Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Solvolysis of Salt of A Weak Acid and Weak Base
Caricato da
Nitty MeYaTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Solvolysis of Salt of A Weak Acid and Weak Base
Caricato da
Nitty MeYaCopyright:
Formati disponibili
Title: solvolysis of the salt of a weak acid and a weak base.
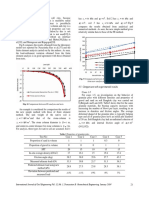
Objective: to determine the extent of solvolysis of ammonium borate in water by calorimetry. Results: 25ml of 1.75M NaOH with 100ml of 0.5M HCl Time, t (sec) 60 120 180 240 309 313 320 348 408 468 528 588 Temperature, T (C) 25.0 25.0 25.0 25.0 27.0 28.0 28.5 29.0 29.0 29.0 29.0 29.0
25ml of 1.75M NaOH with 100ml of 0.5M H3BO3 Time, t (sec) 0 60 120 180 240 306 310 313 336 396 456 516 576 Temperature, T (C) 25.0 25.0 25.0 25.0 25.0 26.0 26.5 27.0 27.5 27.5 27.5 27.5 27.5
25ml of 1.75M NH4OH with 100ml of 0.5M HCl Time, t (sec) 0 60 120 180 240 302 306 308 329 389 449 509 569 Temperature, T (C) 25.0 25.0 25.0 25.0 25.0 25.0 26.0 26.5 27.0 27.0 27.0 27.0 27.0
25ml of 1.75M NH4OH with 100ml of 0.5M H3BO3 Time, t (sec) 0 60 120 180 240 307 313 330 390 450 510 570 830 Temperature, T (C) 25.0 25.0 25.0 25.0 25.0 25.5 26.0 26.5 26.5 26.5 26.5 26.5 26.5
Calculation: Based on the graphs, The values of T in mixtures of; a) 25ml of 1.75M NaOH with 100ml of 0.5M HCl = 4.0C b) 25ml of 1.75M NaOH with 100ml of 0.5M H3BO3 = 2.5C c) 25ml of 1.75M NH4OH with 100ml of 0.5M HCl = 2.0C d) 25ml of 1.75M NH4OH with 100ml of 0.5M H3BO3 = 1.5 C The mole of NaOH used is Mole = MV / 1000 = (1.75M)(25cm3) / 1000 = 0.04375 mol The mole of HCl used is Mole = MV / 1000 = (0.50M)(100cm3) / 1000 = 0.05 mol
Limiting mole is NaOH, hence the enthalpy of neutralisation is as such; -13.36 k cal mol-1 x 0.04375 = -0.5845 k cal Heat released = - q Therefore, -q1 = msolutionCsolution T + Ccalorimeter T - (-0.5845 x 103 cal) = (125g) (1 cal C-1g-1) (4C) + Ccalorimeter (4C) Ccalorimeter = (584.5cal -500cal) / (4C) = 21.125 cal C-1 -q4 = (125g) (1 cal C-1g-1) (2.5C) + (21.125) (2.5C) = 365.3 cal -q5 = (125g) (1 cal C-1g-1)(2.0C) + (21.125)(2.0C) = 292.3 cal q6 = q4 +q5 q1 = (-365.3cal -292.3cal) -(-584.5cal) = -73.1 cal Theoretical q is calculated as such; -q= (125g) (1 cal C-1g-1)(1.5C) + (21.125)(1.5C) = 219.2 cal Fraction of the salt ammonium borate that reacts with water when dissolved in water is calculated as such where is the value when the reactants are mixed in exactly equimolar amount.
