Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Diagnostic Assessment of Patients With Interstitial Lung Disease
Caricato da
phobicmdTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Diagnostic Assessment of Patients With Interstitial Lung Disease
Caricato da
phobicmdCopyright:
Formati disponibili
Copyright PCRS-UK - reproduction prohibited
Prim Care Respir J 2011; 20(2): 120-127
REVIEW
Diagnostic assessment of patients with interstitial lung disease
*Mridu Gulatia
a
Department of Pulmonary & Critical Care, Yale University School of Medicine, New Haven, Connecticut, USA
Originally submitted 20th November 2009; resubmitted 25th January 2010; revised version received 14th July 2010; accepted 20th September 2010; online 20th April 2011
Abstract
The diagnosis of interstitial lung disease (ILD) is frequently delayed because clinical clues are neglected and respiratory symptoms are ascribed to more common pulmonary diagnoses such as chronic obstructive pulmonary disease (COPD) in the primary care setting. While ILD cases ultimately require referral to a pulmonologist, general practitioners can play a crucial role in recognising the need for, and initiating, a diagnostic evaluation. An initial assessment hinges upon a structured history and physical examination with careful attention paid to occupational, environmental and drug exposures as well as a history of symptoms suggesting connective tissue disease. Ultimately a surgical lung biopsy may be indicated, but high resolution computed tomography (HRCT) chest scans are essential to the diagnostic work-up since each ILD form is characterised by a specific pattern of abnormalities. 2011 Primary Care Respiratory Society UK. All rights reserved. M Gulati. Prim Care Respir J 2011; 20(2): 120-127 doi:10.4104/pcrj.2010.00079
Keywords Interstitial Lung disease (ILD), Idiopathic Interstitial Pneumonia, Idiopathic Pulmonary Fibrosis (IPF), Connective Tissue
Disease, Hypersensitivity Pneumonitis, Nonspecific Interstitial Pneumonia (NSIP), High Resolution CT (HRCT) Scan
Contents
Introduction .......................................................................................................................................................................... Definition .............................................................................................................................................................................. Classification ........................................................................................................................................................................ Prevalence ............................................................................................................................................................................. Diagnostic tools .................................................................................................................................................................... Early recognition of an ILD diagnosis .............................................................................................................................. History ................................................................................................................................................................................ 1. General ...................................................................................................................................................................... 2. Occupational & environmental exposures and drug exposures ............................................................................ 3. Connective tissue disease ......................................................................................................................................... Physical examination ......................................................................................................................................................... Pulmonary function testing .............................................................................................................................................. High resolution CT scan .................................................................................................................................................... Blood tests ......................................................................................................................................................................... Biopsy ................................................................................................................................................................................. Conclusion ............................................................................................................................................................................ References ............................................................................................................................................................................
rim R a ep ry ro C du ar ct e R io e n sp pr ir oh at ib ory ite S d o
ci et
120 121 121 121 122 122 122 122 123 124 124 124 125 126 126 127 127
op y
rig
ht P
Introduction
Although interstitial lung disease (ILD) patients account for approximately 15% of the respiratory disease in general practice, ILD diagnosis can be delayed since clinicians often neglect early clinical clues or ascribe respiratory symptoms to more common pulmonary conditions such as chronic
obstructive pulmonary disease (COPD). Primary care providers can play a critical role in expediting an ILD work-up by initiating an appropriate diagnostic evaluation. An initial work-up begins with a comprehensive history and physical examination with a meticulous occupational, environmental and drug history as well as an evaluation of any symptoms
* Corresponding author: Dr Mridu Gulati, Department of Pulmonary & Critical Care, Yale University School of Medicine, 300 Cedar Street, TAC 441S, New Haven Connecticut 06520, USA. Tel: +1 203 785 2509 Fax: +1 203 785 3826 E-mail: elizabeth.tarquino@yale.edu
PRIMARY CARE RESPIRATORY JOURNAL www.thepcrj.org doi:10.4104/pcrj.2010.00079
120
http://www.thepcrj.org
Copyright PCRS-UK - reproduction prohibited
Diagnostic assessment of patients with ILD
suggesting connective tissue disease. Whilst a pulmonlogist may recommend a surgical lung biopsy, referrers can continue a diagnostic assessment by ordering high resolution computed tomography (HRCT) chest scans. Each ILD form is characterised by a specific pattern of HRCT abnormalities.
Figure 1. Classification of interstitial lung disease.
Connective tissue diseases: Sclerodema Polymyositis Occupational: Asbestos Chronic beryllium disease
Definition
Interstitial lung disease (ILD), also referred to as diffuse parenchymal lung disease, refers to a heterogenous group of over 150 unrelated disorders. A distinct clinical presentation as well as radiographic and pathologic findings characterises each ILD form. Several key differences between the clinical presentation of the more frequent obstructive lung disease diagnoses and an ILD diagnosis are due to differences in the anatomic disease distribution. In most cases the pathology of ILD lies in the pulmonary interstitium, which consists primarily of connective tissue and refers to the space between the alveolar epithelial cells and the adjacent capillary endothelial cells. Therefore, in contrast to the wheezing caused by exhaling air through diffusely inflamed airways in asthmatic patients, clinicians detect inspiratory crackles in ILD patients, a finding caused by the opening of collapsed alveolar spaces surrounded by a fibrotic interstitium.
KNOWN
Idiopathic interstitial pneumonia: IPF NSIP Other
UNKNOWN Environmental: Hypersensitivity pneumonitis (mold, birds)
Medications: Amiodarone Nitrofurantoin
Classification
The American Thoracic Society has published an ILD classification scheme.1 While this classification scheme is based on histopathology, the most practical way to classify ILD is to divide between known versus unknown causes, or non-idiopathic versus idiopathic forms (see Figure 1). Referring practitioners can help detect non-idiopathic forms of ILD by reviewing relevant occupational, environmental and drug exposures as well as assessing for the presence of extrapulmonary symptoms suggestive of connective tissue disease. The non-idiopathic forms tend to have a better prognosis and response to therapy for example, for patients with hypersensitivity pneumonitis, cessation of exposure to causative antigens such as avian proteins or mould can lead to immediate substantial improvement. Patients with ILD related to connective tissue disease usually present with the histopathologic entity referred to as nonspecific interstitial pneumonia and have a better response to immunosuppressive therapy than patients with other forms of idiopathic ILD. The idiopathic ILDs encompass several disease categories ranging from granulomatous diseases (which include sarcoidosis) to idiopathic interstitial pneumonias which include the well recognised entity of idiopathic pulmonary fibrosis (IPF). Mastering the ILD alphabet soup is unnecessary, and it is only necessary to remember a few essential points. Sarcoidosis and idiopathic pulmonary fibrosis (IPF) are the two most common forms of idiopathic ILD, and the severity of sarcoidosis ranges
widely. In fact, some sarcoidosis patients may be completely asymptomatic despite the presence of floridly abnormal chest radiographs typically characterised by impressive mediastinal adenopathy. Symptomatic sarcoid patients often respond dramatically to steroids or other immunosuppressive agents. Idiopathic pulmonary fibrosis is another common form of an idiopathic ILD. Rather than being the end stage of all other ILDs, IPF is a specific form of an idiopathic interstitial pneumonia that usually occurs in older patients and has characteristic HRCT findings (peripheral and subpleural bibasilar reticulonodular opacities in association with traction bronchiectasis and honeycombing) and lung biopsy findings (temporal heterogeneity, honeycombing and fibroblastic foci). In general, IPF is characterised by more scarring and less inflammation and has a poorer prognosis than most other ILD forms. Although the sheer number of different types of ILD can be overwhelming, differentiation between the various forms is critical given the significant variability in treatment response and prognosis. While steroid and immunosuppressive therapy are effective for certain ILD forms such as sarcoidosis, no effective drug therapy exists for idiopathic pulmonary fibrosis. Given their poor prognosis and a median survival of three to five years, IPF patients are frequently referred early for lung transplantation evaluation.
