Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
USp Dissolution PVT Revision
Caricato da
mpfebrianDescrizione originale:
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
USp Dissolution PVT Revision
Caricato da
mpfebrianCopyright:
Formati disponibili
STIMULI TO THE REVISION PROCESS 1630
Stimuli articles do not necessarily reect the policies of the USPC or the USP Council of Experts
Pharmacopeial Forum Vol. 34(6) [Nov.Dec. 2008]
Description of the Upcoming Change in Data Analysis for USP Dissolution Performance Verication Tests
Walter W. Hauck,a Anthony J. DeStefano, William E. Brown, Erika S. Stippler, Darrell R. Abernethy, Roger L. Williams, USP, and the Biopharmaceutics Expert Committeeb Stimuli to the Revision Process
ABSTRACT As part of its evaluation of the performance verication tests used periodically to afrm the integrity of the USP Performance test when General Chapter Dissolution h711i is relied upon, the Biopharmaceutics Expert Committee of the Council of Experts, working with staff, decided to change the form of the accept/reject decision from one based on the result for each tablet to one based on the mean and coefcient of variation of results from a set of tablets. This paper describes the new approach. The paper also describes an implementation period for the approach, coupled with a period during which USP will discontinue use of the Salicylic Acid tablet in a performance verication test.
INTRODUCTION USP has embarked on a vigorous program to evaluate its performance verication tests (PVTs) in order to maximize their value in ensuring the integrity of the dissolution and other procedures (e.g., 1, 2). For the dissolution procedure described in General Chapter Dissolution h711i and applied to nonsolution orally administered dosage forms, this has led to two major changes. First, Salicylic Acid tablets will be discontinued as an available ofcial USP Reference Standard (RS) for use in a PVT. The transition is expected at the end of CY 2009. Adequate information will be made available to users to allow a smooth transition, and USPs remaining PVT tablets (Prednisone and Clorpheniramine Maleate Extended-Release RS tablets) will continue to be supplied as before. Second, the form of the accept/reject decision for the PVT will change. A 2007 Stimuli article proposed the change from one based on per-tablet resultswhich correspond to individual positions in an assemblyto one based on the mean and coefcient of variation (CV) of a set of RS tablet results (3). Note that an assembly is the complete dissolution test equipment including 6 to 12 apparatus positions, depending on the manufacturer. Responses to the authors of the ve comments received regarding the Stimuli article were published in a subsequent Stimuli article (4). One comment suggested that the test be done in a two-stage fashion. This is similar to the current procedure for General Chapter Dissolution h711i (5) and for General Chapter Uniformity of Dosage Units h905i (6). A two-stage test is an optional part of the proposal described here. USPs Biopharmaceutics Expert Committee (BPC) reviewed the current proposal and concluded that USP should proceed with implementation of the revised form of the PVT acceptance criteria, as described in the 2007 Stimuli article (3), and include the option of a two-stage test. The purpose of this Stimuli article is to describe the revised approach that will apply to PVTs used to assess the integrity of the dissolution
Correspondence should be addressed to: Walter W. Hauck, PhD, Senior Scientic Fellow, US Pharmacopeia, 12601 Twinbrook Parkway, Rockville, MD 20852-1790; tel. 301.816.8390; e-mail wh@usp.org. b For a list of the members of the Biopharmaceutics Expert Committee, please see Appendix A.
# 2008
a
procedure as described in General Chapter h711i. The background information provided to the BPC, including operating characteristic curves, will be submitted for publication elsewhere (article in preparation). Single-Stage Test The current acceptance criteria for the dissolution PVT are per RS tablet. That is, the result for each tablet/position must fall within the acceptance range, which arises from collaborative studies of RS tablets and is based on both inter- and intralaboratory variability. The proposal is to replace the current approach with one based on the mean and CV of results from a set of tablets judged relative to acceptance ranges obtained from the collaborative study. The new approach will follow ISO International Standard 5725-6 (7). In Technical Specication 21748 ISO recommended a minimum of 15 degrees of freedom for the variability (8). USP elected to increase the number of tablets in the PVT from that currently required but to a lesser extent than that called for by the 15-degreeof-freedom recommendation. The following are step-by-step instructions for the singlestage test. Sufcient detail is provided so readers can both understand the procedure and, if desired, perform the calculations. USP will make available on its Web site a spreadsheet that will accept data from the 12 to 16 individual results from steps 1 and 2 and perform all the calculations.
1.
2.