= = 219.2 cal / 73.1 cal =3 The value of is the fraction of the salt ammonium borate that undergoes solvolysis = 1 - =13 =-2 Hence the value of equilibrium constant can be identified using the relationship between Ksolvolysis and , where; Ksolvolysis =
= = 4/9
Given that KW is 1 x 10-14 and Kb is 1.75 x 10-5 for ammonia, the value of Ka of boric acid can be found as shown below; Ksolvolysis = Ka = (1 x 10-14 ) / ( 4/9 x 1.75 x 10-5) = 1.286 x 10-9
The published value of Ka of boric acid is 7.3 x 10-10 With this, the percentage of error is x 100% = 76.2% Discussion: Based on the results and calculations, the heat released decreases as the strength of acidity and basicity reduces. The heat change of each reaction is tabulated in the table below; Reactions 25ml of 1.75M NaOH with 100ml of 0.5M HCl 25ml of 1.75M NaOH with 100ml of 0.5M H3BO3 25ml of 1.75M NH4OH with 100ml of 0.5M HCl 25ml of 1.75M NH4OH with 100ml of 0.5M H3BO3 Heat Released (Exothermic) (cal) 584.5 365.3 292.3 219.2
Heat evolved during reactions decreases because the reactions are incomplete as the strength of acid or base decreases. The calculated q6 is higher than the observed value of q. The reason is that the reaction does not go to completion so the heat that is supposed to evolve and the observed heat change of reactant is different. According to the published value of Ka of boric acid, the percentage error is high, approximately 7.3 x 10-10 . This is owes to external factors such as the type of material the calorimeter is made up of as well as the efficiency of the calorimeter. Internal factors
such as the temperature change was not clearly recorded together with the mishandling of apparatus would also apply to the inaccuracy in the reading. The results were taken from a group of students in Lab Practical 3 as the results in my group were inaccurate and there were several discrepancies when the values obtained were substituted into the calculations above. Conclusion: 1) 2) 3) The value of calculated Ka of boric acid is 1.286 x 10-9. The heat capacity of calorimeter is 21.125 cal C-1. The equilibrium constant for the solvolysis constant is 0.44
Reference: http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch17/ph.php#ka
Graph: 25ml of 1.75M NaOH with 100ml of 0.5M HCl
temperature (C) against time,t
29.5 29.0 28.5 28.0 27.5 27.0 26.5 26.0 25.5 25.0 24.5 0 100 200 300 400 Time, t (seconds) 500 600 700
25ml of 1.75M NaOH with 100ml of 0.5M H3BO3
temperature (C)
temperature (C) against time,t
28.0
temperature (C)
27.5 27.0 26.5 26.0 25.5 25.0 24.5 0 100 200 300 400 500 600 700 Time, t (seconds)
25ml of 1.75M NH4OH with 100ml of 0.5M HCl
temperature (C) against time,t
27.5
temperature (C)
27.0 26.5 26.0 25.5 25.0 24.5 0 100 200 300 Time, t (seconds) 400 500 600
25ml of 1.75M NH4OH with 100ml of 0.5M H3BO3
temperature (C) against time,t
26.6 26.4 26.2
temperature (C)
26.0 25.8 25.6 25.4 25.2 25.0 24.8 0 100 200 300 400 500 600 700 800 900 Time, t (seconds)
Potrebbero piacerti anche
- Chem 152 Lab 4Documento4 pagineChem 152 Lab 4mifferdk23% (13)
- Heat of Neutralization - Lab ReportDocumento7 pagineHeat of Neutralization - Lab ReportJasmeetSingh56% (9)
- Tutorial 1 - AnswersDocumento8 pagineTutorial 1 - AnswersRaymond Kakala100% (6)
- Adsorption of Acetic Acid On Charcoal SurfaceDocumento3 pagineAdsorption of Acetic Acid On Charcoal SurfaceFrankyFan90% (10)
- Experiment 5Documento8 pagineExperiment 5talaNessuna valutazione finora
- C30 M1 L5 Assn Part 1 Libby FDocumento12 pagineC30 M1 L5 Assn Part 1 Libby Flibby foxNessuna valutazione finora