op y
rig
ht P
PRIMARY CARE RESPIRATORY JOURNAL www.thepcrj.org
rim R a ep ry ro C du ar ct e R io e n sp pr ir oh at ib ory ite S d o
Prevalence
Although less frequent than COPD and asthma, ILD accounts for 15% of the respiratory disease in general practice. Few epidemiological studies exist. However, a frequently cited population-based registry from New Mexico reports a prevalence of 80.9 per 100,000 in males and 67.2 per 100,000 in females.2 IPF and sarcoidosis are the two most common ILD diagnoses. Occupationally-related ILD and connective tissue disease-associated ILD may each account for approximately 15% of all forms of ILD.
121
http://www.thepcrj.org
ci et
Granulomatous: Sarcoidosis
Other: Vasculitides Eosinophilc
Copyright PCRS-UK - reproduction prohibited
M Gulati
Diagnostic tools
Early recognition of an ILD diagnosis
The following section summarises the critical components of an ILD diagnostic work-up (Table 1). As highlighted in this section, general practitioners (GPs) can help expedite an ILD work-up simply by recognising early clues in a patients clinical presentation. Each ILD entity classically presents as an acute, subacute or chronic illness (see Table 2). Many ILD diagnoses such as IPF and sarcoidosis, are chronic diseases, and ILD patients are frequently misdiagnosed as having COPD, particularly if there is a prior smoking history. Progressive dyspnoea and non-productive cough over years despite smoking cessation or in the absence of a smoking history as well as a lack of response to traditional inhaler therapy can suggest ILD. On physical examination, the absence of wheezing and the presence of inspiratory crackles may indicate ILD. IPF but not COPD patients have clubbing. Clinicians routinely order chest radiographs in patients
Table 1. Diagnostic tools in patients with suspected interstitial lung disease history.
Demographics Age >50 years: 20-40 years:
with chronic respiratory symptoms. Since COPD patients have largely normal chest radiographs, the presence of even the most subtle abnormalities such as increased interstitial markings or nodular opacities suggests ILD. Frequently, radiologists mistake chronic radiographic abnormalities in ILD for acute viral or bacterial pneumonias. Persistence of abnormalities despite antibiotic therapy and the lack of infectious symptoms suggest an ILD diagnosis. Patients with chronic respiratory disease or symptoms should undergo full pulmonary function testing; COPD patients have an obstructive pattern while ILD patients have a restrictive pattern. Finally, although ILD patients have normal resting oxygen saturations, ILD patients can become profoundly hypoxaemic on even minimal exertion.
Idiopathic Pulmonary Fibrosis, Cryptogenic Organizing Pneumonia (BOOP)
Sarcoidosis, Connective Tissue Disease Associated ILD, Sarcoidosis, Lymphangioleimyomatosis, Pulmonary Langerhans Cell Histiocytosis Connective Tissue Disease Associated ILD; Lymphangioleiomyomatosis
Gender Female: Male: Family History Exposures
ht P
Occupational Pneumoconiosis; Idiopathic Pulmonary Fibrosis
rim R a ep ry ro C du ar ct e R io e n sp pr ir oh at ib ory ite S d o
Acute (hours to several days) Exposures Acute lung injury Drugs Fumes and toxins Acute interstitial pneumonitis
Once suspected, historical clues alone may implicate a specific ILD diagnosis (see Table 1). Information regarding demographics as well as occupational and environmental exposures, medications, smoking status and connective tissue disease history is invaluable.3,4 Simple demographic data can be useful; for example, IPF patients are typically older and male while patients with connective tissue disease-associated ILD are younger and female. Sarcoidosis patients are younger. There is a higher prevalence of sarcoidosis among African Americans. Extrapulmonary manifestations and chronic progressive disease are also more frequent in African Americans with sarcoidosis.
Table 2. Clinical tempo.
Subacute (weeks to months) Exposures Drug Toxicity Hypersensitivity Pneumonitis Chronic (many months to years) Exposures Drug Toxicity Hypersensitivity pneumonitis Smoking Pneumoconioses Nonspecific interstitial pneumonia (related to CTD, exposure, or Idiopathic) Sarcoidosis Cryptogenic organizing Pneumonia Chronic eosinophilic pneumonia Acute eosinophilic pneumonia Vasculitis Nonspecific interstitial pneumonia (related to CTD, exposure, or Iodiopathic) Sarcoidosis Idiopathic pulmonary fibrosis (usual interstitial pneumonia)
Connective Tissue Disease Diagnosis or Symptoms Occupational Exposures Environmental Exposures Drugs Smoking
Physical Examination
Lung Exam (ie.crackles, wheeze) Clubbing Signs of cor pulmonale Extrapulmonary signs Routine blood work (CBC, Chemistries, Liver function tests) Rheumatologic serologies Hypersensitivity pneumonitis panel Spirometry Lung Volumes Diffusing capacity Resting and ambulatory oxygen saturation Six minute walk distance Arterial blood gas Chest radiograph High Resolution Chest CT Scan Bronchoscopy Surgical Lung Biopsy
Laboratory Testing
Pulmonary Function Testing
op y
rig
Diffuse alveolar haemorrhage
Imaging Biopsy
PRIMARY CARE RESPIRATORY JOURNAL www.thepcrj.org
122
http://www.thepcrj.org
ci et
History 1. General
Copyright PCRS-UK - reproduction prohibited
Diagnostic assessment of patients with ILD
Except for extremely rare ILD forms such as Hermansky Pudlak or metabolic storage diseases, genetic associations only modestly increase the risk for more common ILD forms such as sarcoidosis and IPF. While smoking is a strong risk factor for certain rare forms of ILD such as Respiratory bronchiolitis-Interstitial Lung Disease/Desquamative Interstitial Pneumonia and Pulmonary Langerhans Cell Histiocytosis, smoking only slightly increases the risk for the much more prevalent disease of idiopathic pulmonary fibrosis.5
as causes of hypersensitivity pneumonitis, another nonidiopathic ILD category8 (see Table 5). In a recent Mayo Clinic series, exposure to birds and hot tubs (Mycobacterium avium complex exposure) accounted for 34% and 21% of hypersensitivity pneumonitis cases, respectively. Exposures to
Table 4: Examples of occupational exposures associated with interstitial lung disease.