For each position in the assembly, test one USP PVT RS (Prednisone RS tablets for Apparatus 1 and 2, and Chlorpheniramine Maleate Extended-Release RS tablets for Apparatus 3 at each dip rate), and record the percent dissolved at each sampling time point(s) specied for that apparatus (i.e., 30 min for Apparatus 1 and 2 and each of the times specied for Apparatus 3). After transforming the percent dissolved results to the log scale, determine the mean and variance. For assemblies with 12 positions (12 dissolution vessels), no further testing is required. For assemblies with fewer than 12 positions, repeat Step 1 with an additional set of tablets. Again after transforming the percent dissolved results to the log scale, determine the mean and variance.
The United States Pharmacopeial Convention, Inc.
All Rights Reserved.
STIMULI TO THE REVISION PROCESS
Pharmacopeial Forum Vol. 34(6) [Nov.Dec. 2008]
3.
Stimuli articles do not necessarily reect the policies of the USPC or the USP Council of Experts
1631
4.
5.
Table 1 shows what the acceptance ranges would be for the Calculate the average of the two means and of the two variances obtained in Steps 1 and 2. (Use the results from current lots of Prednisone and Chlorpheniramine Maleate RefStep 1 alone for assemblies that have 12 positions.) erence Standard tablets. Convert the results of Step 3 to a geometric mean (GM) NOTE: The criteria for Prednisone Lot P RS tablets reect and percent coefcient of variation (%CV), and round changed ranges as of 16 July 2007. The decision approach both to one decimal place following USP rounding rules for Apparatus 3 uses 97.5% limits rather than the 95% limits (9). used for the other apparatus in order to adjust for the two inCompare the results of Step 4 to the acceptance ranges on dependent tests at the two dip rates. the data sheet that is shipped with the product. The GM NOTE: These acceptance ranges are not ofcial and are must not fall outside the limits, and the %CV must not be provided for information only. greater than the limit. If both meet the criteria, the assembly has passed the PVT. Table 1. Acceptance Ranges for Basic Test (Single Stage) Not OfcialFor Informational Purposes Only 1A. Acceptance Ranges for Apparatus 1 and 2 Apparatus 2 %CV nmta 11.0% 10.8% 10.6% 10.9% GM on or within 34.549.7 34.549.7 34.649.6 34.549.7 %CV nmt 11.5% 11.2% 11.0% 11.3%
Stimuli to the Revision Process
Apparatus 1 Number of GM on Positions or within 6 53.971.8 7 54.071.7 8 54.071.6 12 53.971.8 nmt = not more than
1B. Acceptance Ranges for Apparatus 3 5 Dips/Min 1 Hour Number of GM on Positions or within 6 27.031.9 7 27.031.9 nlt = not less than 4 Hours %CV nm 6.3% 6.2% GM on or within 59.565.1 59.665.1 %CV nmt 4.7% 4.6% 2 Hours GM on or within 47.664.0 47.763.9 %CV nmt 10.8% 10.5% GM nltb 88.7 88.8 30 Dips/Min 6 Hours %CV nmt 4.4% 4.3%
Figure 1 shows a spreadsheet demonstrating the steps outlined above. The data are from an experiment using Apparatus 1. These data fail the proposed approach because the geometric mean is too low. Note that each result for the rst run
meets the current per-tablet criteria, but one result of the second run does not (the value of 41.1 is below the lower limit of the current range).
Figure 1. Spreadsheet showing implementation of the steps of the basic (single-stage) test.
# 2008
The United States Pharmacopeial Convention, Inc.
All Rights Reserved.
STIMULI TO THE REVISION PROCESS 1632
Stimuli articles do not necessarily reect the policies of the USPC or the USP Council of Experts
Pharmacopeial Forum Vol. 34(6) [Nov.Dec. 2008]
Stimuli to the Revision Process
For laboratories the new approach will mean less work for Apparatus 1 and 2. Because Salicylic Acid tablets are being discontinued, even if the total number of tablets remains unchanged less work remains because the assay for only one compound is required. For Apparatus 3 an increase in work is required because of the doubling in the number of tablets tested. Laboratories can reduce their work for all apparatus by choosing the optional two-stage test (see next section) and controlling their dissolution results so that the data are sufciently tight to meet the accept/reject decision after testing only the rst set of tablets. Optional Two-Stage Test A laboratory may choose to implement the PVT as a twostage test. In the clinical trials literature the underlying idea that supports the two-stage test is termed a group sequential design. That is, the two-stage test is a statistically valid means of allowing the possibility of stopping the test at the rst stage. A group sequential design allows for early stopping of the full 1216 tablet test with a penalty, but the test is still considered a 1216 tablet test. As will be seen below, the accept/reject decision rules are more stringent at the rst stage (the penalty) than at the second and, at the second, slightly different from the single-stage criteria shown in Table 1. Assemblies with 12 positions are not included in the two-stage option because two stages would require users to test 24 tablets, although an assembly with 6 positions would never be required to test more than 12 tablets. Following are step-by-step instructions for the two-stage procedure. As was the case with the single-stage test, USP will make available on its Web site a spreadsheet that practitioners can use to perform the calculations.