- Adsorption of Acetic Acid On Charcoal SurfaceDocumento3 pagineAdsorption of Acetic Acid On Charcoal SurfaceDrGaurav Rajput100% (1)
- Practical 22.1 Iron Wool Redox TitrationDocumento6 paginePractical 22.1 Iron Wool Redox TitrationDanielle CarterNessuna valutazione finora
- Enthalpy of NeutralisationDocumento5 pagineEnthalpy of Neutralisationamykkkk0% (1)
- Hand Warmer LabDocumento5 pagineHand Warmer LabmNessuna valutazione finora
- Format of Lab Report Example 8609Documento14 pagineFormat of Lab Report Example 8609herrk167% (3)
- Experiment Report: Spectrophotometric Analysis of Caffeine and Benzoic Acid in Soft DrinkDocumento12 pagineExperiment Report: Spectrophotometric Analysis of Caffeine and Benzoic Acid in Soft DrinkNitty MeYaNessuna valutazione finora
- To Prove Newton's Second Law Using Fletcher's Trolley: Thomas O'Sullivan's Leaving Cert. Maths & Physics NotesDocumento3 pagineTo Prove Newton's Second Law Using Fletcher's Trolley: Thomas O'Sullivan's Leaving Cert. Maths & Physics NotesDeri Pradana100% (2)
- AP Molar Mass of Calcium Lab Purpose:: Δt = 22.5 CelsiusDocumento4 pagineAP Molar Mass of Calcium Lab Purpose:: Δt = 22.5 Celsiusapi-287656809Nessuna valutazione finora
- Ex.3-Heat of NeutralizationDocumento10 pagineEx.3-Heat of Neutralizationalia2003skNessuna valutazione finora
- My Lab Report For Expt 1Documento11 pagineMy Lab Report For Expt 1Nicklas ReusNessuna valutazione finora
- Purpose:: (S) 3 (Aq) 2 (Aq) 2 (G)Documento5 paginePurpose:: (S) 3 (Aq) 2 (Aq) 2 (G)api-287235370Nessuna valutazione finora
- Chemistry AssignmentDocumento11 pagineChemistry AssignmentAris EahmanNessuna valutazione finora
- Experiment 12Documento9 pagineExperiment 12Sy TamNessuna valutazione finora
- 01 - Ans To Stoichiometry Supplemtary QN - 2012Documento5 pagine01 - Ans To Stoichiometry Supplemtary QN - 2012caspersoongNessuna valutazione finora
- Back TitrationDocumento15 pagineBack TitrationAnis NasuhaNessuna valutazione finora
- CLP302 CLP303 ReportsDocumento7 pagineCLP302 CLP303 ReportsamitNessuna valutazione finora
- FinalDocumento17 pagineFinalMatt Pribadi100% (1)
- Lab Fizikal 2 PDFDocumento17 pagineLab Fizikal 2 PDFAmelia UmangNessuna valutazione finora
- Set 2 SonDocumento4 pagineSet 2 SonJerson Mendoza CNessuna valutazione finora
- Neutralization ReactionDocumento4 pagineNeutralization ReactionNor Ashikin Ismail67% (3)
- Determining The Enthalpy of A Neutralization ReactionDocumento4 pagineDetermining The Enthalpy of A Neutralization ReactionJohn WangNessuna valutazione finora
- Lab ReportDocumento10 pagineLab ReportFatin Fateha71% (7)
- Chm524 Experiment 5Documento26 pagineChm524 Experiment 52022608166Nessuna valutazione finora
- Results CSTR BaruDocumento6 pagineResults CSTR Baruridzuwan rahimiNessuna valutazione finora
- B HeatofPrecipitationDocumento18 pagineB HeatofPrecipitationnoraNessuna valutazione finora
- Cambridge Enthalpy and Calorimetry QuestionsDocumento4 pagineCambridge Enthalpy and Calorimetry QuestionsHakkyu KimNessuna valutazione finora
- 0040 6031 (84) 87153 1Documento10 pagine0040 6031 (84) 87153 1gauravNessuna valutazione finora
- Journal of Colligative PropertiesDocumento9 pagineJournal of Colligative PropertiesMuhammad Baihaqi100% (1)
- Stoich Paper 2Documento56 pagineStoich Paper 2Gangjoon (Ryan) LeeNessuna valutazione finora
- 5.3 Analisis Data 5.3.1 Tabel Data Pengamatan: Naoh NaohDocumento10 pagine5.3 Analisis Data 5.3.1 Tabel Data Pengamatan: Naoh NaohDode AnomNessuna valutazione finora
- Amali Kimia 1 (AutoRecovered)Documento14 pagineAmali Kimia 1 (AutoRecovered)SN2-0618 Muhamad Syahmi Rifqi Bin SharimanNessuna valutazione finora