Agent Fibrous Dust Asbestos Construction, building, mining, shipbuliding, automobile and railroad work, insulation Lower zone predominant reticular opacities, honeycombing, pleural disease Exposure Settings HRCT Findings
2. Occupational & environmental and drug exposures
Primary care clinicians enjoy longstanding relationships with patients and have a rich source of patient information regarding exposures (Table 3). Early detection of potential respiratory toxins can lead to early exposure cessation and clinical improvement. Although often indistinguishable from idiopathic ILD forms, occupational exposures may account for approximately 15% of ILD cases (Table 4). For example, asbestosis in a shipyard worker can be confused with IPF, and chronic beryllium disease in a nuclear industry worker can be mistaken for sarcoidosis. Since disease may present years after exposure cessation, all past and present jobs should be recorded.6,7 The presence of respiratory illnesses in co-workers, inconsistent use of protective respirators as well as poor industrial hygiene controls leading to a dusty work environment are frequently reported. Over 300 environmental exposures have been implicated
Table 3. Principals of occupational and environmental history.
rim R a ep ry ro C du ar ct e R io e n sp pr ir oh at ib ory ite S d o
Coal dust
ci et
Crystalline silica
Mining, construction, sandblasting, granite/stone work, road work, tunneling Coal mining
Inorganic nonfibrous dust
K
Upper zone predominant nodular disease, progressive massive fibrosis, hilar adenopathy Upper zone predominant nodular disease, progressive massive fibrosis Middle and upper zone predominant nodular infiltrates (similar to sarcoidosis)
Metals
Beryllium
Nuclear weapons, electronics, aerospace, ceramics, machining, dental prostheses, alloy machining
ht P
Complete employment history
Job Job activities and tasks Years worked Temporal association between symptoms and work and specific work activities
rig
Table 5. Examples of environmental exposures associated with hypersensitivity pneumonitis.
Disease Antigen Source
op y
Fungal and bacterial Farmers Lung Saccharopolyspora Moldy hay, grain rectivirgula, Thermactinomyces vulgaris Mycobacterium immunogenum Mycobacterium avium complex, Cladosporium Trichosporon cutaneum Bird droppings, serum feathers Isocyanates Metal working fluid Hot tubs, mold on ceiling Contaminated old houses Birds: parakeets, Pigeons, chickens Polyurethane foams Spray paints, Elastomers, special glues
Presence of sick coworkers Exposure Assessment of visible dust or odours on surfaces and in air Hours worked Engineering controls such as local exhaust ventilation Personal protective equipment (respirators) Materials worked (Material Safety Data sheets) Household Exposures or job activities of all household members (exposure to household members dusty work clothes) Hobbies Pets (birds) Water damage (mold exposure) Hot tub usage (Mycobacterium Avium Complex) Metal working fluid Hot tub lung
Summer type pneumonitis Animal proteins Pigeon breeder's disease Chemicals Chemical workers lung
PRIMARY CARE RESPIRATORY JOURNAL www.thepcrj.org
123
http://www.thepcrj.org
Copyright PCRS-UK - reproduction prohibited
M Gulati
Table 6. Symptoms associated with connective tissue disease.
Joint and muscle symptoms Arthritis, arthralgias, synovitis Myalgias, muscle weakness Neuropathy Raynauds Gastrointestinal symptoms (GERD) Sicca symptoms Skin Malar rash/Photosensitivity Machinist hands Gottrons papules Sclerodactyly, digital ulcers Telangiectasias Chest pain Eye abnormalities (uveitis,scleritis, ulcers)
PRIMARY CARE RESPIRATORY JOURNAL www.thepcrj.org
op y
rig
Contrary to sub-specialists who focus on specific organ systems, GPs assess the entire patient. This generalised approach is invaluable in the work-up of the suspected ILD patient since approximately 15-20% of ILD patients have a concomitant diagnosis of connective tissue disease. ILD may also be the initial presentation of connective tissue disease, and systematic manifestations may follow months to years after the appearance of pulmonary disease. GPs should conduct a careful review of extrapulmonary symptoms and assess for the presence of any symptom constellation associated with a particular connective tissue disease (Table 6). For example, scleroderma patients may report skin thickening (i.e. sclerodactyly), Raynauds phenomenon, and gastroesophageal reflux. Polymyositis/dermatomyositis patients may present with typical skin lesions (violaceous scaling papules Gottrons papules and facial heliotrope
ht P
rim R a ep ry ro C du ar ct e R io e n sp pr ir oh at ib ory ite S d o
124
Pulmonary function testing
Primary care clinicians can avoid diagnostic delays in ILD diagnosis by ordering complete pulmonary function testing in patients with chronic respiratory symptoms. Primary care offices frequently administer routine office spirometry. Reduced forced vital capacity (FVC) can suggest the presence of a restrictive lung disease and perhaps ILD, particularly given the appropriate clinical scenario; however, since COPD patients may also have reductions in FVC due to severe air trapping, GPs cannot confidently diagnose restrictive lung disease using office spirometry alone. Full pulmonary function testing, including the measurement of total lung capacity, is necessary to diagnose the presence of the restrictive ventilatory defect typically seen in ILD patients. Available in most pulmonary practices, pulmonary function testing includes spirometry, lung volumes and diffusing capacity and provides critical information regarding disease severity and prognosis.14,15 Whilst COPD patients have an obstructive ventilatory defect with a reduced forced expiratory volume in one second (FEV1) / FVC ratio, ILD patients have a restrictive ventilatory defect with reduced total lung capacity. An impaired diffusion capacity may be the first abnormality
http://www.thepcrj.org
ci et
3. Connective tissue disease
agricultural antigens found on farms and household mould exposure accounted for 11% and 9% of cases, respectively.9 Certain antigens (i.e. avian antigens) are more antigenic than others, and even common household exposures such as mould can cause hypersensitivity pneumonitis. Medications including antibiotics, chemotherapeutic agents and antiarrhythmic agents can cause ILD. Whilst the toxicity of drugs such as amiodarone is known, clinicians overlook the potential toxicity of novel therapeutics such as biologic agents or over-the-counter drugs. While primary care clinicians may easily discern acute temporal associations between drug administration and respiratory symptoms, the relationship between chronic drug exposure and ILD is often less apparent. Reactions can be dose-dependent or idiosyncratic.10 A database of drugs associated with pulmonary toxicity is available at www.pneumotox.com.11
rash), mechanics hands, and muscle disease.12,13 Patients with connective tissue disease-associated ILD often have a better response to immunosuppressive therapy and a better prognosis compared to patients with other ILD forms such as IPF. Furthermore, screening for connective tissue disease may lead to early treatment of other extrapulmonary systemic complications such as renal disease in scleroderma patients.
