Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
PALL ProteinACeramicHyperD
Caricato da
isaacfg1Descrizione originale:
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
PALL ProteinACeramicHyperD
Caricato da
isaacfg1Copyright:
Formati disponibili
PRODUCT
NOTE
Protein A Ceramic HyperD F
BIOSEPRA Protein A Ceramic HYPERD F
Affinity Chromatography Sorbent
Ideal for large-scale antibody purification. High binding capacity for human and murine IgGs. High selectivity with no non-specific binding. Rigid. Low backpressure. Easy to pack.
BioSepra Protein A Ceramic HyperD F sorbent available from Pall is a high capacity affinity sorbent designed for process-scale purification of immunoglobulins G. The sorbent combines ease of use with high binding capacity and excellent scalability. Antibodies of greater than 95% purity are isolated from cell culture supernatant or ascites fluid in a single chromatographic step. Dilute feedstock (~100 g IgG/ml) may be applied without preliminary concentration. BioSepra Protein A Ceramic HyperD F sorbent is prepared using a rigid proprietary ceramic bead. Recombinant Protein A is immobilized to a specially formulated hydrogel within the porous ceramic bead. The ceramic bead, the hydrogel, and the linkage used for the Protein A coupling are chemically stable over a broad range of conditions. Cleaning-in-place and sanitization procedures using sodium hydroxide may be employed with only limited loss of binding capacity. The unique, multi-point coupling chemistry provides a sorbent that exhibits very low leakage of recombinant Protein A. The Protein A ligand is of recombinant origin and is produced in strict compliance with cGMP requirements. The rec Protein A is purified by standard chromatographic processes and is never in contact with immunoglobulins. The rigidity of Protein A Ceramic HyperD F
Table 1: Main Properties of Protein A Ceramic HyperD F sorbent. .
Particle size Dynamic binding capacity for hu IgG* (10% breakthrough, 100 cm/h) Ligand Immobilized Protein A Working pH Cleaning pH Volume changes due to pH and ionic strength Pressure resistance 50 m (av.) > 30 mg/ml
Recombinant Protein A 4 - 5 mg/ml of sorbent 2 - 11 2 - 13 Non compressible 70 bar (1,000 psi)
* Determined using 10 mg/ml hu IgG in PBS, pH 7.4; Elution with 0.1 M sodium citrate, pH 2.5. Column: 4.6 ID x 100 mm.
sorbent facilitates operation at high linear velocity. Similarly, bed depth may be increased to provide increased residence time and enhanced capture efficiency. The sorbent is ideal for production scale purification. The dense ceramic material settles quickly and packs easily, even in large columns. Equally important, the material does not shrink or swell in response to changes in pH, ionic strength or flow rate. The material is shipped in 1 M NaCl containing 20% ethanol and is available in a range of package sizes. Special packaging to meet specific manufacturing requirements is available on request.
PRODUCT
NOTE
Capacity
BIOSEPRA Protein A Ceramic HYPERD F
As with all Protein A sorbents, the dynamic binding capacity is influenced by the composition of the sample, the IgG subclass, and the IgG concentration in the feedstock (figure 1). Nevertheless, Protein A Ceramic HyperD F sorbent exhibits high binding capacities with a variety of samples. Dynamic binding capacity for human IgG exceeds 30 mg/ml (figure 2) at linear velocities ranging from 100 to 300 cm/h. Table 2 shows binding capacity values for different IgG subclasses from ascites or cell culture supernatant. The capacity ranges from 3.6 mg/ml for IgG2a to 19 mg/ml for IgG3. Studies have shown that the best productivity is obtained when the average IgG residence time in the column is about 3-5 minutes (figure 3). A column 15-20 cm deep, operated at 300 cm/h provides a good compromise between high capacity and high linear velocity for process-scale use.
Figure 2. Dynamic hu IgG binding capacity as a function of flow rate (hu IgG: 1 mg/ml).
Dynamic binding capacity (mg/ml)
Linear Velocity (cm/h)
Figure 3. Capacity vs. linear velocity or residence time for hu IgG1.
DBC at 50% breakthrough (mg/ml)
Figure 1. Influence of hu IgG concentration on binding capacity (flow rate: 75 cm/h).
Dynamic binding capacity (mg/ml) DBC at 50% breakthrough (mg/ml)
Linear velocity (cm/h)
Residence time (min) hu lgG concentration (mg/ml)
Experimental: 2.55 mg/ml hu IgG1; Column: 1.0 x 11.7 cm.
Table 2. Binding Capacities for IgG Subclasses at 300 cm/h.
