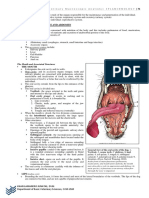
Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Formulation and Evaluation of Microspheres Containing Fluvastatin Sodium
Caricato da
Rajesh KumarTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Formulation and Evaluation of Microspheres Containing Fluvastatin Sodium
Caricato da
Rajesh KumarCopyright:
Formati disponibili
International Journal of Drug Development & Research | April-June 2012 | Vol. 4 | Issue 2 | ISSN 0975-9344 | Available online http://www.ijddr.
in Covered in Official Product of Elsevier, The Netherlands SJR Impact Value 0.03 & H index 2 2012 IJDDR
Formulation and Evaluation of Microspheres Containing Fluvastatin Sodium
Venkatesh D. P1, Roopa Karki1,Sajal Kumar Jha2, Geetha Lakshmi A3, Santha Kumar G.S1, Divakar Goli1 1 Dept. of Pharmaceutics, Acharya & BM Reddy College of Pharmacy, Soldevanahalli, Blore, Karnataka, India 2 Dept. of Pharmaceutics, Bengal College of Pharmaceutical Sciences & Research, Durgapur, West Bengal, India 3 Department of Pharmaceutics, Oxford College of Pharmacy, Bangalore, India.
FULL Length Research Paper Covered in Index Copernicus with IC Value 4.68 for 2010
Abstract
Fluvastatin sodium is a cholesterol lowering agent. It has shorter half life (1.2).It undergoes extensive first pass metabolism. Frequent dosing is required in case of conventional dosage form. The purpose of the study is to formulate microcapsules containing Fluvastatin sodium by complexing with anionic exchange cholestyramine resin Indion-454 by coating with ethyl cellulose, eudragit-RS100 polymers for achieving controlled release in the small intestine. Complexation of drug on the resins by batch method. Compatibility studies were carried out using FTIR, DSC and Xray diffraction studies. Microencapsulation was carried out by w/o/w double emulsion solvent evaporation technique. Characterization of various physicochemical properties like % Yield, % DEE, % Polymer coating and particle size were evaluated. In vitro drug release studies were carried out in USP type I dissolution test apparatus. The accelerated stability studies were carried out on the most satisfactory formulations. FTIR, X-Ray diffraction and DSC spectra of drug, ion-exchange resin and drug-polymers revealed that no chemical interaction. Drug loading was observed in 4 hrs 1:4 ratio. Percentage yield for the formulations observed that for F1 to F8 is varied from 93.45.84 to 98.76.30. Percentage drug entrapment efficiency was observed for the formulations of F1 to F8 are varied from 960% to 98.30.1%. Microcapsules of F4 and F5 prepared with EudragitRS100 and Ethyl cellulose using ion-exchange resins provide a convenient dosage forms for achieving best performance regarding release study, there is no changes in % DEE and %CDR was observed after stability studies.
Key words: Ion exchange resin, Fluvastatin sodium, drugresinates, SEM How to Cite this Paper: Venkatesh D. P1, Roopa Karki1,Sajal Kumar Jha2, Geetha Lakshmi A3, Santha Kumar G.S1, Divakar Goli1 Formulation and Evaluation of Microspheres Containing Fluvastatin Sodium, Int. J. Drug Dev. & Res., April-June 2012, 4(2): 306-314 Copyright 2012 IJDDR, Venkatesh. D. P et al. This is an open access paper distributed under the copyright agreement with Serials Publication, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Article History:-----------------------Date of Submission: 04-05-2012 Date of Acceptance: 02-06-2012 Conflict of Interest: NIL Source of Support: NONE
Introduction: Now a days obesity is more health concern in world *Corresponding author, Mailing address: Venkatesh. D. P Email: venkateshdp@acharya.ac.in wide. Obesity is a national epidemic (1). A controlled release drug delivery system is usually designed to deliver the drug at particular rate controlled release properties can also be imparted to oral dosage
Int. J. Drug Dev. & Res., April-June 2012, 4 (2): 306-314 Covered in Scopus & Embase, Elsevier
306
Venkatesh. D. P et al: Formulation and Evaluation of Microspheres Containing Fluvastatin Sodium
formulations through the formation of resin-drug complexes coated with polymers(2). Fluvastatin sodium, a 3-hydroxy-3 methyl glutaryl co-enzyme (HMG COA) reductase inhibitor, is a lipid regulating drug with actions on plasma lipids, inhibits the HMG-COA reductase leads to reduced cholesterol synthesis in the liver and lower
