Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Combined Spectroscopy Problems1
Caricato da
chem_dream2831Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Combined Spectroscopy Problems1
Caricato da
chem_dream2831Copyright:
Formati disponibili
Combined Spectroscopy Problems
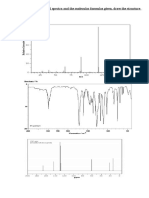
On the following pages you will find spectroscopic data for eight different compounds. The data provided will be some combination of Mass Spec (or molecular formula), 1H-NMR, 13C-NMR, and IR spectroscopy information. Use the data provided to determine the structure of each compound. Each problem has only a single compound that completely consistent with the data provided. 1) Deduce the structure that corresponds to the spectral data. Mass Spectrum: m/z = 86 (M; 20.2%), m/z = 87 (1.2%) IR:
Dont worry about this.
H-NMR: 2.40 ppm (triplet; integral = 1.0), 2.13 ppm (singlet; integral = 1.5), 1.60 ppm (sextet; integral = 1.0) and 0.93 ppm (triplet; integral = 1.5).
13
C-NMR: 208.93 ppm (singlet), 45.71 ppm (triplet), 29.78 ppm (quartet), 17.41 ppm (triplet), 13.70 ppm (quartet).
2) Deduce the structure that corresponds to the spectral data. Mass Spectrum: m/z = 114 (M; 5.2%), m/z = 115 (0.36%) IR:
Dont worry about this.
H-NMR: 2.71 ppm (singlet; integral = 1.0), 2.19 ppm (singlet; integral = 1.5). C-NMR: 206.87 ppm (singlet), 36.96 ppm (triplet), 29.76 ppm (quartet).
13
3) Deduce the structure that corresponds to the spectral data. Molecular Formula: C5H9N IR:
Dont worry about this.
H-NMR: 2.34 ppm (triplet; integral = 1.0), 1.28-1.82 ppm (multiplet; integral = 2.0), 0.96 ppm (triplet; integral = 1.5). C-NMR: 119.84 ppm (singlet), 27.42 ppm (triplet), 21.86 ppm (triplet), 16.81 ppm (triplet), 13.24 ppm (quartet).
13
4) Deduce the structure that corresponds to the spectral data. Mass Spectrum: m/z = 136 (M; 75%), m/z = 137 (6.8%) IR:
H-NMR: 9.86 ppm (singlet; integral = 1.0), 7.82 ppm (multiplet; integral = 2.0), 6.98ppm (multiplet; integral = 2.0), 3.86 (singlet; integral = 3.0). C-NMR: 190.70 ppm (doublet), 164.63 ppm (singlet), 131.93 ppm (doublet), 129.97 ppm (singlet), 114.33 ppm (doublet), 55.33 ppm (quartet).
13
5) Deduce the structure that corresponds to the spectral data. Mass Spectrum: m/z = 136 (M; 75%), m/z = 137 (6.9%) IR:
H-NMR: 12.77 ppm (singlet; integral = 1.0), 7.86 ppm (multiplet; integral = 2.0), 7.31 ppm (multiplet; integral = 2.0), 2.38 (singlet; integral = 3.0). C-NMR: 167.34 ppm (singlet), 142.98 ppm (singlet), 129.37 ppm (doublet), 129.09 ppm (doublet), 128.13 ppm (singlet), 21.10 ppm (quartet).
13
6) Deduce the structure that corresponds to the spectral data. Mass Spectrum: m/z = 170 (M; 100%), m/z = 171 (13.4%) IR:
H-NMR: 7.22 ppm (multiplet; integral = 2.0), 6.98 ppm (multiplet; integral = 2.0), 6.92 ppm (multiplet; integral = 1.0). C-NMR: 157.21 ppm (singlet), 129.64 ppm (doublet), 123.11 ppm (doublet), 118.81 ppm (doublet).
13
7) Deduce the structure that corresponds to the spectral data. Mass Spectrum: m/z = 146 (M; 30.5%), m/z = 147 (2.9%), m/z = 148 (1.4%) Hint: Make sure to consider the M + 2 peak in the mass spectrum, and consider it before the M + 1 peak. IR:
H-NMR: 2.37 ppm (doublet; integral = 2.0), 1.79 ppm (nonet; integral = 1.0), 0.99 ppm (doublet; integral = 6.0). C-NMR: 42.27 ppm (triplet), 28.77 ppm (doublet), 22.09 ppm (quartet).
13
8) Deduce the structure that corresponds to the spectral data. Molecular Formula: C11H20O4 IR:
H-NMR: 4.19 ppm (quartet; integral = 2.0), 3.39 ppm (triplet; integral = 1.0), 1.78 ppm (doublet; integral = 2.0), 1.58 ppm (nonet; integral = 1.0), 1.27 ppm (triplet; integral = 3), 0.93 ppm (doublet; integral = 6.0). C-NMR: 169.69 ppm (singlet), 61.22 ppm (triplet), 50.42 ppm (doublet), 37.63 ppm (triplet), 26.27 ppm (doublet), 22.28 ppm (quartet), 14.12 (quartet).