Source Cell culture supernatant Ascites fluid Ascites fluid Ascites fluid Ascites fluid IgG conc. in the sample (mg/ml) 0.05 6.34 2.43 4.00 4.10 Subclass IgG1 IgG1 IgG2a IgG3 IgG2b Capacity (mg/ml) 13.4 12.7 3.6 19.0 9.5
Column: 3 mm ID x 100 mm; Volume: 0.7 ml; Loading: 1 M glycine / 2 M NaCl, pH 8.9; Elution: 0.1 M acetic acid, pH 3.
Purity During studies with various IgG subclasses and feedstocks, Protein A Ceramic HyperD F sorbent provided high purity product, even at high column loading. Results are summarized in table 3. In all cases, the purity was greater than 98% as determined by SDS-PAGE. No albumin was detected in IgG1 isolated from ascites fluid. Isolation of antibody from serum-containing cell culture supernatant is illustrated in figure 4. Analysis by SDS-PAGE is shown in figure 5. Despite the relatively high selectivity of Protein A, isolation of high purity product, suitable for therapeutic use requires further, orthogonal chromatographic steps. Cation exchange chromatography may be conducted using CM or S Ceramic HyperD F sorbents. Typically, the cation exchange procedure is designed to bind IgG while impurities are selectively desorbed. Anion exchange chromatography on Q or DEAE Ceramic HyperD F sorbents may be employed to bind a variety of relatively acidic impurities while the antibody passes unretained in the flowthrough fraction. Stability BioSepra Protein A Ceramic HyperD F sorbent is a dense, rigid, non compressible material. Packing is faster than for conventional Protein A Agarose. The sorbent typically settles in a few minutes, i.e. less than 10 min are needed to pack a 10 ml column. Protein A Ceramic HyperD F sorbent allows the use of flow rates higher than 300 cm/h at backpressure less than 3 bar. Indeed, the
Table 3. Preparative Purification of Monoclonal IgG on Protein A Ceramic HYPERD F.
Source Ascites fluid Ascites fluid Ascites fluid Ascites fluid Ascites fluid Ascites fluid Cell culture supernatant Cell culture supernatant Origin IgG Subclass Initial IgG Eluted IgG Purity conc. (mg/ml) (mg) SDS-PAGE 6.34 2.26 3.86 2.31 2.43 2.86 0.05 0.20 37 20 31 6 10 28 15 30 > > > > > > > > 98% 98% 98% 98% 98% 98% 98% 98%
murine IgG1 kappa murine IgG1* murine IgG1* murine IgG2b** murine IgG2a** murine IgG2a* murine IgG1* humanized IgG1*
Column: 6.6 mm ID x 120 mm; Volume: 4 ml, 10-30 mg IgG per run; Loading: 1 M glycine / 2 M NaCl (*), pH 8.9 or PBS (**); Elution: 0.1 M acetic acid.
Figure 4. Isolation of IgG1 from serum-containing cell culture supernatant on Protein A Ceramic HyperD F sorbent.
UV
Figure 5. SDS-PAGE analysis of feedstock (1), flowthrough (2), recovered IgG1 (3).
1 2 3
Time (min)
The arrow indicates introduction of elution buffer. Column: 3 mm ID x 100 mm. Loading and wash: 1 M glycine / 2 M NaCl, pH 8.9. Elution: 100 mM acetate buffer, pH 4.6. Linear velocity: 300 cm/h. Sample: 50 ml cell culture supernatant.
Figure 6. Pressure vs. number of cycles.
chromatography described in figure 6 is accomplished at less than 0.4 bar at 300 cm/h. The pressure remained consistent for more than 30 cycles. Pressure/flow rate characteristics of Protein A Ceramic HyperD F sorbent facilitate process-scale application. For example, at large scale, bed heights of 20-50 cm can be used at flow rates up to 200 cm/h to maximize capacity of the sorbent by optimising the residence time.
Pressure (bar)
Number of cycles
hu IgG capacity at 10% breakthrough; Linear flow rate: 300 cm/h; Bed height: 25 cm; Residence time: 5 min; Loading: 35 mg/ml.
