Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
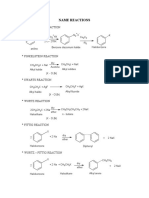
Orgo Reaction Sheet
Caricato da
Kyle BroflovskiTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Orgo Reaction Sheet
Caricato da
Kyle BroflovskiCopyright:
Formati disponibili
Ray
Tasks
1. Carboxylic acids to acyl chlorides O 1. OH 2.
Reactions
SOCL2
Notes O CL
2. Alcohols to alkyl halides
R-OH
O OH
SOCL2 (COCL)2
R-CL
O CL
Addition of Hydrides R Catalytic Hydrogenation
O R O
O1. LiAlH4 R C R H
2. H3O+
OH R C R H
H2, Raney Ni H
OH
Additions of 1 Amines (Imines)
O C
*non Aqueous favors imine
R'NH2
H+ C
R'
*Aqueous favors ketone and amine
Imine Product Derivatives
Ph-HN N
NH2NHPh/H+
N O
NH2OH/H+
OH
O
NH2NH-C-NH2/H+
NH2NH2/H+
O N H N NH2
NH2
Additions of 2 Amines (Enamines)
*follows ZAITSEV'S RULE
(R)2NH
H3O+
(R)2N
Ray
Tasks
Oxidation of Aldehydes to Carboxylic Acids: Silver Reagents O CH2OH H O Carboxylation of Grignard Reagents
X= halide
Reactions O Ag2O/THF/H2O R H R OH O CH2OH OH O O X
1. Mg / ether 2. CO2 / ether 3. H3O+
Notes O
*KMnO4 or *CrO3/H2SO4/H2O/acteone or *Na2Cr2O7/H2SO4/H2O
OR
1. Ag(NH3)2+ OH- (Tollen's Reagent) 2. H+
OH
1 MgX
2 and 3
Hydrolysis of Nitriles
CN
H3O+
heat
O OH O OO
R Ester Formation from Acid Chlorides R Amide Formation from Acid Chlorides O
CN
OHheat
R' OH
CL R O
R'
O CL O CL O CL NH3
O NH2 O
*1
R' NH2
O
N H
R'
!2
!3
R2' NH2
NR2'
Synthesis of Esters: Fischer Esterification R
O OH
1. R'-OH 2. H+ R
O O R'
Ray
Tasks
Esterication with Diazomethane O OH
Reactions
Notes
O CH2N2 OCH3
*SN2 *Great Yield
Direct Amide Synthesis
O OH
R' NH2
NHR'
Reduction of Carboxylic Acids H3C
O OH NH2 O O 1. LiAlH4/Et2O 2. H3O+ 1. LiAlH4/Et2O 2. H3O+ H3C
OH OH CH2NH2
NC
H2NH2C
*reacts with aldehydes, ketones, esters, carboxylic acids, and epoxides (with H3O+ or H2O) *BUT nitriles, amides, and N3 produce R-NH2 (with H2O)
R'
Diborane Reduction H3C NC O Decarboxylation: Hunsdiecker Reaction O O
OR"
R'CH2OH +
O
R"OH
*VERY SELECTIVE!
OH NH2
1. B2H6 / diglyme 2. H3O+
H3C NC O
OH NH2
*Carboxylics reacts faster with B2H6 than any other functional group
1. HgO or Ag2O or Pb(OAC)4 2. heat / Br2 / CCl4 OH O
lose
R Br
*converts Carboxylic acids into alkyl halides with 1 less carbon
R
Carboxylic Acid from Acid Chlorides R
OH
O Cl
H2O R
O OH
Anhydrides from Acid Chlorides R
O O Cl
R'
OR
O O
R'
Ray
Tasks
Cyclic Anhydrides O HO O OH
Reactions O ! O
Notes
*5,6 member rings are best
O Alpha Halogenation of Ketone
*X= Cl2 / I2 / Br2
X2 / CH3COOH
* NO FREAKING ALDEHYDES
*Most SUB ALKENE:MAJOR *Least SUB ALKENE:MINOR
Alpha Bromination of Carboxylic Acid: Hell Volhard Zelinksy
O OH
1. PBr3 / Br2 2. H2O
O OH Br
*carboxylics do not enolize under acidic or basic conditions
Aniline with Nitrous Acid (HNO2)
1. HNO3 / H2SO4
2. Zn, Fe, or Sn / HCl
NO2
NH2
N Cl or Br
3. NaNO2 / HCl
Act.
