Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Radium reaction gas prediction
Caricato da
coughsyrup123Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Radium reaction gas prediction
Caricato da
coughsyrup123Copyright:
Formati disponibili
F321 module 3 Practice 2:
1.
Radium reacts vigorously when added to water.
Ra(s) + 2H2O(l) Ra(OH)2(aq) + H2(g)
(i)
Use the equation to predict two observations that you would see during this
reaction.
.........................................................................................................................
.........................................................................................................................
[2]
(ii)
Predict a pH value for this solution.
.........................................................................................................................
[1]
[Total 3 marks]
2.
Reactions of the Group 2 metals involve removal of electrons. The electrons are
removed more easily as the group is descended and this helps to explain the
increasing trend in reactivity.
(i)
The removal of one electron from each atom in 1 mole of gaseous radium atoms
is called the .....................................................................................................
[2]
The equation for this process in radium is:
.........................................................................................................................
[2]
The King's CE School
(ii)
Atoms of radium have a greater nuclear charge than atoms of calcium.
Explain why, despite this, less energy is needed to remove an electron from a
radium atom than from a calcium atom.
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
[3]
[Total 7 marks]
3.
Chewing chalk has been used for many years to combat excess stomach acid and
indigestion tablets often contain calcium carbonate, CaCO3. Suggest, with the aid of an
equation, how these tablets work.
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
[Total 2 marks]
The King's CE School
4.
Chlorine is used in the preparation of many commercially important materials such as
bleach and iodine.
Bleach is a solution of sodium chlorate(l), NaOCl, made by dissolving chlorine in
aqueous sodium hydroxide.
Cl2(g) + 2NaOH(aq) NaOCl(aq) + NaCl(aq) + H2O(l)
Determine the changes in oxidation number of chlorine during the preparation of bleach
and comment on your results.
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
[Total 3 marks]
5.
Iodine is extracted commercially from seawater with chlorine gas. Seawater contains
very small quantities of dissolved iodide ions, which are oxidised to iodine by the
chlorine gas.
(i)
Write an ionic equation for the reaction that has taken place.
.........................................................................................................................
[2]
(ii)
Use your understanding of electronic structure to explain why chlorine is a
stronger oxidising agent than iodine.
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
[2]
[Total 4 marks]
The King's CE School
6.
The atomic radii of nitrogen and oxygen are shown below.
element
nitrogen
oxygen
atomic radius/nm
0.075
0.073
Explain why a nitrogen atom is larger than an oxygen atom.
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
[Total 4 marks]
The King's CE School
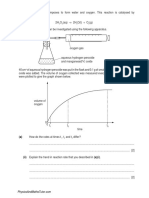
7.
The first ionisation energies of the elements H to K are shown below. Use this diagram
to help with your answers to this question.
2500
1 s t io n is a tio n e n e r g y
/ k J m o l 1
He
N e
2000
1000
Be
M g
Si
500
0
Ar
1500
Li
0
Na
4
Al
10 11 12 13 14 15 16 17 18 19 20
a to m ic n u m b e r
(a)
Define the term first ionisation energy.
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
[3]
(b)
Explain why the first ionisation energies show a general increase across Period 2
(Li to Ne).
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
[2]
[Total 5 marks]
The King's CE School
8.
State and explain the trend in first ionisation energies shown by the elements with the
atomic numbers 2, 10 and 18.
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
[Total 4 marks]
9.
The Group 2 metal strontium, Sr, is very reactive.
Strontium metal is stored under oil and, when exposed to air, the shiny surface of the
strontium becomes dull.
Predict, with an equation, what reaction takes place when strontium is exposed to air.
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
[Total 2 marks]
10.
The reaction of strontium with water is a redox reaction. A student reacted 0.438 g of
strontium with 200 cm3 of water.
Sr(s) + 2H2O(l) Sr(OH)2(aq) + H2(g)
(i)
Use oxidation numbers to show that strontium has been oxidised in this reaction.
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
[2]
The King's CE School
(ii)
Calculate how many moles of Sr were reacted.