1.
2.
For each position in the assembly, test one USP PVT RS (Prednisone RS tablets for Apparatus 1 and 2, and Chlorpheniramine Maleate Extended-Release tablets for Apparatus 3 at each dip rate), and record the percent dissolved at each sampling time point(s) specied for that apparatus (30 min for Apparatus 1 and 2 and each of the times specied for Apparatus 3). After transforming the percent dissolved results to the log scale, determine the mean and variance. Convert the results of Step 1 to a GM and %CV, round both to one decimal place following USP rounding rules (9), and compare to the Stage One acceptance ranges on the data sheet that is shipped with the product. The GM must not fall outside the limits, and the %CV must not be greater than the limit.
If results of Step 2 satisfy both acceptance criteria, stop; the assembly has passed the PVT. Otherwise continue to Step 4. Repeat Step 1 with an additional set of tablets and after transforming the percent dissolved results to the log scale determine the mean and variance for the data obtained at this step.
3. 4.
Average the two means and two variances obtained in Steps 1 and 4. 6. Convert the results of Step 5 to a GM and %CV, and round both to one decimal place following USP rounding rules (9). 7. Compare the results of Step 6 to the Stage 2 acceptance ranges on the data sheet that is shipped with the product. The GM must not fall outside the limits, and the %CV must not be greater than the limit. If both meet the acceptance criteria, the assembly has passed the PVT. Table 2 shows what the acceptance ranges would be for the two-stage test for the current lots of Prednisone and Chlorpheniramine Maleate Reference Standard tablets. [NOTE: These acceptance ranges are not ofcial and are provided for information only].
5.
# 2008
The United States Pharmacopeial Convention, Inc.
All Rights Reserved.
STIMULI TO THE REVISION PROCESS
Pharmacopeial Forum Vol. 34(6) [Nov.Dec. 2008]
Stimuli articles do not necessarily reect the policies of the USPC or the USP Council of Experts
1633
Table 2. Acceptance Ranges for Optional Two-Stage Test Not OfcialFor Informational Purposes Only 2A. Acceptance Ranges for Apparatus 1 and 2 Apparatus 1 Number of Positions 6 7 8 After 1 Stage GM on %CV or within nmt 58.466.3 58.466.2 58.566.2 8.2% 8.2% 8.3%
st
After 2 Stage GM on %CV or within nmt 53.971.8 54.071.7 54.071.6 9.6% 9.5% 9.4%
nd
Apparatus 2 After 1 Stage After 2nd Stage GM on %CV GM on %CV or within nmt or within nmt
st
Stimuli to the Revision Process
38.244.9 38.344.8 38.344.8
8.6% 8.6% 8.6%
34.549.7 34.549.7 34.649.6
11.2% 11.0% 10.9%
2B. Acceptance Ranges for Apparatus 3, 5 Dips/Min 1 Hour Number of Positions 6 7 After 1 Stage GM on %CV or within nmt 28.230.2 28.230.2 4.5% 4.5%
st
4 Hours
nd
After 2 Stage GM on %CV or within nmt 27.031.9 27.031.9 6.2% 6.1%
After 1 Stage GM on %CV or within nmt 60.963.3 60.963.3 3.3% 3.3%
st
After 2nd Stage GM on %CV or within nmt 59.565.1 59.665.1 4.6% 4.5%
2C. Acceptance Ranges for Apparatus 3, 30 Dips/Min 2 Hours Number of Positions 6 7 After 1st Stage GM on %CV or within nmt 51.558.2 51.558.2 7.6% 7.7% After 2nd Stage GM on %CV or within nmt 47.664.0 47.763.9 10.6% 10.3% 4 Hours After 1st Stage After 2nd Stage GM on %CV GM %CV or within nmt nlt nmt 92.498.3 92.498.2 3.1% 3.1% 88.7 88.8 4.3% 4.2%
Figure 2 shows a spreadsheet implementing the procedure outlined above. The data are from Apparatus 1 and are the same as in Figure 1. These data do not meet the acceptance criteria after the rst stage because of a geometric mean that falls below the Stage 1 lower limit in the new acceptance
range, which requires the second stage of testing. They then fail the new acceptance criteria after the second stage because the geometric mean is below the stage 2 lower limit in the new acceptance range.