Physical examination
A thorough ILD work-up requires a comprehensive physical examination. Since ILD patients are often misdiagnosed with COPD, GPs should take note of physical exam findings that can differentiate a diagnosis of ILD from COPD. Most ILD patients do not have wheezing, a finding caused by airway disease, but they do have inspiratory crackles, a finding caused by the opening of collapsed alveolar spaces amidst a fibrotic interstitium. Clubbing is seen in certain forms of ILD such as IPF but is not found in COPD patients. Finally, the presence of extrapulmonary findings argues against a diagnosis of COPD. Certain constellations of findings point to specific diagnoses. For example, lymphadenopathy combined with certain skin findings such as erythema nodosum or lupus pernio suggest sarcoidosis. Arthralgias are common in ILD but active synovitis suggests connective tissue disease. Scleroderma patients typically have sclerodactyly, Raynaud's phenomenon, and telangiectatic lesions. Proximal muscle weakness along with typical skin lesions (violaceous scaling papules Gottrons papules, facial heliotrope rash) and mechanics hands may point to a diagnosis of Polymyositis/dermatomyositis.
Copyright PCRS-UK - reproduction prohibited
Diagnostic assessment of patients with ILD
present. The presence of oxygen desaturation at rest or with ambulation portends a poorer prognosis and can lead to treatment with oxygen supplementation. Six-minute walk tests detect the presence of oxygen desaturation during ambulation and distance walked.
High resolution CT scanning
During a routine work-up of respiratory symptoms, GPs often order plain chest radiographs (CXRs). Routine CXRs in ILD patients often have subtle abnormalities such as ground glass abnormalities, nodular opacities or increased reticular markings, and less experienced radiologists often miss such subtle findings. In addition to complete pulmonary function tests, GPs should order high resolution computed tomography (HRCT) chest scans, if available without specialist referral, for patients with suspected ILD. Compared to a standard CT Scan, HRCT provides superior spatial and contrast resolution.
Table 7: High resolution CT scan features in different forms of interstitial lung disease.
Diagnosis Typical HRCT Features Reticular, honeycombing, traction bronchiectasis
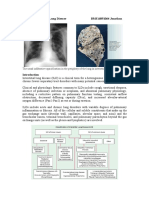
Figure 2. Idiopathic Pulmonary Fibrosis.
Idiopathic Pulmonary Fibrosis/Usual Interstitial Pneumonia Nonspecific Interstitial Pneumonia Cryptogenic Organizing Pneumonia Acute Interstitial Pneumonia Desquamative Interstitial Pneumonia Hypersensitivity Pneumonitis
Ground glass attenuation and reticular lines Consolidation
rig
Consolidation, ground glass Ground glass attenuation, reticular lines
ht P
op y
Ground glass, centrilobular nodules, air trapping (mosaic attenuation)
Sarcoidosis
Hilar adenopathy, nodular Upper and mid opacities, beading and lung zones thickening along peribronchiovascular bundles Reticular, honeycombing, traction bronchiectasis, pleural plaques marker of asbestos exposures Nodular opacities (may coalesce into nodules greater than 10 mm in size progressive massive fibrosis), hilar adenopathy with calcifications Lower lung zones
Asbestosis
Silicosis
PRIMARY CARE RESPIRATORY JOURNAL www.thepcrj.org
rim R a ep ry ro C du ar ct e R io e n sp pr ir oh at ib ory ite S d o
Typical Distribution on CT Peripheral, subpleural basal, lower lung zones Peripheral Peripheral, peribronchial Diffuse Lower lung zones
IPF HRCT: Subpleural reticular markings, traction bronchiectasis and honeycombing in a lower lobe distribution
Upper and mid lung zones
Figure 3. Sarcoidosis.
Upper and mid lung zones
Sarcoidosis HRCT: Hilar and mediastinal lymphadenopathy, beaded appearance and thickening of brochovascular bundles, nodules along bronchi
125
http://www.thepcrj.org
ci et
Abnormalities on HRCT can be assessed for patterns (e.g. reticular markings, consolidation, ground glass, nodules), anatomic distribution (i.e. peripheral vs. central, upper vs. lower), and associated findings (i.e. mosaic attenuation, hilar adenopathy). Intravenous contrast can help distinguish lymphadenopathy from surrounding vasculature in sarcoidosis patients but is not generally needed. Different HRCT patterns (Table 7) suggest different diagnoses.16-18 For example, an elderly patient with an insidious history of dry cough and dyspnoea who has an HRCT with findings classic for IPF the presence of subpleural reticular markings, traction bronchiectasis and honeycombing in a lower lobe distribution does not need a lung biopsy (see Figure 2). The presence of hilar adenopathy and nodules in a perilymphatic distribution in the upper and mid lung zones suggests sarcoidosis (Figure 3).
Copyright PCRS-UK - reproduction prohibited
M Gulati
General radiologists are familiar with radiographic patterns of IPF and sarcoidosis but often neglect subtle findings seen in other ILD forms such as ground glass abnormalities and centrilobular nodular distribution. Appreciating such details is critical since each ILD form has a distinct set of therapeutic options and prognosis. Hence, after ordering HRCTs, GPs should refer to a pulmonologist for a more accurate assessment.
Blood tests
Extensive laboratory testing in the ILD work-up is typically performed by a pulmonologist. Blood testing (Table 8) is useful in demonstrating the presence of systemic involvement, the detection of connective tissue disease, and in establishing a causative antigen in hypersensitivity pneumonitis patients. Initial blood testing includes a full differential blood count, serum urea, electrolytes, creatinine and liver function tests. Hypercalcemia is often seen in sarcoidosis patients. Renal involvement is seen in certain connective tissue diseases such as scleroderma and lupus. Although false negative and positive testing is common, autoantibody testing can help diagnose a coexisting connective tissue disease. A complete blood test work-up includes rheumatoid factor and antinuclear antibodies with extractable nuclear antigens Ro and La (Sjogrens), Scl-70
Table 8. Laboratory studies in the workup interstitial lung disease.
Laboratory Study
(scleroderma), Jo-l (Polymyositis/Dermatomyositis), and RNP (Mixed Connective Tissue Disease). Hypersensitivity pneumonitis bloods test for the presence of IgG antibodies to environmental antigens and differ from the IgE antibody testing performed in standard allergy blood testing. Most outpatient laboratories test for a small specific set of antibodies to several mould species and avian proteins. False positive testing and false negative testing is common. False positive testing may simply be a marker of exposure while false negative testing may be due to testing for the wrong antigen or removal from exposure. Given the high rates of false positives and negatives, the blood test results following autoantibody testing for connective and hypersensitivity pneumonitis should not supplant a clinical diagnosis.
Biopsy
A pulmonologist should address the decision regarding biopsy. Factors to be considered include HRCT findings, history of exposures, and the risks and benefits of an invasive procedure given the patients clinical status and patient preference.19 A diagnosis of IPF can be made without a biopsy in patients with a classic constellation of HRCT findings, including lower lobe predominance of abnormalities, traction bronchiectasis, honeycombing and subpleural reticular markings (Figure 2). Thus, a bird owner with an HRCT showing characteristic hypersensitivity pneumonitis features centrilobular nodules, ground glass opacities, air trapping, and abnormalities in an upper and mid lung zone distribution and positive serum precipitins, does not need a biopsy (Figure 4). If a biopsy is indicated, bronchoscopy with transbronchial biopsy does not usually yield sufficient tissue and a surgical lung biopsy is needed.20 Infrequently, bronchoscopy can be useful, such as in the diagnosis of sarcoidosis or hypersensitivity pneumonitis. When indicated, video assisted thorascopic surgery generally has a relatively low mortality rate of less than 2%.