Ar: Sr, 87.6
answer ............... mol
[1]
(iii)
Calculate the volume, in dm3, of H2(g) produced. You can assume that, under the
experimental conditions, 1.00 mol of H2(g) has a volume of 24.0 dm3.
answer .............. dm3
[1]
(iv)
Calculate the concentration, in mol dm3, of the Sr(OH)2 produced.
answer ............... mol dm3
[1]
[Total 5 marks]
The King's CE School
Potrebbero piacerti anche
- Topic 4 - Group 7Documento9 pagineTopic 4 - Group 7Abirame SivakaranNessuna valutazione finora
- Nitrogen & Fertilisers 4 QPDocumento8 pagineNitrogen & Fertilisers 4 QPUsha PerumalNessuna valutazione finora
- Chemical Energetics 2 QPDocumento11 pagineChemical Energetics 2 QPWilliam TsuiNessuna valutazione finora
- Balanced Equations & Associated Calc's 05 QPDocumento8 pagineBalanced Equations & Associated Calc's 05 QPlmao lmaoNessuna valutazione finora
- Chlorine and Its Compounds Chemistry Form 3 Topical Questions and AnswersDocumento18 pagineChlorine and Its Compounds Chemistry Form 3 Topical Questions and Answersideal writersNessuna valutazione finora
- Kinetics exam questionsDocumento9 pagineKinetics exam questionsridithaNessuna valutazione finora
- Periodic Table, Group 2 and The Halogens 2 QPDocumento14 paginePeriodic Table, Group 2 and The Halogens 2 QPmalakNessuna valutazione finora
- t2 Chem Revision Ex 22Documento19 paginet2 Chem Revision Ex 22Nicholas OwNessuna valutazione finora
- Electroplating steel with silver processDocumento11 pagineElectroplating steel with silver processValerine VictoriaNessuna valutazione finora
- As Level Chemistry: Topic 2 - Amount of Substance TestDocumento10 pagineAs Level Chemistry: Topic 2 - Amount of Substance Testkarima akterNessuna valutazione finora
- AS LEVEL CHEMISTRY REDOX AND GROUP 2 REACTIONSDocumento19 pagineAS LEVEL CHEMISTRY REDOX AND GROUP 2 REACTIONSsemirah anthonyNessuna valutazione finora
- 10 Homework PackDocumento10 pagine10 Homework PackatasayNessuna valutazione finora
- Mixed Topic Revision 1 DiffusionDocumento23 pagineMixed Topic Revision 1 DiffusionYaakkwNessuna valutazione finora
- Born-Haber Cycles 1 QP PDFDocumento9 pagineBorn-Haber Cycles 1 QP PDFTalha KhanNessuna valutazione finora
- A-Level Chemistry: Paper 3 Practice Paper 3Documento20 pagineA-Level Chemistry: Paper 3 Practice Paper 3Jesus ChristNessuna valutazione finora
- Water and HydrogenDocumento7 pagineWater and HydrogenOkumu KevinsNessuna valutazione finora
- BondingDocumento14 pagineBondingMuizzudin AzaliNessuna valutazione finora
- Periodicity Past QuestionsDocumento17 paginePeriodicity Past QuestionsMohammad KhanNessuna valutazione finora
- Reversible Reactions WorksheetDocumento14 pagineReversible Reactions WorksheetShriyaNessuna valutazione finora
- A-LEVEL CHEMISTRY PAPER 3 PRACTICE PAPER 8Documento19 pagineA-LEVEL CHEMISTRY PAPER 3 PRACTICE PAPER 822S48 SUNDARAM RAMASUBBU RAKSHANessuna valutazione finora
- Module 806Documento18 pagineModule 806Hema LataNessuna valutazione finora
- Question Database For Amount of SubstancesDocumento23 pagineQuestion Database For Amount of SubstancesKamrul Alam MasumNessuna valutazione finora
- F321: Atoms, Bonds and Groups Redox: Plymstock School 1Documento7 pagineF321: Atoms, Bonds and Groups Redox: Plymstock School 1Younes AlahmadNessuna valutazione finora
- Rate of Reaction 2 QP (Tomek)Documento9 pagineRate of Reaction 2 QP (Tomek)Tomasz OstrowskiNessuna valutazione finora
- Week 26 A2 Questions Name .. /49 1Documento5 pagineWeek 26 A2 Questions Name .. /49 1Solace HusseinNessuna valutazione finora
- Amount of Substance 1 QPDocumento10 pagineAmount of Substance 1 QPHajhoj CellNessuna valutazione finora
- Rate of Reaction PPsDocumento23 pagineRate of Reaction PPstcs202231Nessuna valutazione finora
