel industry
gas turbines
bio gas burners
burners for hydrogen reformers
burners for CHP units
and others.
1
Introduction
If a combustible mixture of fuel and air is ignited, a flame can develop. In the reaction zone,
called flame front, the temperature rises quickly to temperatures close to the adiabatic
temperature. The flame can be stabilized within or close to the burner, so that the combustion
goes stable and controlled. The different methods for flame stabilization play an important role
in the field of burner development. Examples are baffle and swirl stabilization.
For the required flame supervision, optical and electrical effects of flames are used. Modern
burner designs use UV or ionisation detectors for automatic flame safety systems. In the absence
of a flame signal, the burner is shut off. Therefore it can be said, that flames fullfill two
important functions:
flame stabilisation guarantees a constant and controlled reaction
flame
fronts
flame
stabilization
air fuel
flame
supervision
and ignition
<600 900 1200 1500 1800 2100C
Flamme
and a stable flame provides a steady
reliable signal for flame safety
systems.
The question is why to five up the proven
concept of flames and what are the
advantages of flameless oxidation. The main
important answer to this question is that
flameless oxidation can suppress thermal
NO-formation even when highly preheated
air is used.
The presentation will provide an overview
of the activities around flameless oxidation
from the last decade and it will also give an
outlook on future potentials.
Figure 1: main characteristics of flames
The Starting Point The Eighties
Following the first and second energy crisis in the seventies and early eighties, many R&D
activities focussed on the improvement of energy efficiency. The most effective method for
improving efficiency of combustion
systems, used for high temperature
processes, is combustion air preheating.
Central recuperators reach air preheat
temperatures of up to 600C.
Decentralized air preheat systems allow
for much higher figures. Since the air
preheat temperature increases
approximately linear with the process
temperature, the efficiency of combustion
systems can be shown in a clearly
arranged diagram.
0 250 500 750 1000 1250C 1500
0
0.25
0.5
0.75
1
0
.
2
0
.6
0.8
= 1
=
0
REGEMAT
REKUMAT
0
.4
e
f
f
i
c
i
e
n
c
y
exhaust temperature
(prior to the heat exchanger)
rel. air preheat
exhaust temperature
~
~
central heat exchanger
air preheat temperature
Figure 2: efficiency
2
During the same period, the awareness of
the negative effects of NO
x
-emissions on
human health and the environment put
growing pressure on operators and
producers of combustion equipment. While
NO
x
-emissions were abated with secondary
measures in some technical sectors, e.g.
catalytic converters in automobiles, the
thermal process industry developed and
used widely primary measures like staging
(see Figure 3). Using these NO
x
-reduction
techniques, emission standards like the
German TA-Luft could be met for process
temperatures up to 1200C and relative air
preheat of 0.6 (~700C). To meet more
rigid existing or future limits as well as to
apply higher air preheat temperatures was not
possible.
secondary air
fuel
primary
air
primary
combustion
exhaust gas
for cooling of
combustion products
secondary combustion
Figure 3: burner nozzle of an air staged high velocity
burner
Combustion without a Flame
In 1989, a surprising phenomenon was observed during experiments with a self recuperative
burner. At furnace temperatures of 1000C and about 650C air preheat temperature, no flame
could be seen and no UV-signal could be detected. Despite that, the fuel was completely burnt.
The carbon-monoxide content in the exhaust was below 1ppm. The NO
x
emissions were close to
zero, in the single digits, what was first thought to be a malfunction of the NO-analyser. The
combustion was stable and smooth, there was no lifted flame.
We called that condition flameless oxidation of short FLOX
1
.
air
fuel
reacting fuel & air
1
registered trade name of WS Wrmeprozesstechnik GmbH, Renningen
Figure 4: Flame and FLOX
3
Further experiments were carried out to
determine the essential conditions for
FLOX
. Fuel and air jets have to be
mixed into a strong recirculating flow of
exhaust prior to reaction. Then, no flames
and therefore no high temperature peaks
occur
1
. Air preheat is not a prerequisite
for flameless oxidation. The technique of
flameless oxidation was patented
worldwide
2
.
Flamme
FLOX
<600 900 1200 1500 1800 2100C
temperature
supervision
mixing of air and fuel
with exhaust prior to reaction
(no flame front)
strong
recirculation
air fuel
Investigating the basics The Early
Nineties
While the first FLOX
burners were
developed and the first commercial burner
was sold in 1991, R&D programs wee set
up to investigate the fundamentals of
flameless oxidation
3
4
5
.
Similar to flame combustion, areas of
stable combustion for flameless oxidation
can be described. In between these areas,
unstable and lifted flames occur which are not suitable for technical combustion.