Figure 2. Spreadsheet showing implementation of the steps of the optional two-stage test.
# 2008
The United States Pharmacopeial Convention, Inc.
All Rights Reserved.
STIMULI TO THE REVISION PROCESS 1634
Stimuli articles do not necessarily reect the policies of the USPC or the USP Council of Experts
Pharmacopeial Forum Vol. 34(6) [Nov.Dec. 2008]
Stimuli to the Revision Process
Laboratory personnel should be aware of some special considerations when they choose the two-stage test: 1. First, if a second stage is required, the assembly has not failed the test after the rst stage. It is still a test of 12 to 16 tablets, depending on the number of positions in the assembly. The assembly can be considered to have failed only after the second stage, i.e., only after the full complement of 12 to 16 tablets is tested. 2. The laboratory has the option of stopping the test after the rst stage if it sees a problem in the assemblys operation. It is not a requirement of the PVT to complete the second stage in such a circumstance. However, after taking action(s) to x the problem, the laboratory must start the PVT again at Step 1. 3. The choice of using the basic single-stage test or the twostage test must be made as a matter of company policy and documented in standard procedures for the PVT. The choice of single- or two-stage test can differ with respect to different apparatus. Implementation USP is implementing two changes in its dissolution PVT. First, Salicylic Acid tablets will be discontinued as an ofcial USP RS for use in a PVT. The discontinuation is proposed as a revision to h711i in PF 34(5) with a targeted ofcial date at the end of 2009. USP has sufcient inventory of the Salicylic Acid tablets to meet industry needs during this period. Adequate information will be made available to users to allow a smooth transition, and USPs remaining PVT tablets (Predisone and Clorpheniramine Maleate Extended-Release RS tablets) will continue to be supplied as before. The second is the change in the design of the PVT study, increasing from 612 tablets to 1216, and the form of the accept/reject decision rule described herein. Although these are two separate decisions, USP has chosen to link their implementation in order to simplify the process of changing USP documents and laboratory SOPs. To implement both changes, USP will be publishing changes to General Chapter h711i to remove the test using Salicylic Acid and to remove the per-tablet approach. These changes are to USP text only and do not inuence the harmonized portions of this chapter. A new lot, Q, of the Prednisone RS tablets is in production. As was the case for prior lots, a collaborative study will be conducted to determine the PVT acceptance ranges. Both the new and current (per-tablet) acceptance ranges will be determined for Lot Q and will be proposed to USPs Biopharmaceutics and Reference Standards Expert Committees for endorsement and approval. The current plan is that the per-tablet accept/reject approach will continue as ofcial at the time of release of the Lot Q series of Prednisone RS tablets in early 2009. The new accept/reject approach, as described here, will then become ofcial for Apparatus 1 and 2 in late 2009 after a period of time during which users will have an opportunity to become more familiar with the changes. The ofcial date for the change for Apparatus 3 has not been decided. Once the new approach becomes ofcial for an apparatus, the per-tablet approach will no longer be valid for that apparatus. Actual implementation will be executed when USP states the new approach and acceptance ranges on the data sheets shipped with the reference standards. Removal of Salicylic Acid as a PVT test for Apparatus 1 and 2 will occur on 1 December 2009, the date when changes published in USP 32, Supple-
ment 2, become ofcial. USP will make available spreadsheets (compendial tools) similar to those illustrated by Figures 1 and 2. Laboratory staff will be able to enter their data in the spreadsheet, which will automatically display whether the data meet the criteria or do not meet the criteria. The available USP toolkit (10) also will be modied accordingly. USP may prepare additional training materials to assist users in the transition. SUMMARY In 2009 the accept/reject approach for the dissolution PVT will change from the basis of per-tablet results to the basis of the mean and variability of results from a set of RS tablets. The change brings the USP standard into alignment with ISO International Standard 5725 (7) and advances improvement in measurement science. With the current criteria it is possible for some of the tablets to just pass at the low end of the criteria and the remainder to just pass at the high end (e.g., for Apparatus 2, three results at 30% and three at 57%). Although they technically meet the current PVT acceptance criteria, such results clearly indicate a problem with the assemblys operation/ performance. With the new approach, the requirement regarding the %CV ensures sufcient consistency of results across positions in the assembly. Reliance on the mean ensures that those consistent results are within an acceptable interval of percent dissolved as determined from the RS collaborative study. The new approach will replace the current one, so individual tablet results no longer will be considered in evaluation of whether an assembly satises stipulations of a PVT.