Cleaning Recombinant Protein A, rich in carboxylic side chains, is coupled to the primaryamine-containing hydrogel via stable secondary-amide linkages. The procedure has been tailored to give optimized multi-point attachment, as illustrated in figure 7. This unique multi-point coupling chemistry confers high chemical stability. Cleaning with sodium hydroxide from 1 to 100 mM can be performed repeatedly. Modest decreases in binding capacity can occur over time depending upon the concentration of sodium hydroxide and the total contact time. Alternatively, cleaning may be conducted using 6 M guanidine hydrochloride for some/all cleaning cycles. Ligand leakage As a consequence of the Protein A coupling chemistry, the ligand leakage is low. An independent study of Protein A Ceramic HyperD F sorbent showed less than 10 ng Protein A per mg of purified hu IgG (average value). A non-competitive ELISA procedure was used to determine native Protein A in the IgG eluate. These findings reflect the high stability of Protein A Ceramic HyperD F sorbent. In many pharmaceutical applications, an anion exchange procedure is employed to assure removal of trace quantities of leached rec Protein A. Q Ceramic HyperD F sorbent is well suited to this application. The procedure is conducted under conditions which promote dissociation of the IgG-Protein A complex. As illustrated in figure 8, IgG is unretained, while the relatively acidic Protein A (pI < 5) is bound. The study was performed using IgG1 spiked with 107 g of rec Protein A per mg of IgG1.
Figure 7. Tentative schematic structure of immobilized Protein A on Ceramic HyperD.
The arrows represent proteolysis sensitive locations of Protein A between sub-units responsible for Fc binding.
Figure 8. Separation of spiked Protein A from IgG1 using a Q Ceramic HyperD F column.
UV abosrbance
Time (s)
Sample: IgG1 (7.9 mg) purified from a cell culture supernatant using Protein A Ceramic HyperD F sorbent spiked with 850 g Protein A. As required, the mobile phase can contain up to 2 M urea and 50% ethylene glycol. A sodium chloride gradient up to 2.6 mS can be employed.
Applications
References
1. Boschetti, E., Jungbauer, A., Separation Science & Technology, Academic Press Vol. 2 (2000) 535. 2. Guerrier, L., et al., J. Chromatogr. B 755 (2000) 37. 3. Eliasson, M., et al., J. Biol. Chem. 263 (1988) 4323. 4. Akerstrom, B., J. Biol. Chem. 261 (1986) 10240. 5. Watanabe, M., et al., Japan J. Exp. Med. 225 (1981) 51. 6. Der Balian, G.P., et al., J. Exp. Med. 152 (1980) 209. 7. Ledbetter, J.A., Immunol. Rev. 47 (1979) 63. 8. Medgyesi, G.A., Fust, G., et al., Immunochemistry 15 (1978) 125. 9. Lindmark, R., Movitz, J., Sjoquist, J., Eur. J. Biochem. 74 (1977) 623. 10. Sjoquist, J., Movitz, J., et al., Eur. J. Biochem. 30 (1972) 190. 11. Kronvall, G., Seal, U.S., et al., J. Immunol. 104 (1970), 140.
BIOSEPRA Protein A Ceramic HYPERD F
Optimum binding conditions depend upon the IgG subclass to be purified. In most applications, binding is conducted under physiological conditions. For weakly interacting immunoglobulins (e.g. murine IgG1), efficient binding is accomplished at higher pH (~ 8.5 - 9.0) using a mobile phase augmented with glycine and sodium chloride. Elution is normally accomplished at acidic pH (2.5 - 3.0). Milder pH can be used for weakly adsorbed IgGs such as mouse IgG1. Once conditions have been optimized, scale up is accomplished by increasing the column diameter while maintaining the optimum bed height and linear velocity. Detailed recommendations for use can be found in the Product Insert which is supplied with the product. Protein A Ceramic HyperD F sorbent is well suited to isolation and purification of IgG from ascites fluid, cell culture supernatant, transgenic milk, and various animal sera. Other applications include : - Separation of IgG subclasses (e.g. Protein A does not interact with human IgG3). - Separation of Fc fragments from a mixture of Fc and Fab fragments obtained after enzymatic hydrolysis. - Purification of humanized or mouse monoclonal antibodies. - Separation of immune complexes. - Purification of enzyme conjugates. - Removal of bovine IgG from hybridoma cell culture.