Act.
CuCl or CuBr
I
KI
NCL OH
H3 O+/!
1. HBF4 2. !
CuCN
CN
H3PO2
Ray
Tasks
Cyclic Anhydrides O O O Synthesis and Reactions of Anhydrides O R OH
Reactions O CH3OH O O
NaOH
Notes
OCH3 OH
O R O-
R Cl R
O O O 2x R
O R
*Friedel Crafts acylation: NO DEACTIVATING OR AMINO GROUPS
O R O R O R O R Synthesis and Reactions of Esters R O O O O O O
O R O R O R O R
H2O
OH O R O OH
R' OH
R
O OR'
AlCl3
NH3 or R'NH2 or R'2NH
R O R O NH3 OH R R" OH
1. R"MgX or R"Li 2. H3O+
+ R R"
O OH
O Cl
R'OH R'OH / H+
OH O-
+ R'OH
or
O
H2O H+ or OHR"MgX or R"Li
O OR'
1. DIBAH 2. H3O+
+ R'O-
R
1. LiAlH4 2. H3O+
R"
O R OR''
R"OH / H+ xs
O R
NH3 or R''NH2 or R''2NH
RCH2OH + R'OH
O R H
*5,6 membered ring
NH2
Cyclic Esters O O HO OH H+ O
Ray
Tasks
Cyclic Anhydrides 1. O O OH OH
Reactions O ! >100C O ! ~800C O O O O O CH3OH O O NR2 O NHR O NH2
(3) Br2 / NaOH / H2O
(4) POCl3 or SOCl2 or P2O5
Notes
1. maleic anhydride
2.
2x CH3CO2H
3.
OCH3 OH
Synthesis and Reactions of Amides
*rxn 1 reacts ONLY WITH AMIDES
R
3 2 1
R R R
NR2 NHR NH2
(2) H2O / Heat H+ or OH(1) 1. LiAlH4 2. H2O or H3O+
R
1
*rxn 2 nucleophilic acyl substitution and get AMINE PRODUCT
R C N
*rxn 3 get AMINE PRODUCT
*rxn 4 reacts ALL 1" AMIDES and get NITRILE PRODUCT
ACID PRODUCT:
BASE PRODUCT:
NH4 Synthesis and Reactions of Nitriles
OR
OH
NH2
O1. LiAlH4 2. H2O or H3O+
or
R CH2NH2
LiAlH4
H2 / Pt or Ni
R CH2NH2
H2O / Heat H+ or -OH
ACID PRODUCT: BASE PRODUCT:
NH4
OH
OR
O-
NH3
*REACTS ONLY WITH METHYL KETONES
Haloform Reaction R
CH3
X2 (xs) NaOH (xs)
O R O+ HCX3
Ray
Tasks
Alkylation of Ketones, Esters, and Nitriles: LDA
(lithium diisopropyl amide)
Reactions O H3C CH3
1.
Notes O H3CH2CH2C
2.
1. LDA 2. CH3CH2Br
CH3
OH2C C O O
1. LDA 2. CH3Br
O O
CH3CH2CN
1. LDA 2. CH3Br
CN H3C
O
Ha
O CH3
Hb
1. LDA 2. CH3Br
Ha MAJOR (less bulky)
CH3
+
O
Hb Minor (more bulky)
CH3 CH3
O O Hydrolysis of Nitriles (extended) CHO
1. LDA 2. CH3Br
CN
O O CHO
CN
R CN
OHH2O
R
O
NH2
OHH2O
R
O
O-
*Basic conditions: Carboxolate Anion watch out for dimerization.