REFERENCES
Deng G, Ashley AJ, Brown WE, et al. The USP performance verication test, part I: quality attributes and experimental variables contributing to dissolution variance. Pharm Res . 2008;25(5):11001109. 2. Glasgow M, Dressman S, Brown WE, et al. The USP performance verication test, part II: collaborative study of USPs Lot P Prednisone Tablets. Pharm Res. 2008;25(5):11101115. 3. Hauck WW, Manning RG, Cecil TL, Brown WE, Williams RL. Proposed change to acceptance criteria for dissolution performance verication testing. Pharm Forum. 2007;33(3):574579. 4. Hauck WW, Cecil TL, Brown WE, Abernethy DR, Koch WF, Williams RL. USP responses to comments on Stimuli article, Proposed change to acceptance criteria for dissolution performance verication testing. Pharm Forum. 2008;34(2):474 476. 5. USP. USP 31NF 26, Dissolution h711i. Rockville, MD: USP; 2008:267274. 6. USP. USP 31NF 26, Uniformity of Dosage Units h905i. Rockville, MD: USP; 2008:363369. 7. ISO. ISO 5725-6:1994, Accuracy (Trueness and Precision) of Measurement Methods and ResultsPart 6: Use in Practice of Accuracy Values. Geneva, Switzerland: ISO; 1994. 8. ISO. ISO/TS 21748:2004, Guidance for the Use of Repeatability, Reproducibility, and Trueness Estimates in Measurement Uncertainty Estimation. Geneva, Switzerland: ISO; 2004. 9. USP. USP 31NF 26, General Notices, Signicant Figures, and Tolerances. Rockville, MD: USP; 2008:4. 10. USP. Dissolution Toolkit. Available at: http://www.usp.org/ USPNF/compendialTools.html. Accessed July 16, 2008. 1.
# 2008
The United States Pharmacopeial Convention, Inc.
All Rights Reserved.
STIMULI TO THE REVISION PROCESS
Pharmacopeial Forum Vol. 34(6) [Nov.Dec. 2008]
Stimuli articles do not necessarily reect the policies of the USPC or the USP Council of Experts
1635
APPENDIX A: MEMBERS OF USPS BIOPHARMACEUTICS EXPERT COMMITTEE Thomas S. Foster, PharmD (Chair) Professor, University of Kentucky Medical Center James E. Polli, PhD (Vice Chair) Professor, University of Maryland Diane J. Burgess, PhD Professor of Pharmaceutics, University of Connecticut, School of Pharmacy G. Bryan Crist, BS Scientic Affairs Manager, Varian, Inc. Mario A. Gonzalez, PhD President, PKinetics International, Inc. Vivian A. Gray, BS President, V.A. Gray Consulting, Inc. Johannes Kraemer, PhD CEO, Phast Lewis J. Leeson, PhD President, LJL Associates Inc. Alan F. Parr, PharmD, PhD Director, Biopharmaceutics, GlaxoSmithKline
Stimuli to the Revision Process
Leon Shargel, PhD President, Applied Biopharmaceutics Eli Shefter, PhD Chief Scientic Ofcer, IriSys R & D W. Craig Simon, PhD Manager, Bureau of Pharmaceutical Sciences Health Canada Nhan L.Tran, PhD Senior Associate, Lachman Consultant Services, Inc Clarence T. Ueda, PharmD, PhD Professor of Pharmaceutical Sciences, University of Nebraska College of Pharmacy
# 2008
The United States Pharmacopeial Convention, Inc.
All Rights Reserved.