Ordering Information
Product Cat. No. Size
Protein A Ceramic HYPERD F
20078-036 5 ml 20078-028 25 ml 20078-010 100 ml 20078-044 1L 20078-051 5L 20078-069 10 L
PRODUCT NOTE
New York - USA +1 516.484.5400 phone +1 516.801.9548 fax pharmafilter@pall.com Portsmouth - Europe +44 (0)23 9230 3303 phone +44 (0)23 9230 2506 fax BioPharmUK@europe.pall.com Cergy - France Pall BioSepra +33 (0)1 34 20 78 00 phone +33 (0)1 34 20 78 78 fax bioseprainfo@pall.com
Visit us on the web at www.pall.com/biopharmaceutical
These products are for laboratory research use only and are not intended for human or animal diagnostic, therapeutic, or other clinical uses, unless otherwise stated. The information contained in this brochure are subject to change without notice. Pall Corporation has offices and plants throughout the world in locations including: Argentina, Australia, Austria, Belgium, Brazil, Canada, China, France, Germany, India, Indonesia, Ireland, Italy, Japan, Korea, Malaysia, Mexico, the Netherlands, New Zealand, Norway, Poland, Puerto Rico, Russia, Singapore, South Africa, Spain, Sweden, Switzerland, Taiwan, Thailand, United Kingdom, United States and Venezuela. Distributors are located in all major industrial areas of the world. , Pall, BioSepra, HyperD are trademarks of Pall Corporation. Filtration. Separation. Solution. is a service mark of Pall Corporation. indicates a trademark registered in the U.S. Pall Corporation 2004
LPN PN702-004 - 12/2004
Potrebbero piacerti anche
- Determination of Amount of CaCO3 in Eggshell by Back Titration MethodDocumento5 pagineDetermination of Amount of CaCO3 in Eggshell by Back Titration MethodMg H100% (2)
- Top Steel Companies in IndiaDocumento3 pagineTop Steel Companies in Indiazukmos67% (3)
- BASF Poligen Wax Application GuideDocumento9 pagineBASF Poligen Wax Application Guideneil smithiesNessuna valutazione finora
- Wolkite University Museum Technique Group AssignmentDocumento23 pagineWolkite University Museum Technique Group AssignmentNatnael SisayNessuna valutazione finora
- Glutathione Sepharose High Performance: RuctionsDocumento28 pagineGlutathione Sepharose High Performance: RuctionsBatoNessuna valutazione finora
- Kolom Protein A Sepharose High PerformanceDocumento12 pagineKolom Protein A Sepharose High PerformanceAldi IgnielNessuna valutazione finora
- InTech-Isolation and Purification of Bioactive Proteins From Bovine ColostrumDocumento20 pagineInTech-Isolation and Purification of Bioactive Proteins From Bovine ColostrumDenise NepomucenoNessuna valutazione finora
- International Journal of Pharma and Bio Sciences: Subtilis Dj5 Into Gelatin Film by Glutaraldehyde CrosslinkingDocumento10 pagineInternational Journal of Pharma and Bio Sciences: Subtilis Dj5 Into Gelatin Film by Glutaraldehyde CrosslinkingAnggaAnugrah LiberoeAdjhaNessuna valutazione finora
- Bradford, 1976Documento7 pagineBradford, 1976Jefferson Silva FerreiraNessuna valutazione finora
- Nab™ Spin Columns, 0.2 ML For Antibody Purification: InstructionsDocumento3 pagineNab™ Spin Columns, 0.2 ML For Antibody Purification: InstructionsHiro MurayamaNessuna valutazione finora
- Turb y Nef 4Documento5 pagineTurb y Nef 4Dulce María LópezNessuna valutazione finora
- Hafidz2011 - (48) IFRJ-2010-159 PDFDocumento5 pagineHafidz2011 - (48) IFRJ-2010-159 PDFiulianaNessuna valutazione finora
- Refined Preparation and Use of Anti-Diglycine Remnant (K-Quantification of 10,000s of Ubiquitination Sites in Single Proteomics ExperimentsDocumento7 pagineRefined Preparation and Use of Anti-Diglycine Remnant (K-Quantification of 10,000s of Ubiquitination Sites in Single Proteomics ExperimentsArranegiko FarerasNessuna valutazione finora
- Hitrap Igy Purification HP: Ge Healthcare Life SciencesDocumento4 pagineHitrap Igy Purification HP: Ge Healthcare Life SciencesIsa PachecoNessuna valutazione finora
- Genei: Bacterial Gene Expression Teaching Kit ManualDocumento12 pagineGenei: Bacterial Gene Expression Teaching Kit Manualhar2dikNessuna valutazione finora