Figure 6: stability limits
Figure 5: main characteristics of flameless oxidation
It was found that it is possible to produce highly visible areas with flameless
oxidation when conditions allowed fuel to pyrolise in low oxygen areas to
produce shining soot particles.
During this period, commercial CFD codes (Computational Fluid Dynamics)
became available to run on workstations or PC's. The suitability of CFD codes
to simulate flameless oxidation was part of a R&D project, funded by the
German Ministry of Education and Research. A test furnace was designed and
built to study flameless oxidation and to provide data sets for comparison with
result from computer simulations. A recuperative burner was firing bottom-up
into a cylindrical furnace, minimizing buoyancy effects. The furnace was
equipped with air cooled tubes which were arranged concentric along the
furnace wall. Air cooling, in contrast to water cooling, allows for adjustable
cooling and make it possible to adjust the furnace temperature widely
independent from the burner capacity. The air preheat temperature was
adjusted by controlling the amount of exhaust flow, bypassing the recuperator.
The non-cooled probe was inserted from the top and could be positioned fully
automatic throughout almost the whole furnace. Data collection for several
hundred positions in the furnace was possible in about one hour. The probe
was equipped to measure temperature, using a 50 m PtRh Pt thermocouple,
pressure and gas samples.
4
Figure 7: experimental furnace
Figure 8 shows temperatures from a fixed position, 250 mm away from the burner. NO
x
was
measured in the exhaust. The burner was operated in flame, lifted flame and FLOX
mode.
Flame and flameless oxidation mode show steady temperature conditions. NO
x
and noise are
substantially higher in flame mode, compared to FLOX
mode. NO
x
and noise are inbetween for
the lifted flame but the temperature signal is highly unstable.
igure 9 shows temperature fields for FLOX and flame operation. Clearly visible is the
0
0 2,5 5
Time
probe
7,5 s 10
200
400
600
800
1000
1200
1400
C
1600
lifted flame, x 60ppm, 92 dB(A)
NO
FLOX, x 6ppm, 82 dB(A)
NO
noise of the air alone 78 dB(A)
flame, x 160ppm, 98 dB(A)
NO
T
e
m
p
e
r
a
t
u
r
e
Figure 8: time resolved temperature measurement for FLOX
, flame and lifted flame
F
different characteristics of the temperature field. High temperature close to the burner nozzle in
flame operation (temperature measurements were limited by the thermocouple temperature
range), low temperatures in FLOX
mode.
axialer Abstand z radialer Abstand y
FLOX
Flame
axialer Abstand z radialer Abstand y
Figure 9: measured temperature fields of FLOX
and flame operation
5
After determine the suitable sub models and constants for
the CFD calculations, the data from the computer
simulation and the experiments were compared. The
results showed good agreement over wide range of
parameter variations except changing the fuel. The
predictions of NO
x
predictions were qualitatively and on a
technical level satisfying. This allowed for parameter
variations (see figure 11) to be calculated in a fraction of
time and costs, compared to experiments.
This kind of fundamental inve This kind of fundamental investigations were continued
by WS GmbH and a number of research groups to the
resent
6
7
8
9
. One example in figure 12 shows data
10
l satisfying. This allowed for parameter
variations (see figure 11) to be calculated in a fraction of
time and costs, compared to experiments.
stigations were continued
by WS GmbH and a number of research groups to the
resent
6
7
8
9
. One example in figure 12 shows data
10
p p
obtained by laser measurements techniques .
obtained by laser measurements techniques .
F
l
F
l
Flame
Experiment
F
L
O
X
F
L
O
X
FLOX
distanc
a
m
e
a
m
e
e
m 0
T
e
m
p
e
r
a
t
u
r
C
0
500
1000
1500
2000
T
e
m
p
e
r
a
t
u
r
C
0
500
1000
2000
T
e
m
p
e
r
a
t
u
r
C
0
500
1000
1500
2000
0.2 0.4 0.6
1500
CFD
Brennstoff:
Leistung P :
Brennraumtemperatur :
Luftvorwrmung :
Lambda :
Luftgeschwindigkeit w:
A
Ofen
Luvo
L
Erdgas
25 kW
1000C
0.65
1.15
variiert
Ofen
100m/s 8
0m
/s
6
0
m
/s
4
0
m
/s
2
0
m
/ s
Brennstoff:
LeistungP :
Brennraumtemperatur :
Luftvorwrmung :
A
Ofen
Luvo
Erdgas
25 kW
1000C
variiert
Lambda :
Luftgeschwindigkeit w: L
1.15
100 m/s
20
0.8 1.0
Figure 10: comparison of experiment and model
0C
400C
600C
8
00C
velocity variation
preheat variation
Figure 11: CFD parameter variations
300
900
1500
[K]
2100
0
0.0 5.0 10.0
[mm]
800
1600
2400
[a.U.]