Potrebbero piacerti anche
- Crossover Designs: Testing, Estimation, and Sample SizeDa EverandCrossover Designs: Testing, Estimation, and Sample SizeNessuna valutazione finora
- Dissolution Calibration NetDocumento7 pagineDissolution Calibration Net0921pyNessuna valutazione finora
- Mathematical Models of Drug Dissolution PDFDocumento9 pagineMathematical Models of Drug Dissolution PDFClaudia GarcíaNessuna valutazione finora
- Laboratory Manual PHM 2143 Physical Pharmacy Ii: Department of Pharmaceutical TechnologyDocumento10 pagineLaboratory Manual PHM 2143 Physical Pharmacy Ii: Department of Pharmaceutical TechnologyYuppie RajNessuna valutazione finora
- Users Guide CUDALDocumento52 pagineUsers Guide CUDALdrs_mdu48Nessuna valutazione finora
- S53 - FPSC Batla HouseDocumento1 paginaS53 - FPSC Batla HousenamanNessuna valutazione finora
- S.B. Murray Et Al - The Role of Energy Distribution On The Transmission of DetonationDocumento30 pagineS.B. Murray Et Al - The Role of Energy Distribution On The Transmission of DetonationOlmeaNessuna valutazione finora
- V2 EXP2 Logic Valve and Sequence Control For Multiple Cylinder OperationDocumento12 pagineV2 EXP2 Logic Valve and Sequence Control For Multiple Cylinder OperationGeetha Sai KumarNessuna valutazione finora
- SMART Crack-Growth Assumptions and LimitationsDocumento3 pagineSMART Crack-Growth Assumptions and LimitationsAlexgh1993Nessuna valutazione finora
- Mpif 35-2007 粉末冶金结构零件材料标准简介Documento80 pagineMpif 35-2007 粉末冶金结构零件材料标准简介Jou0411Nessuna valutazione finora
- Mathematical Modeling of An Aqueous Film Coating Process in A Bohle Lab-Coater, Part 1: Development of The ModelDocumento8 pagineMathematical Modeling of An Aqueous Film Coating Process in A Bohle Lab-Coater, Part 1: Development of The Modelmido nasseNessuna valutazione finora
- AADE 03 NTCE 35 PowerDocumento9 pagineAADE 03 NTCE 35 PowerAmalina SulaimanNessuna valutazione finora
- ANSI Gases Combustibles y VaporesDocumento216 pagineANSI Gases Combustibles y VaporesHiram FloresNessuna valutazione finora
- LC Handbook Complete 2Documento163 pagineLC Handbook Complete 2KrishnaNessuna valutazione finora
- MEDDEV 2.71 revision 4 - 국문번역본Documento66 pagineMEDDEV 2.71 revision 4 - 국문번역본박성민Nessuna valutazione finora
- TG 244 Anal Write UpDocumento14 pagineTG 244 Anal Write Upapi-347149977Nessuna valutazione finora
- Chapter 12Documento44 pagineChapter 12Muhammad Nauman UsmaniNessuna valutazione finora
- ZhangHuichen EG4301 Asessment1Documento10 pagineZhangHuichen EG4301 Asessment1ZhangHuiChenNessuna valutazione finora
- Dissolution Profile ComparisonDocumento17 pagineDissolution Profile Comparisondipti_srivNessuna valutazione finora
- Technical Report For SIWES PDFDocumento102 pagineTechnical Report For SIWES PDFTanuj HandaNessuna valutazione finora
- 2.2.48. Raman SpectrometryDocumento2 pagine2.2.48. Raman Spectrometrymihazav100% (1)
- Prospectus Keam12Documento128 pagineProspectus Keam12jacob_thomas_68Nessuna valutazione finora
- Dissolved Gas Analysis of Transformer: Cellulose Insulation Carbon OxidesDocumento2 pagineDissolved Gas Analysis of Transformer: Cellulose Insulation Carbon OxidesheroNessuna valutazione finora
- GC Solution Software User Basics: Real Time AnalysisDocumento7 pagineGC Solution Software User Basics: Real Time AnalysisZetsu MandaNessuna valutazione finora
- Meclizine HCLDocumento10 pagineMeclizine HCLChEng_Nessuna valutazione finora
- Short-Term Forecasting Model For Electric Power Production of Small-HydroDocumento8 pagineShort-Term Forecasting Model For Electric Power Production of Small-HydroNuno LeaoNessuna valutazione finora
- Quality Information Summary (Qis)Documento12 pagineQuality Information Summary (Qis)Arham AhmedNessuna valutazione finora
- Tutorial 5Documento16 pagineTutorial 5anhntran4850Nessuna valutazione finora
- 9 Air in Leakage AilDocumento38 pagine9 Air in Leakage AilprakashNessuna valutazione finora
- Long-Term Stability (Zone IV) 30-75%RHDocumento6 pagineLong-Term Stability (Zone IV) 30-75%RHমোঃ এমদাদুল হকNessuna valutazione finora