- Glutathione Sepharose 4B: GE HealthcareDocumento26 pagineGlutathione Sepharose 4B: GE HealthcareMadstcNessuna valutazione finora
- Isolation and Purification of Polyclonal Igg Antibodies From Bovine Serum by High Performance Liquid ChromatographyDocumento7 pagineIsolation and Purification of Polyclonal Igg Antibodies From Bovine Serum by High Performance Liquid ChromatographyMoody ModmodNessuna valutazione finora
- Preparation and Analysis of Immunoglobin G (IgG) from Chicken SerumDocumento9 paginePreparation and Analysis of Immunoglobin G (IgG) from Chicken SerumKhAi EnNessuna valutazione finora
- CIPAC HANDBOOK Volume H, Glyphosate 284 (M), 1995Documento3 pagineCIPAC HANDBOOK Volume H, Glyphosate 284 (M), 1995Laura Guarguati100% (1)
- Plant Protein Extraction Protocol For SDS PageDocumento7 paginePlant Protein Extraction Protocol For SDS PageAbhinay BatchuNessuna valutazione finora
- Quim. Nova,: A B C DDocumento8 pagineQuim. Nova,: A B C DG8-35 M Faozi Rahman WakhidNessuna valutazione finora
- Bradford 1976Documento7 pagineBradford 1976arvindftNessuna valutazione finora
- Sensors 09 08271Documento7 pagineSensors 09 08271xinying94Nessuna valutazione finora
- Resin - Comparison of JSR Protein A Versus GE MabselectDocumento12 pagineResin - Comparison of JSR Protein A Versus GE MabselectHyunjung KimNessuna valutazione finora
- Or-Amylase Production in Aqueous Two-Phase Systems With: Bacillus SubtilisDocumento6 pagineOr-Amylase Production in Aqueous Two-Phase Systems With: Bacillus SubtilisHong HanhNessuna valutazione finora
- Aasr 2012 3 5 2739 2744Documento6 pagineAasr 2012 3 5 2739 2744ana_amyNessuna valutazione finora
- 7080148A Prospekt Xevonta e 06 10Documento10 pagine7080148A Prospekt Xevonta e 06 10natasa972Nessuna valutazione finora
- Bradford - A Rapid and Sensitive Method For The QuantitationDocumento7 pagineBradford - A Rapid and Sensitive Method For The QuantitationilhambopNessuna valutazione finora
- Bradford Assay 2Documento7 pagineBradford Assay 2GLADYSNessuna valutazione finora
- Purification of A Synthetic Oligonucleotide by Anion Exchange Chromatography Method Optimisation and Scale-UpDocumento10 paginePurification of A Synthetic Oligonucleotide by Anion Exchange Chromatography Method Optimisation and Scale-UpMike BudimanNessuna valutazione finora
- Mini Protean TGX Precast GelsDocumento6 pagineMini Protean TGX Precast GelsKai PacNessuna valutazione finora
- Computer Simulation For Large Scale Bioprocess Design: S.A. Rouf, P.L. Douglas, M. Moo-Young, J.M. ScharerDocumento6 pagineComputer Simulation For Large Scale Bioprocess Design: S.A. Rouf, P.L. Douglas, M. Moo-Young, J.M. Scharerdaimon_pNessuna valutazione finora
- Mutagenesis Screening of High-Yielding GlutathioneDocumento7 pagineMutagenesis Screening of High-Yielding GlutathioneLadys DunlapNessuna valutazione finora
- Canine IgG InsertDocumento2 pagineCanine IgG InsertGomatheeswariNessuna valutazione finora
- AD0012LC Post Column Derivatization AflatoxinDocumento4 pagineAD0012LC Post Column Derivatization Aflatoxinevenspase7859Nessuna valutazione finora
- 5989 0332enDocumento8 pagine5989 0332enJelena DjogovicNessuna valutazione finora
- Development of a strategy for the screening of α-glucosidase-producing microorganismsDocumento10 pagineDevelopment of a strategy for the screening of α-glucosidase-producing microorganismsJose Luis Ponce CovarrubiasNessuna valutazione finora
- 13 5 Allen SecuredDocumento5 pagine13 5 Allen SecuredVenkata Suryanarayana GorleNessuna valutazione finora
- Cap EiaDocumento8 pagineCap EiangathamNessuna valutazione finora
- C18 Spin Column Usage MethodDocumento8 pagineC18 Spin Column Usage Methodphd.data19Nessuna valutazione finora
- Characterization of The Keratinolytic Activity of IndigenousDocumento10 pagineCharacterization of The Keratinolytic Activity of IndigenousFaisal AwanNessuna valutazione finora