cellulose from central drug house Pvt. Ltd, New Delhi. EudragitRS 100 from Micro Labs, Bangalore. Preformulation studies: Drug polymer compatibility studies were carried out using FTIR, DSC and X-ray diffraction studies. Complexation of drug on the resin (preparation of drug-resinate complex): Preparation of drug-resinate was tried by batch method (9). Accurately weighed drug and cholestyramine resins in 1:4 ratios. Then slurry of resin was made by using 100 mL distilled water and stirred half an hour at 500 rpm, in order to allow the polymer structure to swell uniformly. Drug solution was made by using 2 mL 0.1N NaOH, then slowly drug solution was added to resin slurry under stirred condition, The mixture was stirred for 4hrs continuously on magnetic stirrer till equilibrium was established. Drug loading of Fluvastatin sodium was determined spectrophotometrically by at 304nm. Determination of amount of uncomplexed drug by UV: The mixture to be kept aside to allow the particles to sediment and then filtered. From the filtrate 1mL was transferred into volumetric flask and volume was adjusted with 0.1N NaOH. After suitable dilution, drug was determined spectrophotometrically at 304nm (10). Microencapsulation of drug-resinates: To further retard the drug release, the resinate particles were coated with ethyl cellulose, eudragit RS100 with different ratios. Microencapsulation was carried out by w/o/w technique. Drug resin complex 1.0 gram was poured in 20 mL of methylene chloride containing polymers (As shown in the table1). The solution was mixed for 30 sec, using vertex mixer. To make w/o emulsion first by added 1 ml of water. Then added 100mL of 0.01%w/w cold poly vinyl alcohol (PVA) solution to form w/o/w double emulsion. Continuously stirred at room temperature until methylene chloride gets evaporated to form solid microcapsules.
FULL Length Research Paper Covered in Index Copernicus with IC Value 4.68 for 2010
intracellular cholesterol concentrations. The drug has a relatively short half life (1.2) hours and it undergoes extensively first metabolism in the liver (3). The cholestyramine resin is an insoluble strongly basic anion exchange resin in the chloride form supplied as a dry fine powder. Cholestyramine resin is suitable for pharmaceutical applications as carrier for anionic drug substances. When used as an active ingredient, cholestyramine resins a strong pharmacological bile salt-binding agent binds bile acid, this leads to replenishment of the bile acids through increased catabolism of serum cholesterol resulting in lowered serum cholesterol levels (4). Anionic exchange cholestyramine resin Indion-454 as a drug carrier, it complexes with anionic drug. Ion exchange process alone, without any barrier cannot achieve satisfactory controlled release. Thus the resin complexes were coated with ethyl cellulose, eudragitRS100 polymers for achieving controlled release in the small intestine. The drug is released from the resin in vivo as the drug resinates reaches equilibrium with the high electrolyte concentrations typically found in the gastrointestinal tract (2). The drug release from the drug-resin complex occurs by replacement of drug molecule by sodium, potassium and chloride ions in the GI tract and diffusion of drug molecule from the resin (8). Materials & Methods: Fluvastain sodium was gift sample from Biocon Company, Bangalore and Ion exchange resins gift sample from Ion exchange resins Ltd Mumbai. Ethyl
307
Int. J. Drug Dev. & Res., April-June 2012, 4 (2): 306-314 Covered in Scopus & Embase, Elsevier
Venkatesh. D. P et al: Formulation and Evaluation of Microspheres Containing Fluvastatin Sodium
Table 1: Microencapsulation formulation chart
Formulation code Drugresinates taken(g) 1 1 1 1 Eudragit RS100 Polymer coating ratios (%) 5 10 15 20 Formulation code Drugresinates taken(g) 1 1 1 1 Ethyl Cellulose Polymer coating ratios(mg) 5 10 15 20
dichloromethane to remove polymer coating. The remaining drug-resinate core were dried at room temperature for 12 hrs and weighed. Percentage coating polymer =Microcapsules weight Dried complex weight/Microcapsules weight 100 In-vitro drug release study: In vitro drug release studies were carried out in Type: USP type I dissolution test apparatus. Microspheres equivalent to 80 mg of the drug were used for dissolution study. The dissolution test was carried out using 870 ml of dissolution medium at pH 1.2 for first 2 h and volume was made up to 900ml by adjusting the pH 6.8 for remaining 6h, a speed of 100 rpm and temperature 37 0.5C was employed. The amount of dissolved drug was determined using UV spectrophotometer method at 304 nm.