R CN
H+ (non-aqueous)
H+ (non-aqueous)
NH2
OH
*Acidic conditions: must be non-aqueous. nitrile group unaffected if H3O+ is utilized.
Ray
Tasks
Electrophilic Aromatic Substitution of Aniline NH2 1.
Reactions NH2 Br2 / H2O Br Br
Notes
2. NO AMINO NOR DEACTIVATING 3. EXPLODE !
Br NH2 2. O Cl AlCl3 NH2 3. Add Groups to Anilines O Cl NH O Cl AlCl3
-OH
NO F.C.
HNO3 / H2SO4
BOOM!
O NH /!
NH2
O Br2 / FeBr3 NH2
O NH
-OH
NH2 /! Br
Br Hofmann Elimination NH2
1. CH3I (xs) 2. Ag2O / H2O / !
(MAJOR)
* Has to make 3 AMINE and serves as a LG! *Least
+
(minor)
sub alkene is MAJOR
*Most sub Alkene is minor
Formation and Reduction of Azides
Br 1.
1. NaN3 2. LiAlH4 3. H2O
NH2
*Forms 1 amines only
N3
2/3
Ray
Tasks
Hofmann Rearrangement R O
Reactions
Notes
X2 / H2O / NaOH NH2
R NH2
O NH2
X2 / H2O / NaOH O NH2
lose
NH2
Curtius Rearrangement
O Cl 1. NaN3 2. H2O / !
NH2
GOOD LUCK ON CH. 22 REACTIONS!
Potrebbero piacerti anche
- Organic Chemistry Cheat Sheet For Midterm2015Documento1 paginaOrganic Chemistry Cheat Sheet For Midterm2015Norma Leticia Ramos100% (6)
- Oxidation, Reduction, HydrolysisDocumento19 pagineOxidation, Reduction, HydrolysisTEJA SINGHNessuna valutazione finora
- Organic II Reactions BETADocumento8 pagineOrganic II Reactions BETARicky Fontaine100% (9)
- Advanced Organic Reactions 2000 - WarrenDocumento174 pagineAdvanced Organic Reactions 2000 - Warrenshiv57100% (3)
- Sr. No. Reaction Reagent Condition Mechanism Example NoteDocumento3 pagineSr. No. Reaction Reagent Condition Mechanism Example NoteAbbas HaiderNessuna valutazione finora
- Reagent TableDocumento10 pagineReagent Tablebluebeary22Nessuna valutazione finora
- Reagent and The Reactions They CauseDocumento3 pagineReagent and The Reactions They CauseChip Timmons100% (9)
- Organic Chemistry Reacions SummaryDocumento22 pagineOrganic Chemistry Reacions SummaryvgettinfatNessuna valutazione finora
- Organic Chemistry Midterm 1 Dir+eff++keyDocumento1 paginaOrganic Chemistry Midterm 1 Dir+eff++keyNorma Leticia RamosNessuna valutazione finora
- CHEM 215 F12 Chapter 13 Notes UMICHDocumento13 pagineCHEM 215 F12 Chapter 13 Notes UMICHRoxanne IlaganNessuna valutazione finora
- Revision Notes On AlcoholsDocumento13 pagineRevision Notes On AlcoholsMuredzwa MuzendaNessuna valutazione finora
- High Yield Biochemistry PDFDocumento41 pagineHigh Yield Biochemistry PDFKyle Broflovski100% (2)
- More Examples From 10.4: Math 1432 Notes - Week 10Documento17 pagineMore Examples From 10.4: Math 1432 Notes - Week 10Kyle BroflovskiNessuna valutazione finora
- More Examples From 10.4: Math 1432 Notes - Week 10Documento17 pagineMore Examples From 10.4: Math 1432 Notes - Week 10Kyle BroflovskiNessuna valutazione finora
- Chapter-11 Respiration in Plants PDFDocumento26 pagineChapter-11 Respiration in Plants PDFVedavathi100% (1)
- Ald&Ketone IIDocumento51 pagineAld&Ketone IIheraldas2421Nessuna valutazione finora
- Reaction SummaryDocumento5 pagineReaction SummaryShafaqatRahmanNessuna valutazione finora
- Reaction MechanismDocumento68 pagineReaction MechanismSiddarth Singh73% (11)