- The Bradford Method For Protein Quantitation. Nicholas Kruger PDFDocumento7 pagineThe Bradford Method For Protein Quantitation. Nicholas Kruger PDFGise Clara HernandezNessuna valutazione finora
- Sigma Wide MarkerDocumento2 pagineSigma Wide MarkerMarek Adamczak0% (1)
- BRADFORD MECHANISM GrintzalisPapapostrolouGeorgiou2009Documento10 pagineBRADFORD MECHANISM GrintzalisPapapostrolouGeorgiou2009fabiowalligatorNessuna valutazione finora
- TGA Article Print VersionDocumento10 pagineTGA Article Print VersionjenniNessuna valutazione finora
- Total Dietary Fiber: Assay ProcedureDocumento20 pagineTotal Dietary Fiber: Assay ProcedurebeatcookNessuna valutazione finora
- Histochemical and Fluorometric Assays For Iuidai Gus Gene DetectDocumento11 pagineHistochemical and Fluorometric Assays For Iuidai Gus Gene DetectDaviid A'SNessuna valutazione finora
- Elisa TestDocumento9 pagineElisa Testbrkica2011Nessuna valutazione finora
- Bacterial endotoxins test methodsDocumento8 pagineBacterial endotoxins test methodsCeangoNessuna valutazione finora
- Desain Reaktor IsomerisasiDocumento7 pagineDesain Reaktor IsomerisasiIka Y. RachmawatiNessuna valutazione finora
- 2782 FTPDocumento5 pagine2782 FTPJosilene AmaroNessuna valutazione finora
- Estudos de Saccharomyces Cerevisiae Imobilizados. Análise de Fermentação Rápida Contínua de Etanol em Reator de Células ImobilizadasDocumento15 pagineEstudos de Saccharomyces Cerevisiae Imobilizados. Análise de Fermentação Rápida Contínua de Etanol em Reator de Células ImobilizadasMarina Cunha SiebenNessuna valutazione finora
- Immobilization of Protease EnzymeDocumento10 pagineImmobilization of Protease EnzymeSnape the PrinceNessuna valutazione finora
- 316 Report DraftDocumento53 pagine316 Report DraftSimon TsoiNessuna valutazione finora
- International Journal of Biological Macromolecules: Hafsa Sattar, Afsheen Aman, Shah Ali Ul QaderDocumento6 pagineInternational Journal of Biological Macromolecules: Hafsa Sattar, Afsheen Aman, Shah Ali Ul QaderSinethembaNessuna valutazione finora
- Soybean Glucosidase Immobilisated On Chitosan Beads and Its Application in Soy Drink Increase The AglyconesDocumento8 pagineSoybean Glucosidase Immobilisated On Chitosan Beads and Its Application in Soy Drink Increase The AglyconesMaruf MuhammadNessuna valutazione finora
- 932 HK081Documento2 pagine932 HK081rkccharanNessuna valutazione finora
- HPLC Method Detects Fructo-Oligosaccharides in DairyDocumento5 pagineHPLC Method Detects Fructo-Oligosaccharides in DairyAnju DoraisamyNessuna valutazione finora
- Protocol of E.BDocumento7 pagineProtocol of E.BAsimNessuna valutazione finora
- Articulo 1 PDFDocumento19 pagineArticulo 1 PDFJennifer A. PatiñoNessuna valutazione finora
- Fungal EnzymesDocumento9 pagineFungal EnzymesYui Bioscience PalsanNessuna valutazione finora
- Baieli Et Al-2012-Journal of Separation ScienceDocumento8 pagineBaieli Et Al-2012-Journal of Separation ScienceEmanuelMuruagaNessuna valutazione finora
- Soy Protein-Based Blends, Composites and NanocompositesDa EverandSoy Protein-Based Blends, Composites and NanocompositesNessuna valutazione finora
- Experimental approaches to Biopharmaceutics and PharmacokineticsDa EverandExperimental approaches to Biopharmaceutics and PharmacokineticsNessuna valutazione finora
- Acer Software License InformationDocumento7 pagineAcer Software License Informationムキノイ ア乇尺ムズNessuna valutazione finora
- 32 Workbook Answer KeyDocumento16 pagine32 Workbook Answer Keyisaacfg1Nessuna valutazione finora
- Interchange 3ed Teacher Lev1 Unit7Documento6 pagineInterchange 3ed Teacher Lev1 Unit7isaacfg1Nessuna valutazione finora
- Interchange4thEd IntroLevel Unit01 Extra WorksheetDocumento2 pagineInterchange4thEd IntroLevel Unit01 Extra WorksheetZINEB10Nessuna valutazione finora
- Signalling Defects and Disease - 2012Documento75 pagineSignalling Defects and Disease - 2012isaacfg1Nessuna valutazione finora