3200
Height=13.0mm
0.0 6.0 12.0
[mm]
18.0
12.0
6.0
0.0
[mm]
0.0 6.0 12.0
18.0
12.0
6.0
0.0
[mm]
[mm]
18.0
[mm]
0.0 3.0 6.0 9.0 12.0
12.0
6.0
0.0
[mm]
0 800 1600 2400 3200
OH-LIPF Intensitt [a.U.]
0.0 3.0 6.0 9.0 12.0
6.0
0.0
[mm]
[mm]
300 900 1500 2100
Temperature[K]
18.0
12.0
300
900
1500
2100
0
800
1600
2400
0.0 5.0 10.0
[mm]
3200
He
[K]
FLAME
Temperature
OH
LIPF Intensity
FLOX
ight =15.13mm
[a.U.]
Figure 12: temperature and OH data obatained by laser measurement techniques
6
7
Further research will enable predictions of flameless oxidation for a wide range of gaseous,
liquid and solid fuels in a wide variety of set-ups as well as improved predictions on emissions.
Among others, one it is not necessary to
ixed is burnt" approach was used
in the beginning, m ultaneous mixture
of fuel, air and exhaust, suppres ad to new patented burner
designs, called "one-nozzle" FLOX
Exploitation
Steel Industry
While the first rec ners were installed during the early
nineties, several FLOX serial product for a variety of
applications. ating furnaces of the metal
and steel industry
12
ing to develop new burner
designs or to investigate the effects of applying flameless oxidation to certain processes.
, efficiency and NO
x
is an
important issue since internal temperatures in radiant tubes are substantially higher then the
process temperature. A breakthrough was the installation of several hundred ceramic radiant
tubes and FLOX
burners in a silicon steel strip line in 1994. Up to know, several thousand
ceramic burners and radiant tubes are installed
16
. While first designed for high temperature
applications to replace electric heating and short living metallic radiant tubes, ceramic radiant
tubes were used more and more in lower temperature processes, providing approximately double
the heat flux, compared to metallic tubes. Without flameless technology, maintaining acceptable
levels of NO
x
would not be possible in many cases.
very important result of the investigations was, that
inject fuel and oxidant separately into the furnace. While the "m
ore knowledge about the reaction kinetics allowed for a sim
sing reaction by slow kinetics. This le
burners
11
.
uperative and regenerative FLOX
bur
burner designs became a regular
Most of the burners were installed in heating and heat tre
1314
. Several projects were carried which were aim
One of the R&D projects focused on the development of ceramic recuperative burners and
radiant tubes
15
. In radiant tubes, and more so in ceramic radiant tubes
Figure 13: silicon steel strip line
In 1995, a EC funded project started to demonstrate the potentials of a new compact regenerative
burner design. The burner uses ceramic honeycomb regenerators which are integrated into the
s r uire a lot of research and convincing the
. Traditionally, the glass industry applied
ting and sometimes fuel preheating. Drastic
ce there is no shut down of these furnaces for
ects are based on empiricism rather than well
recast what would be the effect of changes of
on process on the product. But considering the high costs for post exhaust gas
easures for NO
x
reduction.
There are highly energy intensive chemical processes. Flameless Oxidation has a large potential,
m eformer processes.
burner.
Figure 16 shows installed regenerative burners in an annealing and pickling line. The specific
energy consumption of the furnace was cut in half while the production capacity was increased
considerably. NO
x
-levels are considerably below 100ppm. The furnace is located the steel works
of Terni, approximately 100km north of Rome
17
. The recent increases in energy prices have led
a growing interest and several new installations.
Ceramic Industry
There is a large potential for new combustion technologies in the ceramic industry. However,
often the processes are highly integrated and change
Figure 15: self regenerative burner
Figure 14: annealing and pickling line in Terni
eq
customers to leave known pathes.
Glass Industry
The glass industry is a substantial emitter for NO
x
regenerators, providing combustion air prehea
changes to glass melting furnaces are not easy sin
modifications. Another factor is that many asp
known and understood facts. So its difficult to fo
the combusti
treating, every effort should be made to find primary m
Chemical Industry
ainly for petrochemical and r
8
Combined Heat and Power
In anothe
r R&D project, a FLOX burner for Stirling engines was developed. Besides low NO
x
-
missions and high efficiency, a compact design and the potential for low production cost, when
units (combined heat and power) for decentralized electric po
ment. Reformer units for the on-site generation
f hydrogen where delivered to two hydrogen fuel stations in Madrid and Munich.
e
produced in large series, were important. The Stirling engines are intended to be used as CHP-
wer generation.