13
Potrebbero piacerti anche
- Columb's LawDocumento9 pagineColumb's LawShahzadNessuna valutazione finora
- Synthetic ReagentsDocumento75 pagineSynthetic ReagentsBapu Thorat100% (1)
- Analytical Techniques in Drug DevelopmentDocumento16 pagineAnalytical Techniques in Drug DevelopmentLeonard Goh Zhong Ning100% (3)
- Spectroscopy Problems Part 1Documento49 pagineSpectroscopy Problems Part 1Partha Samanta100% (1)
- FMO LectureDocumento14 pagineFMO Lecturebooks4free23Nessuna valutazione finora
- NMR Solving StrategyDocumento2 pagineNMR Solving Strategysorrow Lemon100% (1)
- UV Visible Spectroscopy NotesDocumento10 pagineUV Visible Spectroscopy NotesMalik Hamza AslamNessuna valutazione finora
- Chapter 7Documento71 pagineChapter 7cjn999980% (10)
- AromaticityDocumento24 pagineAromaticitymilindthakare75Nessuna valutazione finora
- NMR Organic ChemistryDocumento17 pagineNMR Organic Chemistrysallymoon34Nessuna valutazione finora
- Disconnection in Organic SynthesisDocumento31 pagineDisconnection in Organic SynthesisSwami Prabhu100% (2)
- Three Failures of Classical PhysicsDocumento67 pagineThree Failures of Classical PhysicsAdli Hadiyan Munif50% (2)
- NMR - Multiple Choice QuestionsDocumento71 pagineNMR - Multiple Choice QuestionsOmSilence265171% (31)
- Practice Problems-Pericyclic ReactionsDocumento5 paginePractice Problems-Pericyclic ReactionsJethro Sanz75% (4)
- Applications of NMR Spectroscopy in Inorganic ChemistryDocumento11 pagineApplications of NMR Spectroscopy in Inorganic ChemistryDhanaswamy Ilangeswaran92% (12)
- NMR Multiple Choice Questions PDFDocumento71 pagineNMR Multiple Choice Questions PDFDeepak SinghNessuna valutazione finora
- NMR 1Documento3 pagineNMR 1amitNessuna valutazione finora
- Benzene Derivatives Multiple Choice QuestionsDocumento33 pagineBenzene Derivatives Multiple Choice QuestionsDante Ramoth75% (4)
- Reaction MechanismDocumento68 pagineReaction MechanismSiddarth Singh73% (11)
- CH203 Fall 2014 Exam Two Practice Test With AnswersDocumento10 pagineCH203 Fall 2014 Exam Two Practice Test With AnswersBUCH203Nessuna valutazione finora
- PMR Spectroscopy: Solved Problems Volume : IIDa EverandPMR Spectroscopy: Solved Problems Volume : IIValutazione: 5 su 5 stelle5/5 (3)
- Combined Problems and Solution On Organic Spectros PDFDocumento2 pagineCombined Problems and Solution On Organic Spectros PDFJill29% (7)
- Spectra ProblemsDocumento111 pagineSpectra ProblemsChandra Reddy100% (1)
- The Nature and Origin of ChargeDocumento3 pagineThe Nature and Origin of ChargeFrancesca GenerNessuna valutazione finora
- Protecting Group HandoutDocumento5 pagineProtecting Group HandoutRafaelle Sanvictores SilongNessuna valutazione finora
- Pericyclic Reactions and Organic Photochemistry PDFDocumento347 paginePericyclic Reactions and Organic Photochemistry PDFShouvik Halder100% (3)
- CHAPTER 3 MacromoleculesDocumento8 pagineCHAPTER 3 MacromoleculesajmalshierreNessuna valutazione finora
- IR Spectroscopy Problem Set 2Documento5 pagineIR Spectroscopy Problem Set 2Jules BrunoNessuna valutazione finora
- Organic Structure Elucidation WorkbookDocumento498 pagineOrganic Structure Elucidation WorkbookKajan Muneeswaran75% (4)
- Divine Action in Quantum WorldDocumento346 pagineDivine Action in Quantum WorldOarga Catalin-PetruNessuna valutazione finora
- Report NMRDocumento13 pagineReport NMRsarahNessuna valutazione finora
- 202 Combined Spectroscopy ProblemsDocumento7 pagine202 Combined Spectroscopy Problemschem_dream283175% (4)
- Physical Chemistry FormulasDocumento1 paginaPhysical Chemistry FormulasDelphinacro100% (1)
- Questions-Answers Heterocyclic ChemistryDocumento21 pagineQuestions-Answers Heterocyclic ChemistryNguyên Khang70% (10)