F1 F2 F3 F4
F5 F6 F7 F8
FULL Length Research Paper Covered in Index Copernicus with IC Value 4.68 for 2010
Evaluation of microspheres: Microscopic examination: Particle size and shape of microspheres was observed using compound microscope. Scanning Electron Microscopy (SEM): Dried microspheres were mounted into stubs by using double sided adhesive tape. The microspheres were coated with gold and observed under scanning electron microscope for surface characteristics. Particle size analysis: Particle size analysis was determined using compound microscope with the help of stage micrometer and eye piece micrometer, counted at least 100 microspheres per batch. Yield of microspheres: Yield of microspheres was calculated by actual weight of microspheres divided by total weight of copolymers and drug. Percentage Yield = (Actual weight of microspheres / total weight of copolymers and drug) 100 Percentage (%DEE) Accurately weighed drug resinates equivalent to 10 mg of drug was stirred with 100mL of PH 6.8 for 2hours.The solution was filtered and after suitable dilution drug content was determined spectrophotometrically at 304nm. Percentage DEE = (Amount of drug actually present / theoretical drug load expected) 100 Determination microspheres One gram of the microcapsules was accurately weighed and washed 3 times with 20mL of coating polymer on drug entrapment efficiency
Stability studies: The accelerated stability studies were carried out on the most satisfactory formulations. The formulations sealed in aluminum packaging and kept in humidity chamber maintained at 30 2 C / 65 5 % RH and 40 2 C / 75 5% RH for three months. At the end of studies, samples were analyzed for the drug content, in-vitro dissolution and other physicochemical parameters. Results & Discussion: Drug-resinates were prepared by batch method, it was stirred for 4 h continuously in which optimum drug loading was observed in 1:4 ratio because increase in the amount of resins increases the amount of drug complexed from the solution but decreases in the drug content/gram of resinates. FTIR spectra of pure drug shows the peaks at 1587cm-1 due to C=O streching, 970 cm-1 due to aryl-F functional group, 3335cm-1 due to aryl-H , 3247 cm-1 due to C=C stretching, 3878 cm-1 due to O-H bending which are characteristic band for pure drug Fluvastatin Sodium. After complex with resins,
Int. J. Drug Dev. & Res., April-June 2012, 4 (2): 306-314 Covered in Scopus & Embase, Elsevier
308
Venkatesh. D. P et al: Formulation and Evaluation of Microspheres Containing Fluvastatin Sodium
same pure drug considerable peak was observed from the drug resin complex(Fig1). The X-Ray diffraction shows that crystalline characteristics with drug alone. After complexing with resins diffused peak was observed due to amorphous characteristic (Fig2). DSC spectrum of pure drug, exhibited a sharp exothermic peak at 375.1C, resinates 381.4 C and resinates coated with Eudragit RS100 402.4C & Ethyl cellulose 434.9 C due to complexation, it deviates from pure drug Fluvastain Sodium (Fig 3). Since it can be revealed that there is no interaction between drug resinates and polymers. Morphology of microcapsules observed from compound microscope and SEM as shown in the figure 5&6. In SEM, Number of micropores present on the surface. Various physicochemical properties like % Yield, % Drug entrapment, % Polymer coating and particle size were evaluated as shown in the table 1. Percentage yield for the formulations of F1 to F8 varied from 93.45.84 to 98.76.30 due to increase in polymers thickness. Percentage drug entrapment efficiency for the formulations of F1 to F8 varied from 960.8% to 98.70.1%. In-vitro drug dissolution was carried out for all the formulations as shown in the figure 8 & 9. The drug release in acidic medium (pH1.2) is low due to drug is weak acid and its pKa value is 5.5, but when drug resinates comes in contact with alkali medium (pH 6.8), drug release rate is faster because of due to phosphate ions. The drug release rate from
10 0
the Eudragit RS100 and Ethyl cellulose coated microcapsules was slower than that from the uncoated resinates. The drug release from microcapsules data were fitted into drug release kinetic models such as zero order, first order, higuchi and korsmeyers-peppas equation. The exchange of drug from the uncoated resinate was found to follow the particle diffusion process accordance with the equation proposed by Reichenberg in 1953. However coated resinates was deviated from particle diffusion mechanism. The release of drug across the thin layer around the particle release (membrane system. Hence diffusion data control) was fitted was into predominant mechanism involved in this drug
Tra nsm itta nce [% ]
90
92
94
96
98
FULL Length Research Paper Covered in Index Copernicus with IC Value 4.68 for 2010
korsmeyer-peppas equation Mt/M = Ktn, where K is a constant; n is the release exponent, indicates the drug mechanism and Mt/M fraction of drug released at time t. The values of n varied from 0.07 to 0.31. This indicates that fickian type of mechanism. The release data from the Higuchi equation yielded comparable linearity. Eudragit RS100 and Ethyl cellulose coated microcapsules obeyed diffusion controlled process as shown in the table3. Based on drug release kinetics data microcapsules of F4 and F5 formulations provide a convenient dosage form for achieved controlled release, percentage yield, percentage DEE and obeyed diffusion controlled process. There was negligible change in % DEE and in-vitro drug release was observed after stability studies.