In the last years, the efforts to develop fuel cell systems for combined heat and power units,
increased substantially. Flameless combustion can play an important role in this field because it
seems to be the ideal combustion process for compact reformer units. Reformer units for
combined heat and power units are under develop
Figure 16: burner development for stirling engines
o
FLOX - Reformer in a test rig
exhaust
reformer gas
controls
water pump
gas compressor
air blower
FLOX-Reformer
*
Figure 18: FLOX
Reformer at the Munich Airport
hydrogen Figure 17: FLOX
reformer in a test rig fuel station
9
Another promising technology are micro gas turbines which could benefit from flameless
ombustors.
ecent projects have shown the potential of flameless combustion in the power generation
c
Power Generation
R
industry, namely gas turbines
18
( www.came-gt.com ), coal combustion
19
as well as future
processes including processes using bio fuels and CO
2
sequestration (see www.oxy-coal.de ) .
Bio Fuels
There is a growing interest towards the usage of biofuels for the reasons that they are regrowing
and being CO
2
neutral. However, the fuels are often very inhomogenous. Flameless combustion
enables low emission and trouble free combustion. Since there are no flame stability limitations.
There are a number of research projects, covering these aspects, ongoing right know. (see
www.eu-projects.de Bio-Pro for further information).
more homogenous temperature distribution
reduced thermal stress for the burner
reduced noise
less burner faults
less restrictions on fuels because no flame stability is required
were also important factors. Development will continue to improve recuperative and regenerative
burners. Rising energy costs will favour regenerative concepts due to their higher potential for
efficiency.
For some burner designs, licences were granted and further licences will be offered to enable
other companies or research groups to use the potential of flameless oxidation on a wide variety
of applications.
Conclusions
Up to now, more than thousand FLOX
burners were installed in many different applications.
Often, the reduced NO
x
-emissions were the motivation to apply flameless oxidation, but in many
cases:
10
LITERATURE
nning J ., Flammenlose Oxidation von Brennstoff mit hochvorgewrmter Luft, Chem.-Ing.-
12, S. 1243-1245
ritish and German Sections of the Combustion Institute, Cambridge, 1993
nning J .G., Flammlose Oxidation von Brennstoff, Thesis, Aachen, 1996
Combustion Burner, Combustion and
lame, Volume 124, No. 3, Combustion Institute, February 2001
Theoretical and Numerical Investigation on Flameless Combustion, Combustion
cience Technology, 2001
ig,: experimental characterisation of laboratory furnaces by means of advanced
diag s
9
Coelho P.J ., Peter N., Numerical Simulation of a Flameless Oxidation Combustor, Combustion
and a 03-518
l) on Combustion / The Combustion Institute, 1998, pp. 3197-3204
minar on High Temperature Combustion in Industrial Furnaces, Stockholm, 2000
Wnning J ., Regenerative Burner Using Flameless Oxidation, IGRC 95, Cannes, France
14
Weber R., Verlaan A.L., Orsino S., Lallemant N., On emerging furnace design methodology
that provides substantial energy savings and drastic reductions in CO2, CO and NOx emissions,
2
nd
seminar on High Temperature Combustion in Industrial Furnaces, Stockholm, 2000
15
Flamme M. Bo M., Brune M. et. al., Improvement of Energy Saving with New Ceramic Self-
Recuperative Burners, IGRC, San Diego, 1998
1
W
Tech. 63 (1991), Nr.
2
Patent EP 0463218
3
Woelk G., Wnning J ., Controlled Combustion by Flameless Oxidation, J oint Meeting of the
B
4
W
5
Wnning J .A., Wnning J .G., Flameless Oxidation to Reduce Thermal NO-Formation, Prog.