- Organic Chem Midterm 1mech++keyDocumento1 paginaOrganic Chem Midterm 1mech++keyNorma Leticia RamosNessuna valutazione finora
- How To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inDocumento2 pagineHow To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inmyiitchemistry81% (16)
- Name Reactions-II PDFDocumento32 pagineName Reactions-II PDFEstanislao Amadeo Avogadro100% (1)
- Organic Chemistry I: The Unofficial Reaction SheetDocumento11 pagineOrganic Chemistry I: The Unofficial Reaction SheetKarl WilsonNessuna valutazione finora
- 11.alcohol, Phenol & Ethers Colour BookletDocumento84 pagine11.alcohol, Phenol & Ethers Colour BookletVishal Malik100% (1)
- Chapter 10 Haloalkanes and HaloarenesDocumento24 pagineChapter 10 Haloalkanes and HaloarenesSuhas GowdaNessuna valutazione finora
- Organic I Reactions (Complete) PDFDocumento10 pagineOrganic I Reactions (Complete) PDFStarrx714Nessuna valutazione finora
- Organic II Reactions (Complete) BETADocumento21 pagineOrganic II Reactions (Complete) BETATheoNessuna valutazione finora
- Biochemistry ExamDocumento4 pagineBiochemistry ExamKyle Broflovski100% (1)
- CH 8 Handouts (All)Documento34 pagineCH 8 Handouts (All)Ryan MaNessuna valutazione finora
- Huckel Rule of Aromaticity 2 PDFDocumento25 pagineHuckel Rule of Aromaticity 2 PDFUmar Farooq100% (1)
- PMR Spectroscopy: Solved Problems Volume : IIDa EverandPMR Spectroscopy: Solved Problems Volume : IIValutazione: 5 su 5 stelle5/5 (3)
- Carboxylic AcidsDocumento41 pagineCarboxylic AcidsSazzad TanimNessuna valutazione finora
- 3 - Aldehydes and Ketones (Assignment) Booklet-2Documento15 pagine3 - Aldehydes and Ketones (Assignment) Booklet-2kraken monsterNessuna valutazione finora
- Chapter 1 Summary For BiochemistryDocumento16 pagineChapter 1 Summary For BiochemistryKyle BroflovskiNessuna valutazione finora
- Chapter 1 Summary For BiochemistryDocumento16 pagineChapter 1 Summary For BiochemistryKyle BroflovskiNessuna valutazione finora
- 1 Roh Carboxylic Acids: H CroDocumento15 pagine1 Roh Carboxylic Acids: H CroandrewwrobleNessuna valutazione finora
- Organic Chemistry ChartsDocumento84 pagineOrganic Chemistry ChartsPRIYANSHU KUMARNessuna valutazione finora
- SGDGDDDocumento33 pagineSGDGDDyopoboy100% (1)
- Essential Cell BiologyDocumento7 pagineEssential Cell BiologyKyle Broflovski0% (2)
- Aldehydes and KetonesDocumento9 pagineAldehydes and KetonesCamille AdleNessuna valutazione finora
- Organic Reagents: 1. Alcoholic KOH 2. Aluminium EthoxideDocumento9 pagineOrganic Reagents: 1. Alcoholic KOH 2. Aluminium EthoxideAarya Nandal100% (1)
- Organic Conversion A & B Revise Before NEETDocumento2 pagineOrganic Conversion A & B Revise Before NEETAquib JavedNessuna valutazione finora
- 12 Chemistry Notes ch11 Alcohols Phenols and EthersDocumento8 pagine12 Chemistry Notes ch11 Alcohols Phenols and Ethersmv7602456Nessuna valutazione finora
- Alcohol Phenol & EtherDocumento13 pagineAlcohol Phenol & EtherAbir DuttaNessuna valutazione finora
