Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Ehh
Caricato da
Gunjan SolankiCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Ehh
Caricato da
Gunjan SolankiCopyright:
Formati disponibili
Units: R= Joule/K Mol >Fluid is a substance that does not permanently resist distortion.
>Shear stress during slip depend on the magnitude of viscosity and rate of slidi ng. >Pressure depend upon the direction and orientation. >Mach Mach number is the relative velocity of a fluid compared to its sonic ve locity. Mach numbers less than 1 correspond to sub-sonic velocities, and Mach nu mbers > 1 correspond to super-sonic velocities. >Navier-Stokes Equations The motion of a non-turbulent, Newtonian fluid is governed by the Navier-Stokes equation. The equation can be used to model turbulent flow, where the fluid para meters are interpreted as time-averaged values. >>>>>>The Prandtl number is a dimensionless number; the ratio of momentum diffu sivity (kinematic viscosity) to thermal diffusivity. It is named after the Germa n physicist Ludwig Prandtl. It is defined as: Pr= [Momentum diffusivity/Thermal diffusivity] = viscous diffusion rate / thermal diffusion rate =Cp (mu) / k - mercury has .015 and earth has 10^25, water around 7. >For mercury, heat conduction is very effective compared to convection: thermal diffusivity is dominant. For engine oil, convection is very effective in transfe rring energy from an area, compared to pure conduction: momentum diffusivity is dominant. In heat transfer problems, the Prandtl number controls the relative thickness of the momentum and thermal boundary layers. When Pr is small, it means that the h eat diffuses very quickly comp2ared to the velocity (momentum). This means that for liquid metals the thickness of the thermal boundary layer is much bigger tha n the velocity boundary layer. The mass transfer analog of the Prandtl number is the Schmidt number.
>>>>>schmidt number: ration of momentum diffusivity and mass diffusivity. Sc = (dynamics viscosity)/(density*mass diffusivity) viscosity= Pa. s and N.s/m^2 >>Mean free path in thermodynamics is the distance travelled before it again hit the molecules. >>prandtle mixing length is the distance travelled by the parcel of fluid before changing its property. >>Potential flow: No viscosity and incompressible, no dissipation of heat and n o eddies. >>Froude number: Internial force divided by gravitational force. >>Unit of kinematic viscosity is stoke. >>Limitation of bernouili:
The following two assumptions must be met for this Bernoulli equation to ap ply: the flow must be incompressible even though pressure varies, the density must re main constant along a streamline; friction by viscous forces has to be negligible. In long lines mechanical energy dissipation as heat will occur. This loss can be estimated e.g. using Darcy Weisb ach equation. -If the liquid is of curved path, it does not account for centrifugal force. -Mean velocity is considered. >>>>>>The Grashof number (Gr) is a dimensionless number in fluid dynamics and he at transfer which approximates the ratio of the buoyancy to viscous force acting on a fluid. It frequently arises in the study of situations involving natural c onvection. It is named after the German engineer Franz Grashof. -- For turbulent force Gr is 10^8 to 10^9 >>>In forced convection the Reynolds Number governs the fluid flow. But, in natu ral convection the Grashof Number is the dimensionless parameter that governs th e fluid flow. Using the energy equation and the buoyant force combined with dime nsional analysis provides two different ways to derive the Grashof Number. >>Hydraulic diameter is the ration of area wetted divided by wetted perimeter. -for round tube: Dh = 4A/P. >>>The hydraulic radius is a measure of a channel flow efficiency. Flow speed al ong the channel depends on its cross-sectional shape (among other factors), and the hydraulic radius is a characterisation of the channel that intends to captur e such efficiency. Based on the 'constant shear stress at the boundary' assumpti on,[5] hydraulic radius is defined as the ratio of the channel's cross-sectional area of the flow to its wetted perimeter (the portion of the cross-section's pe rimeter that is "wet") Rh = A/P -- greater that hydraulic radius , greater the efficiency of channel and more vo lume it can carry. >> Fd= 6(pi)(mu)rv >>In fluid dynamics, a stagnation point is a point in a flow field where the loc al velocity of the fluid is zero. Stagnation points exist at the surface of objects in the flow