- In Vitro Filament Like Formation Upon Interaction Between Lens A Crystallin and BL Crystallin Promoted by Stress - 2000Documento5 pagineIn Vitro Filament Like Formation Upon Interaction Between Lens A Crystallin and BL Crystallin Promoted by Stress - 2000isaacfg1Nessuna valutazione finora
- Identification of A Novel Ligand Binding Motif in The Transthyretin Channel - 2010Documento11 pagineIdentification of A Novel Ligand Binding Motif in The Transthyretin Channel - 2010isaacfg1Nessuna valutazione finora
- Docking Tutorial DocumentationDocumento13 pagineDocking Tutorial Documentationisaacfg1Nessuna valutazione finora
- Chaperones and Foldases in Endoplasmic Reticulum Stress Signaling in Plants - 2011Documento5 pagineChaperones and Foldases in Endoplasmic Reticulum Stress Signaling in Plants - 2011isaacfg1Nessuna valutazione finora
- Manual-01 Básico EjDocumento7 pagineManual-01 Básico Ejisaacfg1Nessuna valutazione finora
- In Vitro Filament Like Formation Upon Interaction Between Lens A Crystallin and BL Crystallin Promoted by Stress - 2000Documento5 pagineIn Vitro Filament Like Formation Upon Interaction Between Lens A Crystallin and BL Crystallin Promoted by Stress - 2000isaacfg1Nessuna valutazione finora
- Viewing Molecular Mechanisms of Ageing Through A Lens - 2005Documento15 pagineViewing Molecular Mechanisms of Ageing Through A Lens - 2005isaacfg1Nessuna valutazione finora
- Biological and Chemical Approaches To Diseases of Proteostasis Deficiency - 2009Documento36 pagineBiological and Chemical Approaches To Diseases of Proteostasis Deficiency - 2009isaacfg1Nessuna valutazione finora
- Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCRDocumento7 pagineAnalysis of Relative Gene Expression Data Using Real-Time Quantitative PCRSivapong VsmuNessuna valutazione finora
- Redox Biology: Tobias Jung, Tilman GruneDocumento5 pagineRedox Biology: Tobias Jung, Tilman Gruneisaacfg1Nessuna valutazione finora
- Biophysical Chemistry: Maximilian N. Andrews, Roland WinterDocumento8 pagineBiophysical Chemistry: Maximilian N. Andrews, Roland Winterisaacfg1Nessuna valutazione finora
- Biobanks and Use of Samples of Human Origin For SurgicalDocumento6 pagineBiobanks and Use of Samples of Human Origin For Surgicalisaacfg1Nessuna valutazione finora
- Islet Amyloid Polypeptide and DiabetesDocumento8 pagineIslet Amyloid Polypeptide and Diabetesisaacfg1Nessuna valutazione finora
- Automated Docking For Novel Drug Discovery, 2013Documento14 pagineAutomated Docking For Novel Drug Discovery, 2013isaacfg1Nessuna valutazione finora
- Proteomic Typing of Amyloid Deposits in Systemic Amyloidoses, 2011Documento6 pagineProteomic Typing of Amyloid Deposits in Systemic Amyloidoses, 2011isaacfg1Nessuna valutazione finora
- Inhibition of Amyloid Formation - 2012Documento25 pagineInhibition of Amyloid Formation - 2012isaacfg1Nessuna valutazione finora
- Blockade of Islet Amyloid Polypeptide Fibrillation - 2013Documento6 pagineBlockade of Islet Amyloid Polypeptide Fibrillation - 2013isaacfg1Nessuna valutazione finora
- Animal Models of DM2 With Reduced Pancreatic Β-cell Mass - 2006Documento21 pagineAnimal Models of DM2 With Reduced Pancreatic Β-cell Mass - 2006isaacfg1Nessuna valutazione finora
- Protein Ligand DockingDocumento18 pagineProtein Ligand Dockingisaacfg1Nessuna valutazione finora
- Protein-Misfolding Diseases - 2002Documento2 pagineProtein-Misfolding Diseases - 2002isaacfg1Nessuna valutazione finora
- Folding Proteins in Fatal Ways - 2003Documento6 pagineFolding Proteins in Fatal Ways - 2003isaacfg1Nessuna valutazione finora
- Ultrastructural Organization of Amyloid Fibrils by AFMDocumento12 pagineUltrastructural Organization of Amyloid Fibrils by AFMisaacfg1Nessuna valutazione finora
- Influenza Neuraminidase Inhibitor IC50 Data AnalysisDocumento34 pagineInfluenza Neuraminidase Inhibitor IC50 Data Analysisisaacfg1Nessuna valutazione finora