(a)
309
Int. J. Drug Dev. & Res., April-June 2012, 4 (2): 306-314 Covered in Scopus & Embase, Elsevier
Venkatesh. D. P et al: Formulation and Evaluation of Microspheres Containing Fluvastatin Sodium
1 0 0 .0 T ra n s m itta n c e[% ] 9 8 .0 9 8 .5 9 9 .0 9 9 .5
FULL Length Research Paper Covered in Index Copernicus with IC Value 4.68 for 2010
(b)
100.0 Transm ittance [% ] 98.0
3938.64
98.5
99.0
99.5
3874.46
3737.10
3407.48
3295.64
3211.01
3105.79 3055.19
2914.38
2836.07
2684.40
2337.54 2300.55
1920.06
1787.24
1622.47 1573.42
1482.43
1380.56 1340.10
1218.13
1095.14
976.71 928.29 885.73 832.48
3500
3000
2500 2000 Wavenumber cm-1
1500
1000
(c) Figure 1: FTIR Spectra of a) Fluvastatin drug b) Indion 454 resin and c) Drug resonates
C o u n ts F lu v a s t a - T i n - s o d i u m d r u g 300
200
100
(a)
200
100
(b)
Int. J. Drug Dev. & Res., April-June 2012, 4 (2): 306-314 Covered in Scopus & Embase, Elsevier
736.36 702.94 636.68
310
Venkatesh. D. P et al: Formulation and Evaluation of Microspheres Containing Fluvastatin Sodium
100
FULL Length Research Paper Covered in Index Copernicus with IC Value 4.68 for 2010 311
0 10 20 30 40 50 Position [ 2Theta] 60 70 80 90
(c) Figure 2 : X-Ray diffraction spectra of a) Fluvastatin Sodium b) Indion 454 resin c) Drug- resinates
(a)
(b)
(c)
Int. J. Drug Dev. & Res., April-June 2012, 4 (2): 306-314 Covered in Scopus & Embase, Elsevier
Venkatesh. D. P et al: Formulation and Evaluation of Microspheres Containing Fluvastatin Sodium
FULL Length Research Paper Covered in Index Copernicus with IC Value 4.68 for 2010
(d) Figure 4: DSC Spectra a) Fluvastatin Sodium drug b) Drug-resinates c) Drug-resinates microcapsuled with ERS 100 d) Drug-resinates microcapsuled with ethyl cellulose.
(a)
(b)
Figure 5: Microscopic examination a) Ethyl cellulose coated b) Eudragit RS100 coated microspheres.