- Pdf-Haloalkanes and HaloarenesDocumento159 paginePdf-Haloalkanes and HaloarenesOmkar Singh Shekhawat100% (2)
- Organic Chemistry ReagentsDocumento7 pagineOrganic Chemistry ReagentsRishabhNessuna valutazione finora
- Name Reactions: Sandmeyer'S ReactionDocumento9 pagineName Reactions: Sandmeyer'S ReactionSai Krishnan100% (1)
- Haloalkanes and Haloarenes1Documento15 pagineHaloalkanes and Haloarenes1Poorni RenuNessuna valutazione finora
- Inorganic ChemistryDocumento10 pagineInorganic Chemistrydebraj sethi100% (1)
- 100 Organic Reagentspptx - 230327 - 085539 PDFDocumento15 pagine100 Organic Reagentspptx - 230327 - 085539 PDFHeera MeenaNessuna valutazione finora
- WWW - Crackjee.xyz: Organic ChemistryDocumento9 pagineWWW - Crackjee.xyz: Organic ChemistryRau100% (1)
- Reagent ListDocumento9 pagineReagent ListArka MukhopadhyayNessuna valutazione finora
- Hydrocarbons NotesDocumento13 pagineHydrocarbons NotesShivansh Pundir100% (1)
- Organic Chemistry ReactionDocumento3 pagineOrganic Chemistry ReactionGAMEPORIUMNessuna valutazione finora
- Chem 212 Alkyl Halide Problems 2Documento1 paginaChem 212 Alkyl Halide Problems 2kevinamy100% (1)
- Chemistry - Organic Chemistry MechanismsDocumento2 pagineChemistry - Organic Chemistry Mechanismshelixate100% (3)
- Rules For Organic Chemical ConversionsDocumento4 pagineRules For Organic Chemical ConversionsKamran Maqsood78% (9)
- Conversions (ORGANIC)Documento8 pagineConversions (ORGANIC)Abir Dutta80% (5)
- Alkyl Aryl Halides PDFDocumento21 pagineAlkyl Aryl Halides PDFLakshit SanghrajkaNessuna valutazione finora
- 6carboxylic AcidsDocumento1 pagina6carboxylic AcidssharmimiameerasanadyNessuna valutazione finora
- X UV Light or Heat: Reactions in Topic XIDocumento3 pagineX UV Light or Heat: Reactions in Topic XImichelsonyip100% (1)
- Carboxylic AcidsDocumento26 pagineCarboxylic Acidsapi-3734333100% (1)
- Ethers R-O-R or R-O-R : NomenclatureDocumento17 pagineEthers R-O-R or R-O-R : NomenclatureAbhishek Guddad100% (1)
- Acids, Derivatives and NitrilesDocumento23 pagineAcids, Derivatives and NitrilesLuqman HakimNessuna valutazione finora
- ReductionDocumento7 pagineReductionPranayNessuna valutazione finora
- Hydroxyl at I OnDocumento60 pagineHydroxyl at I OngbgbkrishnaNessuna valutazione finora
- Acfrogcxqpd3dqml GQHMZQ B0c089di81vpcrvgphrwcu4gh Bzezvshldjt4clxztdrke4cieuxds1wlvk6scla 0byn2rmeu4btdaq8ckybm0chweegnztu7u2olcnli2lia5txmf386nyikuDocumento6 pagineAcfrogcxqpd3dqml GQHMZQ B0c089di81vpcrvgphrwcu4gh Bzezvshldjt4clxztdrke4cieuxds1wlvk6scla 0byn2rmeu4btdaq8ckybm0chweegnztu7u2olcnli2lia5txmf386nyikuAchal ParekhNessuna valutazione finora
- Water An An Ether: AlcoholDocumento35 pagineWater An An Ether: AlcoholEshita SharmaNessuna valutazione finora
- Org Chem Alcohol Mechanisms From JasperseDocumento22 pagineOrg Chem Alcohol Mechanisms From JasperseChloe JazminesNessuna valutazione finora
- Biochemistry Chapter Summary 10Documento18 pagineBiochemistry Chapter Summary 10Kyle BroflovskiNessuna valutazione finora