- Unit 3 - Atomic Structure and Chemical Periodicity21Documento129 pagineUnit 3 - Atomic Structure and Chemical Periodicity21Horace RoyalNessuna valutazione finora
- AromaticityDocumento12 pagineAromaticityV G Viju KumarNessuna valutazione finora
- Basic 2D NMR techniques for structure determinationDocumento32 pagineBasic 2D NMR techniques for structure determinationDelicz TanNessuna valutazione finora
- 1H NMR Spectroscopy in Organic Chemistry - MCQDocumento18 pagine1H NMR Spectroscopy in Organic Chemistry - MCQShunmugasundaram ArunachalamNessuna valutazione finora
- Spectroscopy ProblemsDocumento16 pagineSpectroscopy Problemsnvithyarajan6872100% (2)
- 4) Transition Metal Electron Configuration Multiple ChoiceDocumento4 pagine4) Transition Metal Electron Configuration Multiple ChoiceAnonymous pgjIAZoNessuna valutazione finora
- Guide To Solving Spectroscopy ProblemsDocumento4 pagineGuide To Solving Spectroscopy ProblemsJen100% (1)
- Quiz 1: IR Spectroscopy (Chapter 2) Name: Fatin Wahida Binti Hashim Student ID: 2017680232 Group: A4AS1205 - 10Documento5 pagineQuiz 1: IR Spectroscopy (Chapter 2) Name: Fatin Wahida Binti Hashim Student ID: 2017680232 Group: A4AS1205 - 10fatin hashimNessuna valutazione finora
- Csir Model QuestionsDocumento15 pagineCsir Model QuestionsbaluNessuna valutazione finora
- Chapter 9 Organic Chemistry SolomonDocumento6 pagineChapter 9 Organic Chemistry SolomonSukhi Sohal0% (1)
- UV-VIS Organic Spectroscopy MCQDocumento20 pagineUV-VIS Organic Spectroscopy MCQShunmugasundaram Arunachalam100% (2)
- Problems On Named ReactionsDocumento103 pagineProblems On Named ReactionsBapu ThoratNessuna valutazione finora
- MSC Org Chem Notes GDDocumento256 pagineMSC Org Chem Notes GDsalinips350% (2)
- Model Answer: The Following Questions Answer Choose The Correct Answer: (20Documento4 pagineModel Answer: The Following Questions Answer Choose The Correct Answer: (20Khalid AbeedNessuna valutazione finora
- Exams Organic Chemistry MITDocumento333 pagineExams Organic Chemistry MITn2h_spNessuna valutazione finora
- Organic ChemistryDocumento20 pagineOrganic ChemistryGirish RaguvirNessuna valutazione finora
- Molecular Orbital Theory in Homonuclear and Heteronuclear Diatomic MoleculesDocumento12 pagineMolecular Orbital Theory in Homonuclear and Heteronuclear Diatomic MoleculesJeevanantham VelayuthamNessuna valutazione finora
- Spectroscopic Solutions of StructureDocumento21 pagineSpectroscopic Solutions of StructureKassimNessuna valutazione finora
- 13C NMR Spectroscopy in Organic Chemistry MCQDocumento27 pagine13C NMR Spectroscopy in Organic Chemistry MCQShunmugasundaram ArunachalamNessuna valutazione finora
- Unimolecular Surface ReactionDocumento13 pagineUnimolecular Surface ReactionIqbal Siddiquey100% (3)
- Photochemistry of Carbonyl Compounds: Primary ProcessesDocumento16 paginePhotochemistry of Carbonyl Compounds: Primary ProcessesNaveen Agarwal100% (1)
- Retrosynthetic Analysis PDFDocumento6 pagineRetrosynthetic Analysis PDFNoleNessuna valutazione finora
- Molecular OrbitalsDocumento80 pagineMolecular Orbitals1balamanian100% (1)
- COORDINATION CHEMISTRY TITLEDocumento11 pagineCOORDINATION CHEMISTRY TITLESubhasish Sau100% (2)
- Chemistry 344: Spectroscopy and Spectrometry Problem Set 1Documento12 pagineChemistry 344: Spectroscopy and Spectrometry Problem Set 1Fasiha RazaNessuna valutazione finora
- Retrosynthetic AnalysisDocumento6 pagineRetrosynthetic AnalysisSiti Hafidzotur RNessuna valutazione finora
- IR Spectroscopy Problem Set 4 Answer KeyDocumento6 pagineIR Spectroscopy Problem Set 4 Answer KeyJules BrunoNessuna valutazione finora
- مسائلDocumento7 pagineمسائلRawan AdelNessuna valutazione finora
- Problems Related To SpectrosDocumento4 pagineProblems Related To SpectrosAkshay KaushikNessuna valutazione finora