(a)
(b)
Figure 6: Surface electron microscopy (SEM) of a) Ethyl cellulose coated b) EudragitRS100 coated microspheres. Table 2: Physicochemical parameters of developed formulations
Batch F1 F2 F3 F4 F5 F6 F7 F8 Drug-resinates to polymer ratio (%w/w) 5 10 15 20 5 10 15 20 Nature Free flowing Free flowing Free flowing Cohesive Free flowing Free flowing Free flowing Cohesive, lumpy Percentage Yield* 97.63.25 98.76.30 95.6 7.5 96.9 6.75 97.8 8.9 93.45.84 95.93.89 94.83.71 Percentage DEE* 98.70.1 98.60.3 96.90.8 98.70.5 98.50.1 97.71.2 97.10.6 96.00.8 Percentage Coating of Polymer* 98.2 5.6 98.1 7.8 97.4 8.9 96.9 7.6 98.7 8.8 98.6 9.7 98.5 9.6 97.9 7.1 Particle size (m) 45-120 54-164 77-176 85-201 43-105 59-140 71-170 86-230
Int. J. Drug Dev. & Res., April-June 2012, 4 (2): 306-314 Covered in Scopus & Embase, Elsevier
312
Venkatesh. D. P et al: Formulation and Evaluation of Microspheres Containing Fluvastatin Sodium
*All readings are mean of three SD Table 3: Effect of formulation factors on Fluvastatin Sodium release kinetics data from microcapsules
Batch F1 F2 F3 F4 F5 F6 F7 F8 Zero order r2 0.6292 0.973 0.453 0.391 0.8297 0.7845 0.8509 0.779 First order r2 0.736 0.931 0.950 0.968 0.921 0.888 0.893 0.889 Higuchi equation r2 KH 0.737 55.0 0.931 53.5 0.950 52.1 0.969 48.7 0.922 81.4 0.888 75.7 0.894 74.0 0.893 71.2 Korsmeyer-peppas equation r2 n 0.592 0.07 0.974 0.09 0.988 0.10 0.914 0.10 0.7202 0.26 0.669 0.27 0.682 0.29 0.691 0.31
FULL Length Research Paper Covered in Index Copernicus with IC Value 4.68 for 2010 313
Figure 7: Dissolution profiles of drug-resinates
Figure 8: In vitro drug release of resinates microencapsulated with Eudragit RS 100
Int. J. Drug Dev. & Res., April-June 2012, 4 (2): 306-314 Covered in Scopus & Embase, Elsevier
Venkatesh. D. P et al: Formulation and Evaluation of Microspheres Containing Fluvastatin Sodium
FULL Length Research Paper Covered in Index Copernicus with IC Value 4.68 for 2010
Figure 9: Invitro drug release of resinates microencapsulated with Ethyl cellulose Conclusion: Thus drug-resinates coated with Ethyl cellulose and EudragitRS100 has proved to be efficient carrier for diffusion controlled release microspheres of Fluvastatin sodium. Acknowledgement: The authors wish to thank Biocon Ltd, Bangalore and Ion exchange resins Ltd, Mumbai for gift of Fluvastatin sodium and Indion 454 resin respectively. We would like to thank Chairman Mr. Premnath Reddy & Mrs. Shalini Reddy, Acharya and BM Reddy College of Pharmacy for providing facility and encouragement. References:
1) Abcde US, Dept of health and human services, National institutes of health. The practical guide identification, 2) evaluation and treatment of 9) overweight and obesity in adults 2000; 5. Irwin WJR Mac Hale and Watts P J. Drug delivery by ion exchange parts: Release of acidic drugs from anionic exchange resinate complexes. Drug. Dev. Ind.Pharm., 1990; 16(6): 883-898. 3) Kathleen P Ed Martindale. The Complete Drug Reference. 32nd Edn. London: The Pharmaceutical press; 1999: 196-97. 4) Sriwong janya Mongal, Bodimeier. Ronald. Effect of ion exchange resins on the drug release from the 8) 7) 6) 5) matrix tablets.Eur.J.Phar.Bio.Pharm., 1998;
46(3):321-327. Torres D, Garcia Encia G, Seijo JL, Vila Jato. Formulation and in vitro evaluation of HPMCPmicro encapsulated drug-resin complexes for sustained release of Diclofenac. Int. J. Pharm., 1995; 121: 239-243. Jain NK. Advanced in controlled and novel drug delivery. New Delhi: CBS Publishers. 2001:290302. Kadam A U, Sakarkar D M, Kawtikwar P S. Development and Evaluation of Oral Controlled Release Chlorpheniramine-IonExchange Resinate Suspension. Indian J Pharm Sci 2008; 70(4):53134. Varaporn Buraphacheep Junyaprasert, Greepol Manwiwwattanakul. Release profile comparison and stability of diltiazem-resin microcapsules in sustained release suspensions. Int. J. pharm., 2008; 352: 81-91. Bhalekar MR, Avari J and Umalkar RA. Preparation and in vitro Evaluation of Sustained Release Drug delivery System for Verapamil HCl. Indian J .Pharm. Sci., 2007; 69(4):418-422. 10) Venkatesh DP, Geetha Rao CG. Formulation of taste masked oro-dispersible tablets of Ambroxol hydrochloride. Asian J. Pharm., 2010:261-264.