- Exam 1Documento9 pagineExam 1Alejandro DuarteNessuna valutazione finora
- Redox 2 QPDocumento7 pagineRedox 2 QPPramitaNessuna valutazione finora
- Redox Questions Igcse ChemDocumento7 pagineRedox Questions Igcse ChemCaylinNessuna valutazione finora
- Periodicity QsDocumento15 paginePeriodicity QsJack SmitNessuna valutazione finora
- Carbon and Some of Its CompoundsDocumento17 pagineCarbon and Some of Its Compoundsbarus michealNessuna valutazione finora
- Topical Questions For ElectrolysisDocumento6 pagineTopical Questions For Electrolysisokguserfucker idontgiveashitNessuna valutazione finora
- 2 HoursDocumento17 pagine2 HoursOTTO OLIMANessuna valutazione finora
- Energy Changes, Rate, Reversible, NH3, H2SO4Documento5 pagineEnergy Changes, Rate, Reversible, NH3, H2SO4HudaNessuna valutazione finora
- 2.4 2.5 2.6 Assessed HomeworkDocumento7 pagine2.4 2.5 2.6 Assessed HomeworkRabia Rafique100% (1)
- Clour Questions and MarkschemeDocumento13 pagineClour Questions and Markschemesquidthekid2005Nessuna valutazione finora
- PDF DocumentDocumento125 paginePDF Documentnabila OktavianiNessuna valutazione finora
- 4.1 Reactivity of Metals 3 QPDocumento16 pagine4.1 Reactivity of Metals 3 QPDumpsterFireGamingNessuna valutazione finora
- Water 1Documento10 pagineWater 12025svyasNessuna valutazione finora
- Ib PhysicsDocumento10 pagineIb PhysicsAndres LopezNessuna valutazione finora
- Cambridge International AS & A Level: CHEMISTRY 9701/22Documento12 pagineCambridge International AS & A Level: CHEMISTRY 9701/22Spider Gamer22Nessuna valutazione finora
- t2 Chem Revision Ex 12Documento16 paginet2 Chem Revision Ex 12Nicholas OwNessuna valutazione finora
- Aqa A2 Biology LDRDocumento21 pagineAqa A2 Biology LDRjames100% (4)
- Equilibria AsDocumento39 pagineEquilibria AsyousafNessuna valutazione finora
- Electrolysis RevisionDocumento15 pagineElectrolysis RevisionPunitha PanchaNessuna valutazione finora
- Amount of Substance CombinedDocumento23 pagineAmount of Substance CombinedJainam MehtaNessuna valutazione finora
- DY Enthalpy TestDocumento19 pagineDY Enthalpy TestStormzy 67Nessuna valutazione finora
- 1 Hydrogen Peroxide Decomposes To Form Water and Oxygen. This Reaction Is Catalysed byDocumento9 pagine1 Hydrogen Peroxide Decomposes To Form Water and Oxygen. This Reaction Is Catalysed byKelvin DenhereNessuna valutazione finora
- Atomic Physics FundamentalsDocumento254 pagineAtomic Physics FundamentalsAli AhmedNessuna valutazione finora
- Nuclear Physics and Reactor Theory Vol 1 and 2Documento245 pagineNuclear Physics and Reactor Theory Vol 1 and 2kollliNessuna valutazione finora
- The Quantum in Chemistry: An Experimentalist's ViewDa EverandThe Quantum in Chemistry: An Experimentalist's ViewNessuna valutazione finora
- Advanced Synthesis of Gold and Zirconia Nanoparticles and their CharacterizationDa EverandAdvanced Synthesis of Gold and Zirconia Nanoparticles and their CharacterizationNessuna valutazione finora
- Metal Catalysed Carbon-Carbon Bond-Forming ReactionsDa EverandMetal Catalysed Carbon-Carbon Bond-Forming ReactionsNessuna valutazione finora
- Ligand Coupling Reactions with Heteroatomic CompoundsDa EverandLigand Coupling Reactions with Heteroatomic CompoundsValutazione: 4 su 5 stelle4/5 (1)
- Environmental Electrochemistry: Fundamentals and Applications in Pollution Sensors and AbatementDa EverandEnvironmental Electrochemistry: Fundamentals and Applications in Pollution Sensors and AbatementNessuna valutazione finora
- The Magnetic Universe: Geophysical and Astrophysical Dynamo TheoryDa EverandThe Magnetic Universe: Geophysical and Astrophysical Dynamo TheoryNessuna valutazione finora