Energy Combust. Sci. Vol. 23, Seite 81-94, 1997
6
Coelho P.J ., Peters N., Numerical Simulation of a Mild
F
7
Tabacco D.,
S
8
Giammartini S., Girardi G., Cipriani R., Cuoco F., Sica M., Diluted combustion with high air
preheat
no tics, INFUB 2000, Porto, Portugal
Fl me, Vol 124, pp. 5
10
Plessing T., Peters N., Wnning J .G., Laser Optical Investigation of Highly Preheated
Combustion With Strong Exhaust Gas Recirculation, Twenty - Seventh Symposium
(Internationa
11
Patent EP 0685683
12
Massingham J , Evaluation of low NOx regenerative burners for steel industry applications, 2
nd
se
13
11
16
Telger R., Roth W., Betriebserfahrung beim Einsatz von Brennern mit flammloser Oxidation,
Milani A., Salomone GV., Wnning J ., Advanced Regenerative Design Cuts Air Pollution,
99, UK
erence,
Noordwijkerhout, Netherlands 2004
19
Fielenbach C., Holfeld T., Petery C. von, Renz U., Wnning J ., NO
x
-reduction in a
Gas Wrme International 44 (1995), Heft 7/8, S. 323-337
17
Advanced Steel 1998-
18
Flamme M., Al-Halbouni A. Wnning J ., Scherer V., Schlieper M., et al., Low Emission Gas
Turbine Combustor Based on Flameless Oxidation, 14th IFRF Member Conf
pressurized pulverized coal flame by flue gas recirculation, Pittsburgh Coal Conference,
Pittsburgh, USA 2003
12
Potrebbero piacerti anche
- Flameless CombustionDocumento8 pagineFlameless CombustionHasan Kayhan KayadelenNessuna valutazione finora
- Combustion Flameless PDFDocumento2 pagineCombustion Flameless PDFMaryNessuna valutazione finora
- Combination of SNCR and SCR NOx Emission Control SystemsDocumento8 pagineCombination of SNCR and SCR NOx Emission Control SystemssdiamanNessuna valutazione finora
- Abatement TechniquesDocumento24 pagineAbatement TechniquesTian NakNessuna valutazione finora
- SKMM 3443 Heat Transfer Project: Sustainable Energy in A Heat Exchanger System'Documento9 pagineSKMM 3443 Heat Transfer Project: Sustainable Energy in A Heat Exchanger System'Izzat AshraffNessuna valutazione finora
- Che-481 - Fuel and Combustion - Group 09Documento24 pagineChe-481 - Fuel and Combustion - Group 09Osama HasanNessuna valutazione finora
- Fluid DynamicsDocumento149 pagineFluid Dynamicsprasanta_bbsrNessuna valutazione finora
- Acoustic Horn On BoilersDocumento1 paginaAcoustic Horn On BoilersjomoltNessuna valutazione finora
- Kinetic Model of Biomass GasificationDocumento7 pagineKinetic Model of Biomass GasificationjuaanxpoonceNessuna valutazione finora
- Ball mill process parameters and air flow dataDocumento4 pagineBall mill process parameters and air flow dataTIRIANTONessuna valutazione finora
- CFBC Boiler - A SurveyDocumento11 pagineCFBC Boiler - A SurveygjanklesariaNessuna valutazione finora
- 1 212 Air PreheaterDocumento5 pagine1 212 Air Preheaterhanafy_arnandaNessuna valutazione finora
- Application of Measuring Gas Flow in The DowncomerDocumento4 pagineApplication of Measuring Gas Flow in The DowncomerAhmad NilNessuna valutazione finora
- Progressing Cavity Pumps: Colfax AmericasDocumento12 pagineProgressing Cavity Pumps: Colfax AmericasKhairil AjjaNessuna valutazione finora
- Heat ExchangerDocumento9 pagineHeat ExchangerChrissa Villaflores GanitNessuna valutazione finora
- Mill Fire Protection: Minimize RisksDocumento3 pagineMill Fire Protection: Minimize RisksANAND PANDEYNessuna valutazione finora
- Design, Process Simulation and Construction of An Atmospheric Dual Fluidized Bed CombustionDocumento9 pagineDesign, Process Simulation and Construction of An Atmospheric Dual Fluidized Bed Combustionapi-3799861Nessuna valutazione finora
- Study of Circulating Coal Fluidized BoilersDocumento5 pagineStudy of Circulating Coal Fluidized BoilersSwaraj BiswasNessuna valutazione finora
- Nox ControlDocumento9 pagineNox Controlsureshbabu7374Nessuna valutazione finora
- Fuels & Combustion Technology (Major Elective Û I) (Chemical Group)Documento2 pagineFuels & Combustion Technology (Major Elective Û I) (Chemical Group)raumil123759033% (3)
- Coal Feeder PaperDocumento8 pagineCoal Feeder PaperPanimanPalengaiNessuna valutazione finora