Int. J. Drug Dev. & Res., April-June 2012, 4 (2): 306-314 Covered in Scopus & Embase, Elsevier
314
Potrebbero piacerti anche
- Opioid Analgesics - Narcotic Anlagesics - 0Documento6 pagineOpioid Analgesics - Narcotic Anlagesics - 0Rajesh KumarNessuna valutazione finora
- Screening of in Vitro Anti Inflammatory Activity of Some Newly Synthesized Fluorinated Benzothiazolo Imidazole CompoundsDocumento3 pagineScreening of in Vitro Anti Inflammatory Activity of Some Newly Synthesized Fluorinated Benzothiazolo Imidazole CompoundsRajesh KumarNessuna valutazione finora
- Biochemistry: For The Students of Pharmacy Technicians (Category-B)Documento74 pagineBiochemistry: For The Students of Pharmacy Technicians (Category-B)Rajesh Kumar0% (1)
- Families of Flowering Plants-NEETDocumento24 pagineFamilies of Flowering Plants-NEETResonance Dlpd74% (19)
- Mistry 139-150Documento12 pagineMistry 139-150Rajesh KumarNessuna valutazione finora
- Full Paper Novel Sulphamides and Sulphonamides Incorporating The Tetralin Scaffold As Carbonic Anhydrase and Acetylcholine Esterase InhibitorsDocumento9 pagineFull Paper Novel Sulphamides and Sulphonamides Incorporating The Tetralin Scaffold As Carbonic Anhydrase and Acetylcholine Esterase InhibitorsRajesh KumarNessuna valutazione finora
- Flowering Plant Wikipedia The Free EncyclopediaDocumento16 pagineFlowering Plant Wikipedia The Free EncyclopediaRajesh KumarNessuna valutazione finora
- Antidermatophytic Activity of Acacia Concinna: V. Natarajan and S. NatarajanDocumento2 pagineAntidermatophytic Activity of Acacia Concinna: V. Natarajan and S. NatarajanRajesh KumarNessuna valutazione finora
- Desmo. BippinetaDocumento5 pagineDesmo. BippinetaRajesh KumarNessuna valutazione finora
- 2007 12 20 Foam Engl 03Documento48 pagine2007 12 20 Foam Engl 03Rajesh Kumar100% (3)
- Mangifera IndicaDocumento3 pagineMangifera IndicaRajesh KumarNessuna valutazione finora
- 10 761 Guide To CPSRDocumento42 pagine10 761 Guide To CPSRJuanLópezNessuna valutazione finora
- Phytochemical Study of ResinDocumento24 paginePhytochemical Study of ResinRajesh KumarNessuna valutazione finora
- Taat 02 I 1 P 21222Documento5 pagineTaat 02 I 1 P 21222Rajesh KumarNessuna valutazione finora
- Oxalis CorniculataDocumento2 pagineOxalis CorniculataRajesh KumarNessuna valutazione finora
- Herbs Play An Important Role in The Field of Cosmetics: Pandey Shivanand, Meshya Nilam, D.ViralDocumento8 pagineHerbs Play An Important Role in The Field of Cosmetics: Pandey Shivanand, Meshya Nilam, D.ViralRajesh KumarNessuna valutazione finora
- Aasr 2010 2 1 225 229Documento5 pagineAasr 2010 2 1 225 229Rajesh KumarNessuna valutazione finora
- 2014 Adv 1 ApplForm DirectRecDocumento6 pagine2014 Adv 1 ApplForm DirectRecRajesh KumarNessuna valutazione finora
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- Biology The Unity and Diversity of Life 13th Edition Starr Solutions Manual DownloadDocumento9 pagineBiology The Unity and Diversity of Life 13th Edition Starr Solutions Manual DownloadGraham Hissem100% (24)
- Ielts Reading Practice Test 65 With Answers PDFDocumento14 pagineIelts Reading Practice Test 65 With Answers PDFSri KarthickNessuna valutazione finora
- Mob Ot 31753002739420Documento276 pagineMob Ot 31753002739420fabriziozaraNessuna valutazione finora
- BIO270 Prelab 1 Assignment 2014Documento8 pagineBIO270 Prelab 1 Assignment 2014noahyuyanNessuna valutazione finora