field, where the f luid is brought to rest by the object. The Bernoulli equation shows that the sta tic pressure is highest when the velocity is zero and hence static pressure is a t its maximum value at stagnation points. This static pressure is called the sta gnation pressure. >>Superficial velocity (or superficial flow velocity), in engineering of multiph ase flows and flows in porous media, is a hypothetical (artificial) fluid veloci ty calculated as if the given phase or fluid were the only one flowing or presen t in a given cross sectional area. Other phases, particles, the skeleton of the porous medium, etc. present in the channel are disregarded. >>>>>>>>>>>LAWS OF THERMODYNAMICS: --Zeroth law of thermodynamics: If two systems are in thermal equilibrium with a third system, they must be in thermal equilibrium with each other. This law hel
ps define the notion of temperature. --fIRST LAW: The increase in internal energy of a body is equal to the heat supp lied to the body minus work done by the body. For a thermodynamic cycle, the heat supplied to a closed system, minus that remo ved from it, equals the net work done by the system. --SECOND LAW: The second law of thermodynamics asserts the irreversibility of na tural processes, and the tendency of natural processes to lead towards spatial h omogeneity of matter and energy, and especially of temperature. It can be formul ated in a variety of interesting and important ways. --Third law: he entropy of a system approaches a constant value as the temperatu re approaches zero. --SEnsible heat: Sensible heat is heat exchanged by a body or thermodynamic sys tem that has as its sole effect a change of temperature Qs= mc(delta T) --Latent heat is the energy released or absorbed by a body or a thermodynamic sy stem during a constant-temperature process. A typical example is a change of sta te of matter, meaning a phase transition such as the melting of ice or the boili ng of water. L=Q/m --Enthalpy is a measure of the total energy of a thermodynamic system. It includ es the system's internal energy and thermodynamic potential (a state function), as well as its volume and pressure >>equation of thermodynamics: 1) H= U+pV 2)A=U-TS 3)G=H-TS >>In thermodynamics, the Helmholtz free energy is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a const ant temperature. >>In thermodynamics, the Gibbs free energy (IUPAC recommended name: Gibbs energy or Gibbs function; also known as free enthalpy[1] to distinguish it from Helmho ltz free energy) is a thermodynamic potential that measures the "usefulness" or process-initiating work obtainable from a thermodynamic system at a constant tem perature and pressure (isothermal, isobaric). Just as in mechanics, where potent ial energy is defined as capacity to do work, similarly different potentials hav e different meanings. The Gibbs free energy is the maximum amount of non-expansi on work that can be extracted from a closed system; this maximum can be attained only in a completely reversible process. When a system changes from a well-defi ned initial state to a well-defined final state, the Gibbs free energy ?G equals the work exchanged by the system with its surroundings, minus the work of the p ressure forces, during a reversible transformation of the system from the same i nitial state to the same final state.
Potrebbero piacerti anche
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- SDM - 2016-18 - Course OutlineDocumento22 pagineSDM - 2016-18 - Course OutlineGunjan SolankiNessuna valutazione finora
- CASE 6: Hanover Bates Chemical Corporation: Group 5Documento1 paginaCASE 6: Hanover Bates Chemical Corporation: Group 5Gunjan SolankiNessuna valutazione finora
- Floor Pricing of "Plasto Cover" Floor Cover: 140 Price of "Plasto Cover" For Time of 5 Year in Case of Optimistic SellingDocumento4 pagineFloor Pricing of "Plasto Cover" Floor Cover: 140 Price of "Plasto Cover" For Time of 5 Year in Case of Optimistic SellingGunjan SolankiNessuna valutazione finora
- Mont BlancDocumento1 paginaMont BlancGunjan SolankiNessuna valutazione finora
- 507419143 (1)Documento2 pagine507419143 (1)Gunjan Solanki0% (1)
- Salary Slip Sept 2014 PDFDocumento2 pagineSalary Slip Sept 2014 PDFGunjan SolankiNessuna valutazione finora
- Body BoiloutDocumento1 paginaBody BoiloutGunjan SolankiNessuna valutazione finora
- Unesco - Eolss Sample Chapters: Electrostatic PrecipitatorsDocumento9 pagineUnesco - Eolss Sample Chapters: Electrostatic PrecipitatorsGunjan SolankiNessuna valutazione finora