- Optimizing Steam Boiler Operations to Reduce CostsDocumento10 pagineOptimizing Steam Boiler Operations to Reduce Costsrashm006ranjanNessuna valutazione finora
- Laser Ignition SystemDocumento6 pagineLaser Ignition SystemAkshay KakaniNessuna valutazione finora
- Design and Analysis of Fire Tube Boiler With Heat Flow AnalysisDocumento3 pagineDesign and Analysis of Fire Tube Boiler With Heat Flow Analysisbez100% (1)
- TP CFB 12 03Documento16 pagineTP CFB 12 03Harish MechNessuna valutazione finora
- Seminar On LASER IgnitionDocumento17 pagineSeminar On LASER IgnitionAnonymous IohZ9Vi100% (1)
- Study The Efficiency of Air PeheaterDocumento3 pagineStudy The Efficiency of Air PeheaterManoj PatilNessuna valutazione finora
- Combustion Basics ExplainedDocumento48 pagineCombustion Basics ExplainedJeevanandam ShanmugasundaramNessuna valutazione finora
- Energy Engineering: 4th Term, B.Sc. Chemical Engineering Session 2008 Delivered byDocumento13 pagineEnergy Engineering: 4th Term, B.Sc. Chemical Engineering Session 2008 Delivered bymmm2025Nessuna valutazione finora
- Experimental Model of Industrial Fire Tube BoilerDocumento11 pagineExperimental Model of Industrial Fire Tube BoilerVignesh AlagesanNessuna valutazione finora
- Unit 3 Power Plant EngineeringDocumento49 pagineUnit 3 Power Plant EngineeringNaren NathanNessuna valutazione finora
- 1010750-Steam Quality TestingDocumento11 pagine1010750-Steam Quality TestingHendra Hadriansyah100% (1)
- "Laser Ignition System": A Seminar Report ONDocumento11 pagine"Laser Ignition System": A Seminar Report ONSACHIN SHAH0% (1)
- CFBC BoilersDocumento11 pagineCFBC BoilersManoj DesaiNessuna valutazione finora
- COMBUSTION PROCESS IN CI ENGINESDocumento11 pagineCOMBUSTION PROCESS IN CI ENGINESSathistrnpcNessuna valutazione finora
- Nemo Pumps - The Perfect Pipeline PumpDocumento6 pagineNemo Pumps - The Perfect Pipeline PumpDiana Lucía PadillaNessuna valutazione finora
- Laser Ignition ReportDocumento26 pagineLaser Ignition ReportRaHul100% (2)
- Industrial BurnersDocumento23 pagineIndustrial Burnerskarthikeyan5000Nessuna valutazione finora
- Assessment of Venturi Nozzle For Filter Bag Cleaning in PDFDocumento0 pagineAssessment of Venturi Nozzle For Filter Bag Cleaning in PDFUmanath R PoojaryNessuna valutazione finora
- BROCHURE - Institucional UTIS - V5 ENDocumento28 pagineBROCHURE - Institucional UTIS - V5 ENsalahromdhani89Nessuna valutazione finora
- CFD Analysis of A 210 MW Tangential Fired BoilerDocumento6 pagineCFD Analysis of A 210 MW Tangential Fired BoilerInnovative Research PublicationsNessuna valutazione finora
- Elliott Turbine-Generator ConfigurationsDocumento8 pagineElliott Turbine-Generator Configurationskishwar999100% (1)
- Lec. 5 CyclonesDocumento50 pagineLec. 5 CyclonesJesusNessuna valutazione finora
- Grafik Excess AirDocumento3 pagineGrafik Excess AirKhairil MunawirNessuna valutazione finora
- Steam Power Plant (PLTU)Documento5 pagineSteam Power Plant (PLTU)Aldi ErzanuariNessuna valutazione finora
- Boiling and CondensationDocumento5 pagineBoiling and CondensationChaubey AjayNessuna valutazione finora
- Static Pitot Tube: Flow MeasurementDocumento23 pagineStatic Pitot Tube: Flow MeasurementMuthu KarthikNessuna valutazione finora
- Computational CombustionDocumento33 pagineComputational CombustionMaria RomeroNessuna valutazione finora
- Westinghouse-Design Steam Chest PowerGen-2012FinalDocumento0 pagineWestinghouse-Design Steam Chest PowerGen-2012FinalSuphi YükselNessuna valutazione finora
- Bed MAterial ChemistryDocumento9 pagineBed MAterial Chemistryyogeshmangal1317Nessuna valutazione finora
- CreepDocumento26 pagineCreepOsu AmpawanonNessuna valutazione finora
- Air Pollution Prevention and Control: Dr. Wesam Al MadhounDocumento52 pagineAir Pollution Prevention and Control: Dr. Wesam Al MadhounAIZAZ SHAIKHNessuna valutazione finora
- Experts - SSO - Manual - Industrial Steam System Optimization.Documento148 pagineExperts - SSO - Manual - Industrial Steam System Optimization.Jaime AndrewsNessuna valutazione finora
- Gas Turbine Components and Brayton CycleDocumento30 pagineGas Turbine Components and Brayton CycleFaisal KhanNessuna valutazione finora