- The Smoking Scare De-Bunked William T WithbyDocumento61 pagineThe Smoking Scare De-Bunked William T WithbyPietje PukNessuna valutazione finora
- The Muscular System: Powerpoint Lecture SlidesDocumento130 pagineThe Muscular System: Powerpoint Lecture SlidesTricia Mae CorpuzNessuna valutazione finora
- Report On The New Zealand Connemara Pony Population.Documento14 pagineReport On The New Zealand Connemara Pony Population.Sheila RamsayNessuna valutazione finora
- 1.wall of ThoraxDocumento14 pagine1.wall of ThoraxChandru ANessuna valutazione finora
- Grammar Meets Conversation Do Does Did Are Is Was Fun Activities Games - 2965Documento3 pagineGrammar Meets Conversation Do Does Did Are Is Was Fun Activities Games - 2965Yendy GutierrezNessuna valutazione finora
- Genetic Disorders in Arab Populations: Qatar: Tawfeg Ben-Omran, Atqah Abdul WahabDocumento6 pagineGenetic Disorders in Arab Populations: Qatar: Tawfeg Ben-Omran, Atqah Abdul WahabUmair ZubairNessuna valutazione finora
- In Vitro Propagation Ludwigia - EgatiDocumento12 pagineIn Vitro Propagation Ludwigia - EgatiendangNessuna valutazione finora
- Bat AnatomyDocumento10 pagineBat AnatomyAlejo RuilovaNessuna valutazione finora
- Botany B.SCDocumento2 pagineBotany B.SCAkash Deep PandeyNessuna valutazione finora
- Week 4 SimDocumento3 pagineWeek 4 SimJEZUE REMULTANessuna valutazione finora
- Substance Id enDocumento118 pagineSubstance Id ensmaps001Nessuna valutazione finora
- Heritability of Angular Leaf Spot Resistance in Populations of Common Bean Developed Using g5686, Mexico 54, Amendoim and Bat 332, As Donor ParentsDocumento5 pagineHeritability of Angular Leaf Spot Resistance in Populations of Common Bean Developed Using g5686, Mexico 54, Amendoim and Bat 332, As Donor ParentsijsidonlineinfoNessuna valutazione finora
- AdatinaDocumento1 paginaAdatinaCRISTIELI IZABEL FOGUESATTONessuna valutazione finora
- My Hometown EssayDocumento6 pagineMy Hometown Essayfz67946y100% (2)
- Module 5 Anatomy I Splanchnology 1Documento17 pagineModule 5 Anatomy I Splanchnology 1neannaNessuna valutazione finora
- Descendants of Joshua Seaver - 8 GenerationsDocumento199 pagineDescendants of Joshua Seaver - 8 GenerationsRandy SeaverNessuna valutazione finora
- Autonomic ReflexesDocumento21 pagineAutonomic ReflexesJoe WeahNessuna valutazione finora
- Cointegrated VectorsDocumento2 pagineCointegrated Vectorsdevikamurugan124206Nessuna valutazione finora
- Rolling Advertisement For FacultyDocumento6 pagineRolling Advertisement For Facultymanahar bakshiNessuna valutazione finora
- Disorders of PigmentationDocumento45 pagineDisorders of PigmentationMoayad NawaflehNessuna valutazione finora
- Complete Abstract Book ASTMH - Atlanta 2012-USADocumento3 pagineComplete Abstract Book ASTMH - Atlanta 2012-USAPercy Lezama VigoNessuna valutazione finora
- Vitamin B6 Deficiency As A Cause of Polyneuropathy in POEMS Syndrome Rapid Recovery With Supplementation in Two CasesDocumento7 pagineVitamin B6 Deficiency As A Cause of Polyneuropathy in POEMS Syndrome Rapid Recovery With Supplementation in Two CasesYaseen MohamnadNessuna valutazione finora
- Chelex 100-Instruction ManualDocumento14 pagineChelex 100-Instruction Manualu77Nessuna valutazione finora
- ScienceDocumento7 pagineScienceDhwani ChitrodaNessuna valutazione finora
- Craetagusarticle PDFDocumento7 pagineCraetagusarticle PDFKatty AcostaNessuna valutazione finora