- Physical Principles of Medical UltrasonicsDa EverandPhysical Principles of Medical UltrasonicsC. R. HillNessuna valutazione finora
- Unit Level Raw Mark and Ums Grade Boundaries June 2014Documento39 pagineUnit Level Raw Mark and Ums Grade Boundaries June 2014coughsyrup123Nessuna valutazione finora
- UKMT Senior Challenge 2012Documento2 pagineUKMT Senior Challenge 2012Daniel CarpenterNessuna valutazione finora
- Lifestyle, Health & Risk Factors for Cardiovascular DiseaseDocumento11 pagineLifestyle, Health & Risk Factors for Cardiovascular Diseasenimz1992Nessuna valutazione finora
- Edexcel C3 Summary NotesDocumento5 pagineEdexcel C3 Summary Notesbloodyinspired50% (2)
- F321 Module 3 Practice 5 AnswersDocumento4 pagineF321 Module 3 Practice 5 Answerscoughsyrup123Nessuna valutazione finora
- RES9 ChemflashcardsDocumento4 pagineRES9 Chemflashcardspleb123Nessuna valutazione finora
- AS Edexcel Biology Topic 1 Lifestyle, Health and Risk 1.2 The Heart and HealthDocumento17 pagineAS Edexcel Biology Topic 1 Lifestyle, Health and Risk 1.2 The Heart and HealthSammy MacuraNessuna valutazione finora
- Further Mechanics Exam Pack MSDocumento45 pagineFurther Mechanics Exam Pack MScoughsyrup123Nessuna valutazione finora
- C1 Gold 3Documento15 pagineC1 Gold 3coughsyrup123Nessuna valutazione finora
- Mark Scheme June 2007 6683 Statistics S1Documento8 pagineMark Scheme June 2007 6683 Statistics S1Ting Phin YuanNessuna valutazione finora
- F321 Module 3 Practice 4 AnswersDocumento4 pagineF321 Module 3 Practice 4 Answerscoughsyrup123Nessuna valutazione finora
- F321 Module 3 Practice 3Documento10 pagineF321 Module 3 Practice 3coughsyrup123Nessuna valutazione finora
- RES5 Bio Revision PlannerDocumento3 pagineRES5 Bio Revision PlannerKawthar SmithNessuna valutazione finora
- F321 Module 3 Practice 4Documento7 pagineF321 Module 3 Practice 4coughsyrup123Nessuna valutazione finora
- F321 Module 3 Practice 5Documento4 pagineF321 Module 3 Practice 5coughsyrup123Nessuna valutazione finora
- F321 Module 3 Practice 2 AnswersDocumento4 pagineF321 Module 3 Practice 2 Answerscoughsyrup123Nessuna valutazione finora
- F321 Module 3 Practice 1 AnswersDocumento6 pagineF321 Module 3 Practice 1 Answerscoughsyrup123Nessuna valutazione finora
- F321 Module 3 Practice 3 AnswersDocumento4 pagineF321 Module 3 Practice 3 Answerscoughsyrup123Nessuna valutazione finora
- F321 Module 3 Practice 1Documento10 pagineF321 Module 3 Practice 1coughsyrup123Nessuna valutazione finora
- F321 Module 2 Practice 5Documento5 pagineF321 Module 2 Practice 5coughsyrup123Nessuna valutazione finora
- F321 Module 2 Practice 6 AnswersDocumento2 pagineF321 Module 2 Practice 6 Answerscoughsyrup123Nessuna valutazione finora
- F321 Module 2 Practice 6Documento2 pagineF321 Module 2 Practice 6coughsyrup123Nessuna valutazione finora
- F321 Module 2 Practice 4Documento6 pagineF321 Module 2 Practice 4coughsyrup123Nessuna valutazione finora
- F321 Module 2 Practice 5 AnswersDocumento3 pagineF321 Module 2 Practice 5 Answerscoughsyrup123Nessuna valutazione finora
- F321 Module 2 Practice 2Documento8 pagineF321 Module 2 Practice 2coughsyrup123Nessuna valutazione finora
- F321 Module 2 Practice 3 AnswersDocumento4 pagineF321 Module 2 Practice 3 Answerscoughsyrup123Nessuna valutazione finora
- F321 Module 2 Practice 4 AnswersDocumento4 pagineF321 Module 2 Practice 4 Answerscoughsyrup123Nessuna valutazione finora
- F321 Module 2 Practice 3Documento7 pagineF321 Module 2 Practice 3coughsyrup123Nessuna valutazione finora
- F321 Module 2 Practice 2 AnswersDocumento5 pagineF321 Module 2 Practice 2 Answerscoughsyrup123Nessuna valutazione finora
- External Pressure Pipe Thickness CalcDocumento3 pagineExternal Pressure Pipe Thickness Calcreach_arindomNessuna valutazione finora