- State Bank of India ReciptDocumento1 paginaState Bank of India ReciptGunjan SolankiNessuna valutazione finora
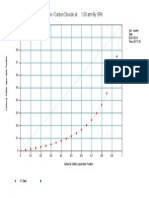
- Carbonyl Sulfide / Carbon Dioxide at 1.00 Atm by SRK: Job: Reactor Date: 05/21/2014 Time: 00:17:30Documento1 paginaCarbonyl Sulfide / Carbon Dioxide at 1.00 Atm by SRK: Job: Reactor Date: 05/21/2014 Time: 00:17:30Gunjan SolankiNessuna valutazione finora
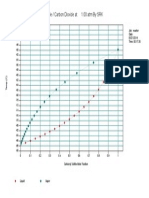
- Carbonyl Sulfide / Carbon Dioxide at 1.00 Atm by SRK: Job: Reactor Date: 05/21/2014 Time: 00:17:30Documento1 paginaCarbonyl Sulfide / Carbon Dioxide at 1.00 Atm by SRK: Job: Reactor Date: 05/21/2014 Time: 00:17:30Gunjan SolankiNessuna valutazione finora
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- EN 288-3xDocumento38 pagineEN 288-3xSyah Reza Maulana0% (1)
- Amit Goswami Quantum Mechanics, Second Edition 2003Documento577 pagineAmit Goswami Quantum Mechanics, Second Edition 2003Solange Ev75% (4)
- Valves: Oscar Mauricio Cala Camacho - 2152815 Iván Darío Nova Uribe - 2142795 Facilidades de Superficie Grupo H1 2020-1Documento34 pagineValves: Oscar Mauricio Cala Camacho - 2152815 Iván Darío Nova Uribe - 2142795 Facilidades de Superficie Grupo H1 2020-1Oscar CalaNessuna valutazione finora
- Dive Scubapro - Mk2+, Mk2 - Maintenance ProcedureDocumento4 pagineDive Scubapro - Mk2+, Mk2 - Maintenance ProceduremanonpomNessuna valutazione finora
- Maxon DC MotorDocumento16 pagineMaxon DC MotorteguhNessuna valutazione finora
- Ne6 3911gb-MidlumDocumento234 pagineNe6 3911gb-MidlumDinu GabrielNessuna valutazione finora
- I.S. Code - 456 Recommendation:: 1. For ConcreteDocumento4 pagineI.S. Code - 456 Recommendation:: 1. For ConcreteSonu PanwarNessuna valutazione finora
- Deformacao AxialDocumento31 pagineDeformacao AxialANTONIONessuna valutazione finora
- CE142P 2 Lab Report 1 Soberano PDFDocumento14 pagineCE142P 2 Lab Report 1 Soberano PDFqwert qwertyNessuna valutazione finora
- Boiling and CondensationDocumento13 pagineBoiling and CondensationPrithvi de VilliersNessuna valutazione finora
- Section 32150 - Bonded Epoxy LiningDocumento11 pagineSection 32150 - Bonded Epoxy LininghelalsolimanNessuna valutazione finora
- WRC 302-1985Documento38 pagineWRC 302-1985CarlosNessuna valutazione finora
- Extensive Applications of PM Gears: FocusDocumento4 pagineExtensive Applications of PM Gears: FocusAAAHNessuna valutazione finora
- MINI Clubman Mobile Vehicle RepairDocumento5 pagineMINI Clubman Mobile Vehicle RepairJamal NoorNessuna valutazione finora
- Riwayat Pemeliharaan Alat Wirtgen 2Documento2 pagineRiwayat Pemeliharaan Alat Wirtgen 2Aulia Rizeky JanuaryNessuna valutazione finora
- 3 Cross Wheel LacingDocumento41 pagine3 Cross Wheel LacingbobNessuna valutazione finora
- Steering Gear TestingDocumento9 pagineSteering Gear TestingArun GK100% (1)
- DRG ListDocumento272 pagineDRG ListVijay PalNessuna valutazione finora
- CF L3 84 EE66011542888876-1Documento8 pagineCF L3 84 EE66011542888876-1Mauli Dudhmogare PatilNessuna valutazione finora
- Cutting Edges & End Bits: Bolt-On & Weld-In Cutting Edges For Buckets & BladesDocumento32 pagineCutting Edges & End Bits: Bolt-On & Weld-In Cutting Edges For Buckets & Blades8897477809Nessuna valutazione finora
- B10 1130 Boron Steel Heattreated Before and After StampingDocumento15 pagineB10 1130 Boron Steel Heattreated Before and After Stamping3MECH015 Bhavatharan SNessuna valutazione finora
- MEC331 - Revision Gear FinalDocumento9 pagineMEC331 - Revision Gear FinalDiana PinkyNessuna valutazione finora
- Catalogo de Partes Blue BirdDocumento112 pagineCatalogo de Partes Blue Birdjhongua7Nessuna valutazione finora
- Measuring InstrumentsDocumento10 pagineMeasuring Instrumentsmohsin931Nessuna valutazione finora
- 03 FloWatch HGVF Input and OutputDocumento21 pagine03 FloWatch HGVF Input and Outputedwin_triana_9Nessuna valutazione finora
- Sharkbite Evopex Product Catalog 08 2020Documento21 pagineSharkbite Evopex Product Catalog 08 2020Arq Cesar Augusto Mejia GNessuna valutazione finora
- Bomba A2fo Bosch RexrothDocumento3 pagineBomba A2fo Bosch RexrothHIDRAFLUIDNessuna valutazione finora
- Syllabus Mechanical Qaqc Course Sdlinc 9600162099Documento3 pagineSyllabus Mechanical Qaqc Course Sdlinc 9600162099Sridurgha Lakshmi Inc SDLINC NDT QA QC INSTITUTE100% (3)
- ROHR2fesu FeaturelistDocumento20 pagineROHR2fesu FeaturelistJohan ConradieNessuna valutazione finora
- Revision Status: Details Accepted Prepared Rev Date Rev NRDocumento49 pagineRevision Status: Details Accepted Prepared Rev Date Rev NRRupam BaruahNessuna valutazione finora