- Biochemistry Chapter Summary 10Documento18 pagineBiochemistry Chapter Summary 10Kyle BroflovskiNessuna valutazione finora
- 05Documento18 pagine05gatototNessuna valutazione finora
- Chapter 5 Biochemistry Text SummaryDocumento18 pagineChapter 5 Biochemistry Text SummaryKyle BroflovskiNessuna valutazione finora
- WK 11 S1Documento16 pagineWK 11 S1Kyle BroflovskiNessuna valutazione finora
- M"is#an#upper%bound"for#S"if#x"≤#M"for#all#x"ε#S." "Documento11 pagineM"is#an#upper%bound"for#S"if#x"≤#M"for#all#x"ε#S." "Kyle BroflovskiNessuna valutazione finora
- WK 11 S1Documento16 pagineWK 11 S1Kyle BroflovskiNessuna valutazione finora
- Chapter30 TestBankDocumento7 pagineChapter30 TestBankKyle BroflovskiNessuna valutazione finora
- WK 8 S1 PDFDocumento13 pagineWK 8 S1 PDFKyle BroflovskiNessuna valutazione finora
- Math 1432 Notes - Week 12 Some Review From Last WeekDocumento11 pagineMath 1432 Notes - Week 12 Some Review From Last WeekKyle BroflovskiNessuna valutazione finora
- WK 6 S2Documento20 pagineWK 6 S2Kyle BroflovskiNessuna valutazione finora
- 9.3-9.4 - More Examples Recall:: X R y R X y RDocumento15 pagine9.3-9.4 - More Examples Recall:: X R y R X y RKyle BroflovskiNessuna valutazione finora
- WK 6 S2Documento20 pagineWK 6 S2Kyle BroflovskiNessuna valutazione finora
- Math 1432 Notes - Week 12 Some Review From Last WeekDocumento11 pagineMath 1432 Notes - Week 12 Some Review From Last WeekKyle BroflovskiNessuna valutazione finora
- M"is#an#upper%bound"for#S"if#x"≤#M"for#all#x"ε#S." "Documento11 pagineM"is#an#upper%bound"for#S"if#x"≤#M"for#all#x"ε#S." "Kyle BroflovskiNessuna valutazione finora
- 9.3-9.4 - More Examples Recall:: X R y R X y RDocumento12 pagine9.3-9.4 - More Examples Recall:: X R y R X y RKyle BroflovskiNessuna valutazione finora
- Chapter 5 Lecture NotesDocumento24 pagineChapter 5 Lecture NotesKyle BroflovskiNessuna valutazione finora
- Chapter 11 HW KeyDocumento5 pagineChapter 11 HW KeyKyle BroflovskiNessuna valutazione finora
- Morning Exam For Biol 3301Documento9 pagineMorning Exam For Biol 3301Kyle BroflovskiNessuna valutazione finora
- Math 1432 Notes - Week 8Documento13 pagineMath 1432 Notes - Week 8Kyle BroflovskiNessuna valutazione finora
- Chapter 4 BiostatisticsDocumento32 pagineChapter 4 BiostatisticsKyle BroflovskiNessuna valutazione finora
- Chapter8 HW SolutionsDocumento8 pagineChapter8 HW SolutionsKyle BroflovskiNessuna valutazione finora
- Confidence IntervalsDocumento3 pagineConfidence IntervalsKyle BroflovskiNessuna valutazione finora
- Ames TestDocumento14 pagineAmes TestrupinisinnanNessuna valutazione finora
- Chemistry 120.1 - Organic Chemistry Laboratory Laboratory ReportDocumento3 pagineChemistry 120.1 - Organic Chemistry Laboratory Laboratory Reportkat katNessuna valutazione finora
- Fatty Acid Oxidation Part Two Illustration AtfDocumento1 paginaFatty Acid Oxidation Part Two Illustration AtfDivyaa VisvalingamNessuna valutazione finora
- One Carbon MetabolismDocumento3 pagineOne Carbon MetabolismDipesh ShresthaNessuna valutazione finora