Figure 4. Hypersensitivity Pneumonitis.
Most Common Disease Association
All Interstitial Lung Disease Patients Routine blood tests Full blood count Urea and creatinine Electrolytes Liver Function tests Bone screening including serum calcium level
Suspected Connective Tissue Disease ESR Any Connective Tissue Disease CRP Any Connective Tissue Disease ANA Any Connective Tissue Disease Anti ds-DNA (SLE) Systemic Lupus Erythematosis Anti-Smith Systemic Lupus Erythematosis Rheumatoid Factor Rheumatoid Arthritis Anti-cyclic citrullinated Rheumatoid Arthritis peptide antibody Anti-SSA/Ro Sjogrens Anti-SSB/La Sjogrens Anti Scl-70 Scleroderma Anti-RNP Mixed CTD Aldolase and myositis Polymyositis panel C, P-ANCA Wegeners, Microscopic polyangiitis Anti-GBM Goodpastures Hypersensitivity Pneumonitis Serum precipitins Hypersensitivity pneumonitis
op y
rig
ht P
PRIMARY CARE RESPIRATORY JOURNAL www.thepcrj.org
rim R a ep ry ro C du ar ct e R io e n sp pr ir oh at ib ory ite S d o
126
HP HRCT: Ground glass and centrilobular nodules in an upper mid lung zone distribution
http://www.thepcrj.org
ci et
Copyright PCRS-UK - reproduction prohibited
Diagnostic assessment of patients with ILD
rim R a ep ry ro C du ar ct e R io e n sp pr ir oh at ib ory ite S d o
Surgeons should obtain large lung tissue sample sizes and specimens should be reviewed by experienced pulmonary pathologists. Although the current ILD classification scheme is based on specific histopathology, problems with even this gold standard exist. Firstly, occupational, environmental and drug exposures have been associated with the development of histopathologic forms of ILD that are indistinguishable from the idiopathic ILDs for example, an asbestos worker may develop histopathologic findings similar to those found in IPF patients, and workers exposed to beryllium may develop a syndrome identical to sarcoidosis. Another challenge facing the histopathologic classification scheme is the substantial interobserver variability existing between pathologists, particularly with diagnoses other than sarcoidosis and IPF.21 Furthermore, biopsies from different lobes may reveal different histopathologies. This occurs typically in patients who have evidence of nonspecific interstitial pneumonia in one lobe and findings consistent with idiopathic pulmonary fibrosis in another lobe; the prognosis of these patients is similar to that seen in IPF patients.22 The limitations of the present classification scheme underscore the fact that even histopathology does not supplant a comprehensive history and physical examination that assesses for relevant exposures or a concomitant connective tissue disease diagnosis.
Committee, June 2001. Am J Respir Crit Care Med 2002;165(2):277-304. 2. 3. Coultas DB, Zumwall RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 1994;150(4):967-72. King TE Jr. Clinical advances in the diagnosis and therapy of the interstitial lung diseases. 4. Am J Respir Crit Care Med 2005;172(3):268-79. http://dx.doi.org/10.1164/rccm.200503-483OE Schwarz MI, King TE, Raghu G. Approach to the evaluation and diagnosis of interstitial lung disease. In: King TE, Schwarz MI, eds. Interstitial Lung Disease. Ontario, Canada: BC Decker; 2003:1-30. 5. Caminati A, Harari S. Smoking-related interstitial pneumonias and pulmonary Langerhans cell Histiocytosis. 6. 7. Proc Am Thorac Soc 2006;3(4):299-306. http://dx.doi.org/10.1513/pats.200512-135TK Glazer CS, Newman LS. Occupational interstitial lung disease. Clin Chest Med 2004;25(3):467-78. Neurnberg AM, Christiani DC. The future of occupationally related diffuse lung 8. 9. disease. Semin Respir Crit Care Med 2008;29(6):680-4. http://dx.doi.org/10.1055/s-0028-1101278 Chest Med 2004;25:531. http://dx.doi.org/10.1016/j.ccm.2004.04.001
Proceedings 2007;82(7):812-16. http://dx.doi.org/10.4065/82.7.812 10. Camus P, Bonniaud P, Fanton A, Camus C, Baudaun N, Foucher P. Druginduced and iatrogenic infiltrative lung disease. 2004;25(3):479-519. Clin Chest Med
11. www.pneumotox.com 12. Tzelespis GE, Toya SP. Moustopoulos HM. Occult connective tissue diseases mimicking idiopathic interstitial pneumonias. Eur Respir J 2008;31(1):11-20. Interstitial lung disease in the patient who has Clin Chest Med 2004;25(3)549-59. connective tissue disease. 13. Strange C, Highland KB.
http://dx.doi.org/10.1016/j.ccm.2004.05.009 Clin Chest Med 2004;25:435. http://dx.doi.org/
14. Lama VN, Martinez FJ. Resting and exercise physiology in interstitial lung diseases. 10.1016/j.ccm.2004.05.005 diseases. Respiration 2004;71:209. http://dx.doi.org/10.1159/000077416 tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med 2005;172(4):488-93. http://dx.doi.org/
Conclusions
Interstitial lung diseases encompass a heterogeneous collection of lung diseases and include diseases with known and unknown causes. While ILD cases often require referral to a pulmonologist, GPs can prevent diagnostic delays by recognising clinical clues, reviewing relevant exposures, and uncovering the presence of possible connective tissue disease, as well as ordering HRCT chest scans and pulmonary function tests if possible. Although GPs often first consider idiopathic ILD forms such as IPF, early recognition of possible non-idiopathic ILD can lead to the early cessation of possible toxic exposures as well as therapy with immunosuppressive therapy for patients with connective tissue disease-related ILD.
15. Chetta A, Marangio E, Olivieri D. Pulmonary function testing in interstitial lung 16. Lynch DA, David Godwin J, Safrin S, et al. High-resolution computed
rig
ht P
op y
10.1164/rccm.200412-1756OC 17. Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001;164(2):193-6. 18. Johkoh T, Muller NL, Cartier Y, et al. Idiopathic interstitial pneumonias: Diagnostic accuracy of thin-section CT in 129 patients. Radiology 1999; 211:555. 19. Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001;164(2):193-6. 20. King TE, Jr. Bronchoscopy in Interstitial Lung Disease. In: Textbook of Bronchoscopy, Feinsilver, SH, Fein, AM (Eds), Williams & Wilkins, Baltimore, 1995, p. 185. 21. Nicholson A, Addis B, Bharucha H, et al. Inter-observer variation between pathologists in diffuse parenchymal lung disease. Thorax 2004;59(6):500-05. http://dx.doi.org/10.1136/thx.2003.011734 22. Flaherty KR, Travis WD, Colby TV, et al. Histopathologic variability in usual and nonspecific interstitial pneumonia. Am J Respir Crit Care Med 2001;164(9): 1722-7.