- 263-Article Text-921-2-10-20190727Documento9 pagine263-Article Text-921-2-10-20190727ankit AcharyaNessuna valutazione finora
- Iso Astm 52701-13 PDFDocumento10 pagineIso Astm 52701-13 PDFAhmed LabibNessuna valutazione finora
- Analytical Profile Index: Product Characterisation of TestDocumento7 pagineAnalytical Profile Index: Product Characterisation of Testndunghua100% (1)
- 〈665〉 Plastic Components and Systems Used to Manufacture PharmaceuticalDocumento9 pagine〈665〉 Plastic Components and Systems Used to Manufacture Pharmaceuticalmehrdarou.qaNessuna valutazione finora
- Hydrogen Content of Gases by Gas ChromatographyDocumento3 pagineHydrogen Content of Gases by Gas ChromatographyDavinNessuna valutazione finora
- Saving Time in The HPLC Lab: SoftwareDocumento12 pagineSaving Time in The HPLC Lab: SoftwareMerck Millipore Brasil - Lab Supply BrasilNessuna valutazione finora
- ACTD PartIVClinical Nov05Documento50 pagineACTD PartIVClinical Nov05TueNessuna valutazione finora
- Pharma - 2015-16 PDFDocumento108 paginePharma - 2015-16 PDFclive.mascarenhas909Nessuna valutazione finora
- APSSSR, 96 AtthapuDocumento64 pagineAPSSSR, 96 AtthapuAtthapu ThirupathaiahNessuna valutazione finora
- Inive-IBPSA-BS05 0603 608Documento6 pagineInive-IBPSA-BS05 0603 608Palwasha MalikNessuna valutazione finora
- Comparison of The Solubilization Effect of Micronized PoloxamersDocumento1 paginaComparison of The Solubilization Effect of Micronized Poloxamerssaeedazadi1352Nessuna valutazione finora
- European Journal of Biomedical AND Pharmaceutical SciencesDocumento14 pagineEuropean Journal of Biomedical AND Pharmaceutical SciencesSACHIN BHASKAR NARKHEDENessuna valutazione finora
- Adsorption and Desorption of Carbon Dioxide and Nitrogen On Zeolite 5A PDFDocumento19 pagineAdsorption and Desorption of Carbon Dioxide and Nitrogen On Zeolite 5A PDFBình Tân LêNessuna valutazione finora
- 5977 MSD and MassHunter Acquisition EFamiliarization ChecklistDocumento10 pagine5977 MSD and MassHunter Acquisition EFamiliarization ChecklistcamiloviviNessuna valutazione finora
- Dissoultion Profile ComparisonDocumento2 pagineDissoultion Profile ComparisonSondellNessuna valutazione finora
- Final CTRL-2 ReportDocumento26 pagineFinal CTRL-2 ReportKongWeiHernNessuna valutazione finora
- CEIC3002 Project Description 2011Documento6 pagineCEIC3002 Project Description 2011MF_WANZNessuna valutazione finora
- Prosonix - Sonocrystallization Proven Across Scale - 2009Documento10 pagineProsonix - Sonocrystallization Proven Across Scale - 2009Joshua JohnsonNessuna valutazione finora
- Tutorial On PLS and PCADocumento17 pagineTutorial On PLS and PCAElsa George100% (1)
- Applied Biopharmacy Exercises by MteDocumento19 pagineApplied Biopharmacy Exercises by MteMINANI TheobaldNessuna valutazione finora
- CPK Guide 0211 TECH1Documento11 pagineCPK Guide 0211 TECH1Mark LacroNessuna valutazione finora
- Calcipotriol+Betamethasone OintmentDocumento50 pagineCalcipotriol+Betamethasone OintmentJai MurugeshNessuna valutazione finora
- BCH314 Tutorial#1Documento1 paginaBCH314 Tutorial#1mondeNessuna valutazione finora
- How Do I Develop Analytical HPLC MethodDocumento25 pagineHow Do I Develop Analytical HPLC MethodDrkrishnasarma pathyNessuna valutazione finora
- Spectrophotometric Method For Simultaneous Estimation of Levofloxacin and Ornidazole in Tablet Dosage FormDocumento14 pagineSpectrophotometric Method For Simultaneous Estimation of Levofloxacin and Ornidazole in Tablet Dosage FormSumit Sahu0% (1)
- 130801512SHA-001 TRDocumento17 pagine130801512SHA-001 TRUsman AliNessuna valutazione finora
- Weaning From Mechanical Ventilation: Moderator - Dr. Arvind Khare Presented by - Dr. Aji Kumar JLN Medical College, AjmerDocumento28 pagineWeaning From Mechanical Ventilation: Moderator - Dr. Arvind Khare Presented by - Dr. Aji Kumar JLN Medical College, AjmerDiadema BorjaNessuna valutazione finora
- Application For Local Transaction of Dangerous Drugs New 23aug2016Documento1 paginaApplication For Local Transaction of Dangerous Drugs New 23aug2016SHARIE GRACE A. RAJIS100% (2)