- Maximized Heat Transfer with TEC Meal Splash BoxDocumento1 paginaMaximized Heat Transfer with TEC Meal Splash BoxBerkan FidanNessuna valutazione finora
- 2 ERG 401 2015 Energy Performance Analysis of BOILERDocumento57 pagine2 ERG 401 2015 Energy Performance Analysis of BOILERnaveenNessuna valutazione finora
- New Aspects of Spillover Effect in Catalysis: For Development of Highly Active CatalystsDa EverandNew Aspects of Spillover Effect in Catalysis: For Development of Highly Active CatalystsNessuna valutazione finora
- Combustion of Natural Gas with High-Temperature Air and Large Quantities of Flue GasDocumento7 pagineCombustion of Natural Gas with High-Temperature Air and Large Quantities of Flue GasMihirduttaNessuna valutazione finora
- Highly Preheated Air Combustion Research in Sweden: Blasiak W, Dong W, Lille SDocumento18 pagineHighly Preheated Air Combustion Research in Sweden: Blasiak W, Dong W, Lille SSaptarshi SenguptaNessuna valutazione finora
- Experimental Evaluation of Flameless Combustor PerformanceDocumento11 pagineExperimental Evaluation of Flameless Combustor PerformancejayakrishnatejaNessuna valutazione finora
- Oi Mate C Maintenance ManualDocumento774 pagineOi Mate C Maintenance ManualDamy ManesiNessuna valutazione finora
- Hotel Registration Form SEODocumento4 pagineHotel Registration Form SEODamy Manesi50% (2)
- Airport FacilitiesDocumento15 pagineAirport FacilitiesDamy Manesi100% (3)
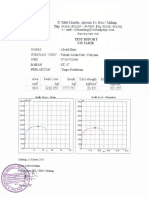
- Test ReportDocumento11 pagineTest ReportDamy ManesiNessuna valutazione finora
- Astm A1007Documento3 pagineAstm A1007Damy ManesiNessuna valutazione finora
- International Journal ReviewDocumento6 pagineInternational Journal ReviewDamy ManesiNessuna valutazione finora
- Set Up Dialog MillingDocumento19 pagineSet Up Dialog MillingDamy ManesiNessuna valutazione finora
- TopCAM CAD IntroductionDocumento14 pagineTopCAM CAD IntroductionDamy ManesiNessuna valutazione finora
- Kurva Tegangan Regangan Kawat Gitar Akustik: Strees (KG/MM )Documento8 pagineKurva Tegangan Regangan Kawat Gitar Akustik: Strees (KG/MM )Damy ManesiNessuna valutazione finora
- Advance Thermodynamics 1Documento16 pagineAdvance Thermodynamics 1T975Nessuna valutazione finora
- From This MomentDocumento1 paginaFrom This MomentDamy ManesiNessuna valutazione finora
- Teknik P & G - 1 - GhurriDocumento42 pagineTeknik P & G - 1 - GhurriDamy ManesiNessuna valutazione finora
- Graduate Courses Faq (English-Korean)Documento10 pagineGraduate Courses Faq (English-Korean)Damy ManesiNessuna valutazione finora
- Advance Thermodynamics 1Documento16 pagineAdvance Thermodynamics 1T975Nessuna valutazione finora
- Matlab Programming Strategy For Vle With EosDocumento7 pagineMatlab Programming Strategy For Vle With Eosmatheus735Nessuna valutazione finora
- A Space Tribology Handbook: ESTL, AEA Technology, Warrington, UKDocumento5 pagineA Space Tribology Handbook: ESTL, AEA Technology, Warrington, UKDamy ManesiNessuna valutazione finora
- Step 1 Engine Electrical Bhs Indo)Documento105 pagineStep 1 Engine Electrical Bhs Indo)rrhakim100% (6)
- AdvanceThermodynamics Materi 6Documento28 pagineAdvanceThermodynamics Materi 6Damy ManesiNessuna valutazione finora
- Strategy For Solving Vle Problems With PR - EosDocumento13 pagineStrategy For Solving Vle Problems With PR - EosDamy ManesiNessuna valutazione finora
- Advance Thermodynamics 1Documento16 pagineAdvance Thermodynamics 1T975Nessuna valutazione finora
- Frekuensi: Senar Notasi Saintis Notasi UmumDocumento2 pagineFrekuensi: Senar Notasi Saintis Notasi UmumDamy ManesiNessuna valutazione finora
- Love EeeeDocumento580 pagineLove EeeeDamy ManesiNessuna valutazione finora
- Momen Dan KopelDocumento14 pagineMomen Dan KopelDamy ManesiNessuna valutazione finora
- Two Tank Study MaterialDocumento28 pagineTwo Tank Study MaterialLi XueNessuna valutazione finora
- Aircraft EngineDocumento37 pagineAircraft EngineDamy Manesi100% (1)
- Keseimbangan PartikelDocumento11 pagineKeseimbangan PartikelDamy ManesiNessuna valutazione finora