Conflict of interest declaration
None.
References
1. American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive
Available online at http://www.thepcrj.org
PRIMARY CARE RESPIRATORY JOURNAL www.thepcrj.org
127
http://www.thepcrj.org
ci et
consecutive patients with hypersensitivity pneumonitis. Mayo Clinic
Hanak V, Golbin JM, Ryu JH et al. Causes and presenting features in 85
Selman M. Hypersensitivity pneumonitis: a multifaceted deceiving disorder. Clin
Potrebbero piacerti anche
- Pulmonary Manifestations of Primary Immunodeficiency DiseasesDa EverandPulmonary Manifestations of Primary Immunodeficiency DiseasesSeyed Alireza MahdavianiNessuna valutazione finora
- SDL 18 Interstitial Lung Disease BMS16091064Documento8 pagineSDL 18 Interstitial Lung Disease BMS16091064Jonathan YeohNessuna valutazione finora
- Diagnosis of Interstitial Lung Diseases: ReviewDocumento11 pagineDiagnosis of Interstitial Lung Diseases: ReviewMatías Jesús Flamm ZamoranoNessuna valutazione finora
- Fibrotic Interstitial Lung Diseases - A Current OutlookDocumento8 pagineFibrotic Interstitial Lung Diseases - A Current OutlookIJAR JOURNALNessuna valutazione finora
- Pulmonary RehabilitationDocumento8 paginePulmonary RehabilitationEduardo BolleguiNessuna valutazione finora
- Approach To The Adult With Interstitial Lung Disease Clinical Evaluation UpToDateDocumento31 pagineApproach To The Adult With Interstitial Lung Disease Clinical Evaluation UpToDatePablo Souza100% (1)
- Diagnosis and Treatment of Diffuse Interstitial Lung DiseasesDocumento21 pagineDiagnosis and Treatment of Diffuse Interstitial Lung Diseaseswajeeh rehmanNessuna valutazione finora
- Interstitial Lung DiseasesDocumento80 pagineInterstitial Lung Diseasespavithranmbbs100% (1)
- Wu 2021Documento8 pagineWu 2021Pulmonologi Dan Kedokteran Respirasi FK UNRINessuna valutazione finora
- Interstitial Pneumonia Seminar PaperDocumento9 pagineInterstitial Pneumonia Seminar PaperLamy SNessuna valutazione finora
- Pi Is 2949788423000205Documento12 paginePi Is 2949788423000205Giancarlo SanteNessuna valutazione finora
- Chapter 10 Interstitial Lung DiseaseDocumento10 pagineChapter 10 Interstitial Lung DiseaseAleksandar Tasic100% (1)
- Description of DiseaseDocumento10 pagineDescription of DiseaseRajaNessuna valutazione finora
- Chronic Interstitial Lung Diseases in Children: Diagnosis ApproachesDocumento30 pagineChronic Interstitial Lung Diseases in Children: Diagnosis Approachesgustavo adolfo rodriguez alzateNessuna valutazione finora
- Comprehensive Review of Current Diagnostic and Treatment Approaches To Interstitial Lung Disease Associated With Rheumatoid ArthritisDocumento10 pagineComprehensive Review of Current Diagnostic and Treatment Approaches To Interstitial Lung Disease Associated With Rheumatoid ArthritisskoamdaskondiajosNessuna valutazione finora
- 2004 Interstitial Lung DiseaseDocumento2 pagine2004 Interstitial Lung Disease나방근Nessuna valutazione finora
- Approach To The Adult With Interstitial Lung Disease Diagnostic Testing UpToDateDocumento44 pagineApproach To The Adult With Interstitial Lung Disease Diagnostic Testing UpToDatePablo SouzaNessuna valutazione finora
- Interstitial Lung Diseases ILD MichaelDocumento77 pagineInterstitial Lung Diseases ILD Michaelmekonnenchirotaw11Nessuna valutazione finora
- PenatalaksanaanDocumento37 paginePenatalaksanaanIndra kusuma mardiaNessuna valutazione finora
- Atlas of Interstitial Lung Disease PathologyDocumento253 pagineAtlas of Interstitial Lung Disease PathologyDANIEL IZE RONCHI100% (1)
- Andrew Churg MD-Atlas of Interstitial Lung Disease Pathology - Pathology With High Resolution CT Correlations-LWW (2013)Documento252 pagineAndrew Churg MD-Atlas of Interstitial Lung Disease Pathology - Pathology With High Resolution CT Correlations-LWW (2013)jorgechungNessuna valutazione finora
- Interstitial Lung Diseases ILD AbebeDocumento85 pagineInterstitial Lung Diseases ILD Abebemekonnenchirotaw11Nessuna valutazione finora
- Role of Multi-Detector Computed Tomography in Evaluation of Lung AnomaliesDocumento16 pagineRole of Multi-Detector Computed Tomography in Evaluation of Lung AnomaliesIJAR JOURNALNessuna valutazione finora
- Interstitial Lung Disease in Connective Tissue Disease: A Common Lesion With Heterogeneous Mechanisms and Treatment ConsiderationsDocumento18 pagineInterstitial Lung Disease in Connective Tissue Disease: A Common Lesion With Heterogeneous Mechanisms and Treatment ConsiderationsskoamdaskondiajosNessuna valutazione finora
- Adult Interstitial Lung Diseases and Their Epidemiology 2019Documento46 pagineAdult Interstitial Lung Diseases and Their Epidemiology 2019Mary CogolloNessuna valutazione finora
- Manajemen Klinis Rumah Sakit Bagi Pasien Dengan Dugaan Terkena COVID-19 (Bahasa Inggris)Documento13 pagineManajemen Klinis Rumah Sakit Bagi Pasien Dengan Dugaan Terkena COVID-19 (Bahasa Inggris)PERDHAKINessuna valutazione finora
- Idiopathic Pulmonary Fibrosis: Diagnosis, Epidemiology and Natural HistoryDocumento11 pagineIdiopathic Pulmonary Fibrosis: Diagnosis, Epidemiology and Natural HistorySuwandi AlghozyNessuna valutazione finora
- 1 s2.0 S0140673622010522 MainDocumento18 pagine1 s2.0 S0140673622010522 MainMirella Rugel SocolaNessuna valutazione finora
- Clinical Management of Persons Admitted To Hospita v1 19 March 2020 PDFDocumento13 pagineClinical Management of Persons Admitted To Hospita v1 19 March 2020 PDFAndreea DobreNessuna valutazione finora
- Atlas of Interstitial Lung Disease Pathology Pathology With High Resolutio PDFDocumento253 pagineAtlas of Interstitial Lung Disease Pathology Pathology With High Resolutio PDFAmilcar Romero100% (1)
- 12 RLDDocumento43 pagine12 RLDNur akilaNessuna valutazione finora