- Mechanisms of Protein Circular Dichroism Insights From Computational ModelingDocumento31 pagineMechanisms of Protein Circular Dichroism Insights From Computational Modelingisaacfg1Nessuna valutazione finora
- RAPID DETECTION OF ALDEHYDE FLAVOURDocumento5 pagineRAPID DETECTION OF ALDEHYDE FLAVOURwahyuningsihNessuna valutazione finora
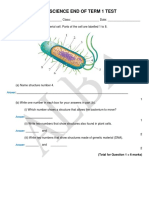
- Year 8 Science End of Term 1 Test: AnswerDocumento11 pagineYear 8 Science End of Term 1 Test: Answerchan myaeNessuna valutazione finora
- MECANIQUE - Construction Practice Onshore SteelDocumento54 pagineMECANIQUE - Construction Practice Onshore SteelYaser ShabasyNessuna valutazione finora
- Kinetics of The Selective Hydrogenation of Phenol To Cyclohexanone Over A Pd-Alumina CatalystDocumento8 pagineKinetics of The Selective Hydrogenation of Phenol To Cyclohexanone Over A Pd-Alumina CatalystTaylor PennaNessuna valutazione finora
- The Radio Chemistry of Mercury - Us AECDocumento211 pagineThe Radio Chemistry of Mercury - Us AEClondonbluetopazNessuna valutazione finora
- MIL-DTL-24441D, TYPE IV: Protective & Marine CoatingsDocumento4 pagineMIL-DTL-24441D, TYPE IV: Protective & Marine CoatingsEduardo Antonio Burgos RuidíasNessuna valutazione finora
- Solid Hollow Plug PDFDocumento3 pagineSolid Hollow Plug PDFSandra DevannyNessuna valutazione finora
- Chapter 2-The Column in GCDocumento92 pagineChapter 2-The Column in GCkhanhvan2105Nessuna valutazione finora
- I ObjectivesDocumento3 pagineI ObjectivesEdelyn Dimatulac TorrelizaNessuna valutazione finora
- Manufacturing Method For CompositesDocumento41 pagineManufacturing Method For CompositestpmendozaNessuna valutazione finora
- Analisis de Gas de Aceite de Transformador Utilizando Una Columna de Separacion Astm d3612 KasalabDocumento2 pagineAnalisis de Gas de Aceite de Transformador Utilizando Una Columna de Separacion Astm d3612 KasalabJohnatan HernándezNessuna valutazione finora
- Journal of Controlled Release: Alexander Wei, Jonathan G. Mehtala, Anil K. PatriDocumento11 pagineJournal of Controlled Release: Alexander Wei, Jonathan G. Mehtala, Anil K. Patriprakush_prakushNessuna valutazione finora
- Belonio, Roi Emman E.Documento4 pagineBelonio, Roi Emman E.Adrian Nazrene BitoonNessuna valutazione finora
- Fluid Properties and Unit Conversions ProgramDocumento13 pagineFluid Properties and Unit Conversions ProgramNawaz KhanNessuna valutazione finora
- AKL10 Laser Technology LiveDocumento49 pagineAKL10 Laser Technology LiveXin ChenNessuna valutazione finora
- Wieland CatalogueDocumento40 pagineWieland Catalogueazb00178Nessuna valutazione finora
- Formulation and Evaluation of Orally Disintegrating Tablets of SertralineDocumento7 pagineFormulation and Evaluation of Orally Disintegrating Tablets of SertralineDinesh BabuNessuna valutazione finora
- Protect and seal concrete floors with PAVIMYC HB 2/CDocumento6 pagineProtect and seal concrete floors with PAVIMYC HB 2/CDory KiblawiNessuna valutazione finora
- Ultraviolet RaysDocumento17 pagineUltraviolet RaysZUBAIR SHAHNessuna valutazione finora
- RHOPLEX™ EC-3000: 100% Acrylic Polymer For The Roof Coatings MarketDocumento4 pagineRHOPLEX™ EC-3000: 100% Acrylic Polymer For The Roof Coatings MarketLong An DoNessuna valutazione finora
- Nova Hunting The Elements Video GuideDocumento3 pagineNova Hunting The Elements Video GuideJaclyn Dugger100% (1)
- Cryocap Air Liquide - BrochureDocumento20 pagineCryocap Air Liquide - BrochurePriyanshi VNessuna valutazione finora
- Environmental Toxicants and Infant Mortality in The UsaDocumento26 pagineEnvironmental Toxicants and Infant Mortality in The UsaRobert E. WaltonNessuna valutazione finora
- SDS for DichloromethaneDocumento33 pagineSDS for DichloromethaneAlexNessuna valutazione finora
- Astm D4053Documento3 pagineAstm D4053AndygarciaNessuna valutazione finora
- Life's Origin TheoriesDocumento8 pagineLife's Origin TheoriesSazzad NiloyNessuna valutazione finora