- CO 301 Heterocyclic ChemistryDocumento31 pagineCO 301 Heterocyclic ChemistryMohini BajajNessuna valutazione finora
- 02ABiomolecules and ThermodynamicsDocumento40 pagine02ABiomolecules and ThermodynamicsAtirahNessuna valutazione finora
- Chm3270 Hw2 KeyDocumento4 pagineChm3270 Hw2 KeyTaiNessuna valutazione finora
- SocialDocumento7 pagineSocialShaif uddinNessuna valutazione finora
- Jurnal Erisanti Octavia R PDFDocumento8 pagineJurnal Erisanti Octavia R PDFErisanti OctaviaNessuna valutazione finora
- The Nucleic Acids: © 2016 Paul BillietDocumento14 pagineThe Nucleic Acids: © 2016 Paul BillietCarah Jean Hurtado GillegaoNessuna valutazione finora
- DNA - The Double Helix - Coloring WorksheetDocumento4 pagineDNA - The Double Helix - Coloring WorksheetANGEL GUZMAN HERNANDEZNessuna valutazione finora
- Vco Philippine National StandardDocumento8 pagineVco Philippine National StandardTeenahNessuna valutazione finora
- Usp39 Nf34 Index Supplement1Documento71 pagineUsp39 Nf34 Index Supplement1Sandip Mehta100% (1)
- Nirogyam Health PackagesDocumento1 paginaNirogyam Health PackagesUNIQUE DIAGNOSTICNessuna valutazione finora
- Protecting Groups Alcohols and AldehydesDocumento18 pagineProtecting Groups Alcohols and AldehydesNicksonNessuna valutazione finora
- Worksheet16 Transcription To TranslationDocumento3 pagineWorksheet16 Transcription To Translationliterally deadNessuna valutazione finora
- Pharmacognosy Lecture # 7+8 (Lipids) (By, Sir Tanveer Khan)Documento40 paginePharmacognosy Lecture # 7+8 (Lipids) (By, Sir Tanveer Khan)Arslan AbdullahNessuna valutazione finora
- Fischer and Schrock CarbenesDocumento8 pagineFischer and Schrock Carbenesharmanpreet kaurNessuna valutazione finora
- Oxidation ReactionDocumento21 pagineOxidation ReactionNor AzilaNessuna valutazione finora
- Enzymes 2023Documento43 pagineEnzymes 2023sanjay jaiswalNessuna valutazione finora
- Cauliflower: CabbageDocumento18 pagineCauliflower: CabbageGabitzzu AnonimNessuna valutazione finora
- Practice Packet: Chapter 8:organicchemistryDocumento45 paginePractice Packet: Chapter 8:organicchemistryKerala MekuriyaNessuna valutazione finora
- Chapter 17 Lipids: 17.4 Chemical Properties of TriacylglycerolsDocumento20 pagineChapter 17 Lipids: 17.4 Chemical Properties of TriacylglycerolsAser SerNessuna valutazione finora
- Chaitanya Deemed University: Food and NutritionDocumento14 pagineChaitanya Deemed University: Food and NutritionVenkatasairamreddy KandulaNessuna valutazione finora
- Valores Orientadores EPADocumento87 pagineValores Orientadores EPAJailson SilvaNessuna valutazione finora
- Lesson Plan HydrocarbonDocumento7 pagineLesson Plan Hydrocarbonedgardo mirandaNessuna valutazione finora
- Jurnal Penelitian SriDocumento8 pagineJurnal Penelitian SriSriNessuna valutazione finora
- Antioxidants and Antioxidant Methods An Updated OverviewDocumento65 pagineAntioxidants and Antioxidant Methods An Updated Overviewalice adanNessuna valutazione finora
- Carbon Compounds (From Google)Documento27 pagineCarbon Compounds (From Google)Luzel AnferneeNessuna valutazione finora