- "Imitators" of The ARDS Implications For Diagnosis and Treatment Acute Lung Injury (ALI) and ARDS (ALI/ARDS)Documento22 pagine"Imitators" of The ARDS Implications For Diagnosis and Treatment Acute Lung Injury (ALI) and ARDS (ALI/ARDS)Odett EzzatNessuna valutazione finora
- Hamman Rich SyndromeDocumento7 pagineHamman Rich SyndromeGerardo Armando EsparzaNessuna valutazione finora
- Nur 111 Session 1 Sas 1Documento8 pagineNur 111 Session 1 Sas 1Zzimply Tri Sha UmaliNessuna valutazione finora
- Interstitial Lung Diseases (ILD)Documento17 pagineInterstitial Lung Diseases (ILD)Rashed ShatnawiNessuna valutazione finora
- 1 s2.0 S0140673622010522 MainDocumento18 pagine1 s2.0 S0140673622010522 MainsilviaNessuna valutazione finora
- Pulmonary Tuberculosis Presenting With Acute Respiratory Distress Syndrome (ARDS) : A Case Report and Review of LiteratureDocumento5 paginePulmonary Tuberculosis Presenting With Acute Respiratory Distress Syndrome (ARDS) : A Case Report and Review of LiteratureCimol AgustinaNessuna valutazione finora
- Printed MaterialsDocumento10 paginePrinted Materialsjesiegutay21Nessuna valutazione finora
- 127+Layout+JR+8 (2) +DR +Budi+Yanti+ (P 87-93) +Documento7 pagine127+Layout+JR+8 (2) +DR +Budi+Yanti+ (P 87-93) +Julian HutabaratNessuna valutazione finora
- Interstitial Lung Diseases RadiologyDocumento26 pagineInterstitial Lung Diseases RadiologyDaniel AshooriNessuna valutazione finora
- Kubo, 2013 PDFDocumento19 pagineKubo, 2013 PDFBimx MolitNessuna valutazione finora
- Pulmonary FibrosisDocumento4 paginePulmonary FibrosisDimpal Choudhary100% (2)
- Interstitial Lung Diseases Radiology 22222Documento26 pagineInterstitial Lung Diseases Radiology 22222Daniel AshooriNessuna valutazione finora
- Interstitiallungdiseases Inchildren, Adolescents, AndyoungadultsDocumento16 pagineInterstitiallungdiseases Inchildren, Adolescents, Andyoungadultsgustavo adolfo rodriguez alzateNessuna valutazione finora
- A Case Presentation On Chronic Obstructive Disease (COPD)Documento18 pagineA Case Presentation On Chronic Obstructive Disease (COPD)Harvey T. Dato-onNessuna valutazione finora
- Idiopathic Pulmonary Fibrosis: Interstitial Lung DiseaseDocumento5 pagineIdiopathic Pulmonary Fibrosis: Interstitial Lung DiseaseAmjaSaudNessuna valutazione finora
- Acute Respiratory Distress SyndromeDocumento70 pagineAcute Respiratory Distress SyndromeAndrea Del Villar100% (1)
- Pulmonary Tuberculosis Presenting With Acute Respiratory Distress Syndrome (Ards) : A Case Report and Review of LiteratureDocumento5 paginePulmonary Tuberculosis Presenting With Acute Respiratory Distress Syndrome (Ards) : A Case Report and Review of Literatureamelya asryNessuna valutazione finora
- Eman YousifDocumento9 pagineEman Yousifsheka mNessuna valutazione finora
- Gupta 2015Documento13 pagineGupta 2015Juan Diego CamposNessuna valutazione finora
- Latest IipDocumento7 pagineLatest IipPULMONOLOGI 10 USKNessuna valutazione finora
- Interstitial Pneumonia With Autoimmune Features Challenges and ControversieDocumento19 pagineInterstitial Pneumonia With Autoimmune Features Challenges and ControversieVitória WasserNessuna valutazione finora
- Smoking-Related Lung DiseaseDocumento49 pagineSmoking-Related Lung DiseaseAnkita ShahNessuna valutazione finora
- Pulmonary FibrosisDocumento39 paginePulmonary FibrosisMarcoNessuna valutazione finora
- ID Faktor Faktor Yang Berhubungan Dengan Buang Air Besar Di Jamban Di Desa GunungsaDocumento14 pagineID Faktor Faktor Yang Berhubungan Dengan Buang Air Besar Di Jamban Di Desa GunungsaRSU DUTA MULYANessuna valutazione finora
- Hide 1 Description 2 Classification 2.1 Chronic Bronchitis 2.2 Emphysema 3 Pathophysiology 4 EpidemiologyDocumento29 pagineHide 1 Description 2 Classification 2.1 Chronic Bronchitis 2.2 Emphysema 3 Pathophysiology 4 EpidemiologyAngie Mandeoya100% (1)
- Restrictive Lung DiseaseDocumento21 pagineRestrictive Lung DiseaseSerena MogniNessuna valutazione finora
- Interstitial Lung Disease: Clinical Year in ReviewDocumento7 pagineInterstitial Lung Disease: Clinical Year in ReviewAnonymous qwH3D0KrpNessuna valutazione finora
- Chronic Obstructive Pulmonary Disease Diagnosis and StagingDocumento50 pagineChronic Obstructive Pulmonary Disease Diagnosis and StagingSensing NonsenseNessuna valutazione finora
- Benefit of CT Scanning For Assessing Pulmonary Disease in The Immunodepressed PatientDocumento6 pagineBenefit of CT Scanning For Assessing Pulmonary Disease in The Immunodepressed PatienthilalNessuna valutazione finora
- Lec 2 - Respiratory DistressDocumento4 pagineLec 2 - Respiratory DistressphobicmdNessuna valutazione finora
- Philppines Consonet Form180613Documento3 paginePhilppines Consonet Form180613erwinmiranda20033Nessuna valutazione finora
- 52 HypoglycemiaDocumento2 pagine52 HypoglycemiaphobicmdNessuna valutazione finora
- Pneumococcal Conjugate Vaccine (PCV) Catch-Up Guidance For Children 4 Months Through 18 Years of Age 2015Documento3 paginePneumococcal Conjugate Vaccine (PCV) Catch-Up Guidance For Children 4 Months Through 18 Years of Age 2015phobicmdNessuna valutazione finora
- Peds Basic Principles Mechanical VentilationDocumento39 paginePeds Basic Principles Mechanical VentilationNav KovNessuna valutazione finora
- Oxaciline PDFDocumento7 pagineOxaciline PDFamatoryfictionliteraNessuna valutazione finora
- Spirometer Handbook NacaDocumento24 pagineSpirometer Handbook NacaebrycNessuna valutazione finora
- Paediatric Empyema: A Case Report and Literature ReviewDocumento5 paginePaediatric Empyema: A Case Report and Literature ReviewphobicmdNessuna valutazione finora
- Protocol 1 Hypoglycemia 2006Documento7 pagineProtocol 1 Hypoglycemia 2006Aprilian Ayu SitaNessuna valutazione finora
- Wheezing in Infancy: Epidemiology, Investigation, and TreatmentDocumento8 pagineWheezing in Infancy: Epidemiology, Investigation, and TreatmentphobicmdNessuna valutazione finora
- Left Bronchus SyndromeDocumento3 pagineLeft Bronchus SyndromephobicmdNessuna valutazione finora