- Problems Related To Spectroscopy (Solution)Documento9 pagineProblems Related To Spectroscopy (Solution)Nana BadrNessuna valutazione finora
- CY4104Documento3 pagineCY4104Aakash BanerjeeNessuna valutazione finora
- 2423L10Documento10 pagine2423L10chem_dream2831Nessuna valutazione finora
- Chem 14C Spring 2012 Lecture Exam ScheduleDocumento1 paginaChem 14C Spring 2012 Lecture Exam Schedulechem_dream2831Nessuna valutazione finora
- Waste TreatmentDocumento18 pagineWaste TreatmentDeji 'Allen Walker' OlapoNessuna valutazione finora
- Problem Set 1Documento1 paginaProblem Set 1Luvita MaritshaNessuna valutazione finora
- Modern Physics 1Documento2 pagineModern Physics 1Ramesh BadamNessuna valutazione finora
- Ch. 10 Homework Models For Conjugated SystemsDocumento3 pagineCh. 10 Homework Models For Conjugated SystemsASUPREMEANessuna valutazione finora
- Early atomic theories developed through experimentationDocumento3 pagineEarly atomic theories developed through experimentationTinray ReyesNessuna valutazione finora
- Introduction to MagnetismDocumento110 pagineIntroduction to MagnetismAhmad AwadallahNessuna valutazione finora
- Department of Mathematics and Natural Sciences CHE 101: Introduction To Chemistry Dr. Zayed Bin Zakir ShawonDocumento13 pagineDepartment of Mathematics and Natural Sciences CHE 101: Introduction To Chemistry Dr. Zayed Bin Zakir ShawonSYEDA UMME SALMANessuna valutazione finora
- Quantum Mechanics THIRD EDITION Eugene MerzbacherDocumento670 pagineQuantum Mechanics THIRD EDITION Eugene MerzbachersplouvrosNessuna valutazione finora
- 11 QuantumDocumento54 pagine11 QuantumVinod RajNessuna valutazione finora
- PYL100 Electromagnetic Waves and Quantum Mechanics Exercise SheetDocumento2 paginePYL100 Electromagnetic Waves and Quantum Mechanics Exercise SheetFranklin GarysonNessuna valutazione finora
- Phys 454Documento75 paginePhys 454huihuan5zhaoNessuna valutazione finora
- Richard FeynmanDocumento2 pagineRichard Feynmanmuzammalsafdar100% (1)
- Seiber Go LogyDocumento155 pagineSeiber Go LogyLambertNessuna valutazione finora
- Physics 30 Practice DiplomaDocumento29 paginePhysics 30 Practice DiplomaAlejandro Romero MejiaNessuna valutazione finora
- Weak InteractionDocumento95 pagineWeak Interactionsimran mishraNessuna valutazione finora
- Electron Correlation: MBPT2, CASPT2, CC, CPF.: Static Dynamic Mrpt2Documento24 pagineElectron Correlation: MBPT2, CASPT2, CC, CPF.: Static Dynamic Mrpt2Sandeep KumarNessuna valutazione finora
- L3 - Atomic Structure PDFDocumento30 pagineL3 - Atomic Structure PDFOnkar DharmadhikariNessuna valutazione finora
- Chapter 6: The Fermi Liquid: L.D. Landau December 22, 2000Documento55 pagineChapter 6: The Fermi Liquid: L.D. Landau December 22, 2000padma princessNessuna valutazione finora
- Chapter #20 Nuclear RadiationsDocumento4 pagineChapter #20 Nuclear RadiationsMuhammad TaahaNessuna valutazione finora
- Neet Ug Physics Photoelectric Effect Final 1Documento60 pagineNeet Ug Physics Photoelectric Effect Final 1Johm WayneNessuna valutazione finora
- End of Unit 5: Answers To Examination-Style QuestionsDocumento13 pagineEnd of Unit 5: Answers To Examination-Style QuestionsjasbirsinghNessuna valutazione finora
- Structure of Atom: Discovery and CharacteristicsDocumento38 pagineStructure of Atom: Discovery and Characteristicskavithanakkiran_3003Nessuna valutazione finora
- I Danaila Bose EinsteinDocumento38 pagineI Danaila Bose EinsteincocoaramirezNessuna valutazione finora
- Can Fermi Energy Be Determined by Heating And/or Cooling A Copper Wire?Documento5 pagineCan Fermi Energy Be Determined by Heating And/or Cooling A Copper Wire?ken adamsNessuna valutazione finora
- Feynman Rules For Electroweak TheoryDocumento5 pagineFeynman Rules For Electroweak TheoryDouglasNessuna valutazione finora