- Management and Energy OptimizationDocumento13 pagineManagement and Energy OptimizationDamy ManesiNessuna valutazione finora
- Aircraft EngineDocumento37 pagineAircraft EngineDamy Manesi100% (1)
- Steam TablesDocumento16 pagineSteam TablesSantosh KumarNessuna valutazione finora
- Boiler CalculationsDocumento16 pagineBoiler CalculationsBilly Moerdani100% (1)
- Company Profile NecarrDocumento10 pagineCompany Profile Necarranescobar0001Nessuna valutazione finora
- Soc300 Final Study GuideDocumento3 pagineSoc300 Final Study GuidenoudlezzNessuna valutazione finora
- Seven QC Tools Tool #5: Part 1-Run ChartDocumento6 pagineSeven QC Tools Tool #5: Part 1-Run ChartAnkur DhirNessuna valutazione finora
- Line Follower Robot ProjectDocumento8 pagineLine Follower Robot ProjectPuneeth ShettigarNessuna valutazione finora
- OQ Task 2 Hein Htet NaingDocumento3 pagineOQ Task 2 Hein Htet NaingHein Htet NaingNessuna valutazione finora
- Infinite SummerDocumento145 pagineInfinite SummermarcelbocalaoNessuna valutazione finora
- 425 TR - Chiller Data SheetSpecDocumento2 pagine425 TR - Chiller Data SheetSpecjohnsvjNessuna valutazione finora
- Biomedical Anna 27Documento30 pagineBiomedical Anna 27Manoj GuruNessuna valutazione finora
- Piaggio Fly 125 I.E - 150 I.E Leader MY 2011 (EN)Documento231 paginePiaggio Fly 125 I.E - 150 I.E Leader MY 2011 (EN)ManuallesNessuna valutazione finora
- Intel® Core™2 Quad Processor Q9550 (12M Cache, 2.83 GHZ, 1333 MHZ FSB) SpecificationsDocumento5 pagineIntel® Core™2 Quad Processor Q9550 (12M Cache, 2.83 GHZ, 1333 MHZ FSB) SpecificationsRobert FarkasNessuna valutazione finora
- Chapter 3 Developing The Whole PersonDocumento15 pagineChapter 3 Developing The Whole PersonRocel DomingoNessuna valutazione finora
- Action Paper g2 Final OutputDocumento67 pagineAction Paper g2 Final Outputjay diazNessuna valutazione finora
- Quality Executive resume highlighting MHA and NABH experienceDocumento3 pagineQuality Executive resume highlighting MHA and NABH experiencesherrymattNessuna valutazione finora
- Machine Learning - Problem Setup, Conditional Probability, MLEDocumento6 pagineMachine Learning - Problem Setup, Conditional Probability, MLEtarun guptaNessuna valutazione finora
- 5-сынып КТП 2023-2024 English PlusDocumento12 pagine5-сынып КТП 2023-2024 English Plusalenova.ddNessuna valutazione finora
- The Spaceships of EzekielDocumento187 pagineThe Spaceships of EzekielRussell Runge100% (1)
- Propp MorphologyDocumento10 paginePropp MorphologyFilippo CostanzoNessuna valutazione finora
- Liebert Deluxe System 3 Chilled Water Installation ManualDocumento36 pagineLiebert Deluxe System 3 Chilled Water Installation Manualhaizad7cosNessuna valutazione finora
- Sajjad Hussain Sociology 2021 Iiui IsbDocumento323 pagineSajjad Hussain Sociology 2021 Iiui IsbTaskeen MansoorNessuna valutazione finora
- Evidence DisputeDocumento10 pagineEvidence DisputeAlexPamintuanAbitanNessuna valutazione finora
- Context CluesDocumento4 pagineContext CluesSherelyn BaidoNessuna valutazione finora
- Young Pianist CompetitionDocumento2 pagineYoung Pianist CompetitionAleksa SarcevicNessuna valutazione finora
- The Deer and The CrocodilesDocumento4 pagineThe Deer and The CrocodilesM Rifky FauzanNessuna valutazione finora
- CPSA Sales Institute Key Competencies in SalesDocumento1 paginaCPSA Sales Institute Key Competencies in Salesmiguel8blancoNessuna valutazione finora
- Astm D-3300Documento4 pagineAstm D-3300Ovi Nafisa Zabeen OviNessuna valutazione finora
- Conceptualizing Wellbeing in The Workplace: Stefania de SimoneDocumento5 pagineConceptualizing Wellbeing in The Workplace: Stefania de SimoneMariaP12Nessuna valutazione finora
- Soil Classification PDFDocumento12 pagineSoil Classification PDFbishry ahamedNessuna valutazione finora
- Ariston Ecosf109 19509154400 - GB CZ SK GRDocumento60 pagineAriston Ecosf109 19509154400 - GB CZ SK GRionNessuna valutazione finora
- XC Assignments SheetDocumento10 pagineXC Assignments Sheetapi-207116966Nessuna valutazione finora
- Bcoc 138Documento2 pagineBcoc 138Suraj JaiswalNessuna valutazione finora