- 141 fullRRRDocumento6 pagine141 fullRRRphobicmdNessuna valutazione finora
- Ios 67Documento5 pagineIos 67phobicmdNessuna valutazione finora
- Research Article: Near Fatal Asthma: Clinical and Airway Biopsy CharacteristicsDocumento7 pagineResearch Article: Near Fatal Asthma: Clinical and Airway Biopsy CharacteristicsphobicmdNessuna valutazione finora
- Left Bronchus SyndromeDocumento3 pagineLeft Bronchus SyndromephobicmdNessuna valutazione finora
- 1B Draft Annce-Wshop 020113.amendedDocumento1 pagina1B Draft Annce-Wshop 020113.amendedphobicmdNessuna valutazione finora
- Diagnosis of Tuberculosis in Children: Increased Need For Better MethodsDocumento9 pagineDiagnosis of Tuberculosis in Children: Increased Need For Better MethodsphobicmdNessuna valutazione finora
- AsthmaDocumento17 pagineAsthmaphobicmdNessuna valutazione finora
- MG SulphateDocumento6 pagineMG SulphatephobicmdNessuna valutazione finora
- Wet CoughDocumento2 pagineWet CoughphobicmdNessuna valutazione finora
- Empyema 2Documento9 pagineEmpyema 2phobicmdNessuna valutazione finora
- Pediatrics 2013 Lantos Peds.2013 1292Documento5 paginePediatrics 2013 Lantos Peds.2013 1292phobicmdNessuna valutazione finora
- The Difficult Coughing Child: Prolonged Acute Cough in ChildrenDocumento5 pagineThe Difficult Coughing Child: Prolonged Acute Cough in ChildrenphobicmdNessuna valutazione finora
- 1A Draft Annce-CME Course 020113.amendedDocumento1 pagina1A Draft Annce-CME Course 020113.amendedphobicmdNessuna valutazione finora
- Pediatrics 2013 Schroeder E1194 201Documento10 paginePediatrics 2013 Schroeder E1194 201phobicmdNessuna valutazione finora
- Pediatrics 2013 Okelo 517 34Documento21 paginePediatrics 2013 Okelo 517 34phobicmdNessuna valutazione finora
- Pediatrics 2013 Biondi Peds.2013 1759Documento9 paginePediatrics 2013 Biondi Peds.2013 1759phobicmdNessuna valutazione finora
- Pediatrics 2013 Harutyunyan Peds.2012 2351Documento12 paginePediatrics 2013 Harutyunyan Peds.2012 2351phobicmdNessuna valutazione finora
- Pediatrics 2013 Feuille S6 7Documento4 paginePediatrics 2013 Feuille S6 7phobicmdNessuna valutazione finora
- A Cleaning Protocol For Niti Endodontic Instruments - ParashosDocumento8 pagineA Cleaning Protocol For Niti Endodontic Instruments - ParashosRens RuedaNessuna valutazione finora
- PIDSR Manual of ProceduresDocumento55 paginePIDSR Manual of ProceduresOrlea FranciscoNessuna valutazione finora
- Acitrom - The No.1 Oral AnticoagulantDocumento31 pagineAcitrom - The No.1 Oral AnticoagulantNishant SagarNessuna valutazione finora
- TutankamonDocumento10 pagineTutankamonlauraNessuna valutazione finora
- Knowledge and Awareness On Hiv-Aids of Senior High School Students Towards An Enhanced Reproductive Health Education ProgramDocumento20 pagineKnowledge and Awareness On Hiv-Aids of Senior High School Students Towards An Enhanced Reproductive Health Education ProgramGlobal Research and Development ServicesNessuna valutazione finora
- DOH Food HandlersDocumento15 pagineDOH Food HandlersmorneNessuna valutazione finora
- Learning ActivitiesDocumento4 pagineLearning ActivitiesNikka AntoqueNessuna valutazione finora
- NCP PTBDocumento9 pagineNCP PTBecbarticanNessuna valutazione finora
- Thesis TitleDocumento15 pagineThesis TitleAnonymous OvzTRpx100% (3)
- Hyperemesis GravidarumDocumento34 pagineHyperemesis GravidarumSiti Nurhayati100% (1)
- Different Types of Urine TestDocumento2 pagineDifferent Types of Urine TestRamon AzurNessuna valutazione finora
- Uganda Health Sector Budget Framework Paper FY 2018/19 - FY 2022/23Documento168 pagineUganda Health Sector Budget Framework Paper FY 2018/19 - FY 2022/23African Centre for Media Excellence100% (1)
- Community Medicine Module Book PDFDocumento37 pagineCommunity Medicine Module Book PDFLiya SuwarniNessuna valutazione finora
- ACTIVITY 4 - Albarico JuanDocumento3 pagineACTIVITY 4 - Albarico JuanKynah AmorNessuna valutazione finora
- 2 Sho Exam-June 2012Documento6 pagine2 Sho Exam-June 2012Hafiz SabriNessuna valutazione finora
- Transformation and ImmortalizationDocumento21 pagineTransformation and ImmortalizationAnupam JainNessuna valutazione finora
- National Action Plan - Viral Hepatitis Control Programmelowress - Reference File PDFDocumento52 pagineNational Action Plan - Viral Hepatitis Control Programmelowress - Reference File PDFFranklin ThomasNessuna valutazione finora
- Bacterial Men CPGDocumento42 pagineBacterial Men CPGKarl Jimenez SeparaNessuna valutazione finora
- Mini Project OfficialDocumento15 pagineMini Project OfficialJaveria A.PNessuna valutazione finora
- Hiv & Aids Awareness ProgramDocumento8 pagineHiv & Aids Awareness ProgramKenzie WalshNessuna valutazione finora
- Case PresentationDocumento31 pagineCase PresentationRifka WindiputriNessuna valutazione finora
- Physical Examination of The SkinDocumento4 paginePhysical Examination of The SkinJessica Febrina WuisanNessuna valutazione finora
- 1 HMN Assessment Tool Version 4 0 Eng With SwitchboardDocumento156 pagine1 HMN Assessment Tool Version 4 0 Eng With SwitchboardjonidacekreziNessuna valutazione finora
- Pathogenic e ColiDocumento4 paginePathogenic e ColiRia MicuaNessuna valutazione finora
- Heprrp DilasagDocumento92 pagineHeprrp DilasagAlbert100% (1)
- Staphylococcus Epidermidis MeningitisDocumento11 pagineStaphylococcus Epidermidis MeningitisEkhi RezkiNessuna valutazione finora
- Life-Changing Journey: AfricaDocumento5 pagineLife-Changing Journey: AfricaBobby Mathew ZNessuna valutazione finora
- IntroductionDocumento2 pagineIntroductionHarvey Gian JavierNessuna valutazione finora
- ECPE All-Star Practice Tests New Cloze TestDocumento13 pagineECPE All-Star Practice Tests New Cloze Testsiana_shNessuna valutazione finora
- Parasitology Guide Questions 1Documento2 pagineParasitology Guide Questions 1Cess LacatangoNessuna valutazione finora