- Chm2045 Final ADocumento2 pagineChm2045 Final AChelsea LawrenceNessuna valutazione finora
- 068 ProNetDocumento117 pagine068 ProNethuynhhaichauchauNessuna valutazione finora
- CHAPTER 8 f4 KSSMDocumento19 pagineCHAPTER 8 f4 KSSMEtty Saad0% (1)
- ProAct ISCDocumento120 pagineProAct ISCjhon vergaraNessuna valutazione finora
- Om Deutz 1013 PDFDocumento104 pagineOm Deutz 1013 PDFEbrahim Sabouri100% (1)
- POB Ch08Documento28 paginePOB Ch08Anjum MalikNessuna valutazione finora
- Precision Medicine Care in ADHD The Case For Neural Excitation and InhibitionDocumento12 paginePrecision Medicine Care in ADHD The Case For Neural Excitation and InhibitionDaria DanielNessuna valutazione finora
- Unmasking Pleural Mesothelioma: A Silent ThreatDocumento11 pagineUnmasking Pleural Mesothelioma: A Silent ThreatCathenna DesuzaNessuna valutazione finora
- Cataloge ICARDocumento66 pagineCataloge ICARAgoess Oetomo100% (1)
- The Preparation of Culture MediaDocumento7 pagineThe Preparation of Culture MediaNakyanzi AngellaNessuna valutazione finora
- Sociology/Marriage PresentationDocumento31 pagineSociology/Marriage PresentationDoofSadNessuna valutazione finora
- 1979 The Cult Phenomenon in The United States - DR John Gordon ClarkDocumento8 pagine1979 The Cult Phenomenon in The United States - DR John Gordon ClarkFrederick BismarkNessuna valutazione finora
- DIP Lecture1Documento12 pagineDIP Lecture1Manish SandilyaNessuna valutazione finora
- Estrogen Dominance-The Silent Epidemic by DR Michael LamDocumento39 pagineEstrogen Dominance-The Silent Epidemic by DR Michael Lamsmtdrkd75% (4)
- Bartos P. J., Glassfibre Reinforced Concrete - Principles, Production, Properties and Applications, 2017Documento209 pagineBartos P. J., Glassfibre Reinforced Concrete - Principles, Production, Properties and Applications, 2017Esmerald100% (3)
- HEAS 1000 Health Assessment: Reflection of PracticeDocumento4 pagineHEAS 1000 Health Assessment: Reflection of PracticePreet ChahalNessuna valutazione finora
- Celitron ISS 25L - Product Spec Sheet V 2.1 enDocumento9 pagineCelitron ISS 25L - Product Spec Sheet V 2.1 enyogadwiprasetyo8_161Nessuna valutazione finora
- S108T02 Series S208T02 Series: I (RMS) 8A, Zero Cross Type Low Profile SIP 4pin Triac Output SSRDocumento13 pagineS108T02 Series S208T02 Series: I (RMS) 8A, Zero Cross Type Low Profile SIP 4pin Triac Output SSRnetiksNessuna valutazione finora
- Practice Test For Exam 3 Name: Miguel Vivas Score: - /10Documento2 paginePractice Test For Exam 3 Name: Miguel Vivas Score: - /10MIGUEL ANGELNessuna valutazione finora
- Evidence Based DesignDocumento4 pagineEvidence Based Designmartinadam82Nessuna valutazione finora
- InotroposDocumento4 pagineInotroposjuan camiloNessuna valutazione finora
- Meditran SX Sae 15w 40 API CH 4Documento1 paginaMeditran SX Sae 15w 40 API CH 4Aam HudsonNessuna valutazione finora
- LYON Conditions of Secondment 3500EUR enDocumento4 pagineLYON Conditions of Secondment 3500EUR enabdu1lahNessuna valutazione finora
- TCM Reach TrucksDocumento5 pagineTCM Reach TrucksMuhammad SohailNessuna valutazione finora
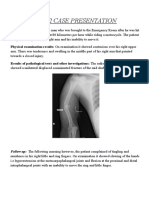
- PBL 2 Case PresentationDocumento12 paginePBL 2 Case PresentationRamish IrfanNessuna valutazione finora
- NCP Ineffective Breathing ActualDocumento3 pagineNCP Ineffective Breathing ActualArian May Marcos100% (1)
- ZL Ap381Documento10 pagineZL Ap381micyNessuna valutazione finora
- DET Tronics: Unitized UV/IR Flame Detector U7652Documento2 pagineDET Tronics: Unitized UV/IR Flame Detector U7652Julio Andres Garcia PabolaNessuna valutazione finora
- Laboratory Medicine Internship BookletDocumento95 pagineLaboratory Medicine Internship BookletMuhammad Attique100% (1)
- We Find The Way: Shipping InstructionsDocumento10 pagineWe Find The Way: Shipping InstructionsLuke WangNessuna valutazione finora
- CatalogDocumento12 pagineCatalogjonz afashNessuna valutazione finora
- Dif Stan 3-11-3Documento31 pagineDif Stan 3-11-3Tariq RamzanNessuna valutazione finora