- Atomic Particles Chart: + o - ST ND RD THDocumento2 pagineAtomic Particles Chart: + o - ST ND RD THZexdxrNessuna valutazione finora
- How I was floored by a tick reveals Lyme disease diagnosis journeyDocumento12 pagineHow I was floored by a tick reveals Lyme disease diagnosis journeyKylie GolindangNessuna valutazione finora
- 24150Documento3 pagine24150B.yaswanth KumarNessuna valutazione finora
- SGM1 - Study Guide For Module 1 PDFDocumento25 pagineSGM1 - Study Guide For Module 1 PDFEj ParañalNessuna valutazione finora
- Food Research International: SciencedirectDocumento7 pagineFood Research International: SciencedirectFausto GuasguaNessuna valutazione finora
- Houghton Rust Veto 4222 S Spec SheetDocumento4 pagineHoughton Rust Veto 4222 S Spec SheetSuprastowo Bin SarinoNessuna valutazione finora
- GDL in Meat ProcessingDocumento3 pagineGDL in Meat Processingadiy0n9100% (1)
- Plan-J: Chemistry Form 5Documento12 paginePlan-J: Chemistry Form 5Mohd HafiezNessuna valutazione finora
- MeteorologyDrivesAmbientAirQua MISHRA2016Documento17 pagineMeteorologyDrivesAmbientAirQua MISHRA2016KAVHALE DHANANJAY PARMESHWARNessuna valutazione finora
- Earth Science Quarter 2 Module 1Documento4 pagineEarth Science Quarter 2 Module 1Rhianne Grace CastroNessuna valutazione finora
- Is 5382 - 1998 Specification For Rubber, Sealing Rings For Gas Mains, Water Mains and SewageDocumento23 pagineIs 5382 - 1998 Specification For Rubber, Sealing Rings For Gas Mains, Water Mains and Sewagecevivek100% (4)
- Six-Membered Pyridine (Azine) : Aromatic HeterocylsDocumento21 pagineSix-Membered Pyridine (Azine) : Aromatic HeterocylsManmood ShakerNessuna valutazione finora
- FirstDefender RMX SpecSheetDocumento2 pagineFirstDefender RMX SpecSheetMario OrdenanaNessuna valutazione finora
- SDS Unleaded Gasoline RON 95Documento19 pagineSDS Unleaded Gasoline RON 95syazani salin71% (7)
- Wastewater Treatment ActivityDocumento4 pagineWastewater Treatment Activityapi-330049991Nessuna valutazione finora
- Enzyme KinecticsDocumento25 pagineEnzyme KinecticsRhia80% (5)
- Pharmaceutical Ultrapure Water Systems - : Igor GorskyDocumento36 paginePharmaceutical Ultrapure Water Systems - : Igor GorskyunknownNessuna valutazione finora
- Interpreting Carburized Case DepthsDocumento4 pagineInterpreting Carburized Case Depthsmp87_ingNessuna valutazione finora
- FS Ii Question Bank PDFDocumento11 pagineFS Ii Question Bank PDFARYAN RATHORENessuna valutazione finora
- Structure of Atoms: Long Answers QuestionsDocumento7 pagineStructure of Atoms: Long Answers QuestionsMussadiq RehmanNessuna valutazione finora
- Stanyl TW200F6: PA46-GF30Documento3 pagineStanyl TW200F6: PA46-GF30Paul JosephNessuna valutazione finora
- Baden Forster Chem ReportDocumento9 pagineBaden Forster Chem ReportWillNessuna valutazione finora
- Performance of Heat ExchangersDocumento10 paginePerformance of Heat ExchangersJusztinAquinoNessuna valutazione finora
- Ceramic Waste Aggregate Improves Asphalt Mixture PropertiesDocumento12 pagineCeramic Waste Aggregate Improves Asphalt Mixture Propertiesnw__ayNessuna valutazione finora
- Investigatory Project On Prep of CementDocumento9 pagineInvestigatory Project On Prep of CementKrish JaiswalNessuna valutazione finora
- Evaluation of Glass-to-Metal Headers Used in Electron DevicesDocumento5 pagineEvaluation of Glass-to-Metal Headers Used in Electron DevicesRed RedNessuna valutazione finora
- Catalog Cleaning - 2020 Eng - CompressedDocumento30 pagineCatalog Cleaning - 2020 Eng - CompressedSalkic HamdijaNessuna valutazione finora
- Alkaline Phosphatase: Test Principle: Enzymatic ColorimetricDocumento2 pagineAlkaline Phosphatase: Test Principle: Enzymatic ColorimetricValdez Francis ZaccheauNessuna valutazione finora