- Enthalpy of ProtonationDocumento9 pagineEnthalpy of ProtonationMalik Alnabhani0% (1)
- Quantitative Determination of Soda Ash Composition by Double Indicator TitrationDocumento3 pagineQuantitative Determination of Soda Ash Composition by Double Indicator TitrationRain Y.Nessuna valutazione finora
- Result:: Volume of Naoh 250 ML Volume of HCL 10 ML Volume of Ethyl Acetate 250 MLDocumento4 pagineResult:: Volume of Naoh 250 ML Volume of HCL 10 ML Volume of Ethyl Acetate 250 MLmujahid alkolaibiNessuna valutazione finora
- Chem 36: General ChemistryDocumento13 pagineChem 36: General ChemistryAbdulhakeemSolimanNessuna valutazione finora
- Acfrogcs7fbqjvsonty9-Var8pzflplnmzq7jlvwswtshzsfuf2bbnb4h01iqlzkrtfbriym9 Qou Ckabf3ezbeowett03wcfpb H66xigpu0o6kv2fyb3v36xwmqonjtn8wxpteloiewhjiupDocumento9 pagineAcfrogcs7fbqjvsonty9-Var8pzflplnmzq7jlvwswtshzsfuf2bbnb4h01iqlzkrtfbriym9 Qou Ckabf3ezbeowett03wcfpb H66xigpu0o6kv2fyb3v36xwmqonjtn8wxpteloiewhjiupحسين عمار محسن سالمNessuna valutazione finora
- Calorimetry Lab ReportDocumento7 pagineCalorimetry Lab ReportSarah B - she herNessuna valutazione finora
- '16-'17-1T-CHEM 5 PtsDocumento21 pagine'16-'17-1T-CHEM 5 PtsLorenz BerroyaNessuna valutazione finora
- Abstract/Summary Aims/Objectives Theory Experimental Procedure Results Calculations Discussion Conclusion Recommendation References AppendicesDocumento10 pagineAbstract/Summary Aims/Objectives Theory Experimental Procedure Results Calculations Discussion Conclusion Recommendation References AppendicesNabilla NaharuddinNessuna valutazione finora
- Amali Kimia 1 (AutoRecovered)Documento17 pagineAmali Kimia 1 (AutoRecovered)Syahmi RifqiNessuna valutazione finora
- Chloroalkali Process: Membrane Cell: Process Synthesis Term ProjectDocumento14 pagineChloroalkali Process: Membrane Cell: Process Synthesis Term ProjectAndrés Camilo Regino RamirezNessuna valutazione finora
- S.no. Name of The Experiment Date of Conduction Date of Submission P2 Cascade CSTR 4 February, 2021 9 February, 2021Documento14 pagineS.no. Name of The Experiment Date of Conduction Date of Submission P2 Cascade CSTR 4 February, 2021 9 February, 2021DEEPSHIKA DUTTANessuna valutazione finora
- Lab 3 NewerDocumento12 pagineLab 3 NeweraskjdglaskjgdaNessuna valutazione finora
- Kinetics 1Documento3 pagineKinetics 1JuarezNessuna valutazione finora
- 5.4.1 INV5.4.1HessLawLab - Sem2 2017-HaleemMohamedAli EditDocumento7 pagine5.4.1 INV5.4.1HessLawLab - Sem2 2017-HaleemMohamedAli EditHaleem MohamedNessuna valutazione finora
- Exercise 4Documento32 pagineExercise 4Mas IzyanNessuna valutazione finora
- 2 Heat of PrecipitationDocumento22 pagine2 Heat of PrecipitationSyawal AnizamNessuna valutazione finora
- A Fixed Quantity of Gas at 21Documento8 pagineA Fixed Quantity of Gas at 21nonoytagupa3Nessuna valutazione finora
- Methanol Production From Syngas Reactor DesignDocumento48 pagineMethanol Production From Syngas Reactor DesignJasonNtsako100% (2)
- Molar Enthalpy of A Chemical ChangeDocumento2 pagineMolar Enthalpy of A Chemical ChangeSourabh Das100% (2)
- Exercises-Topic 5Documento5 pagineExercises-Topic 5Arturo AtienzaNessuna valutazione finora
- Sample ProblemsDocumento18 pagineSample ProblemsEggy ThreekingsNessuna valutazione finora
- Expt01 HCL and NaOH AnsDocumento3 pagineExpt01 HCL and NaOH AnsaragpdNessuna valutazione finora
- Chemistry ReportDocumento7 pagineChemistry ReportAlasdair McFadzeanNessuna valutazione finora
- Cutting-Edge Technology for Carbon Capture, Utilization, and StorageDa EverandCutting-Edge Technology for Carbon Capture, Utilization, and StorageKarine Ballerat-BusserollesNessuna valutazione finora
- Application FormDocumento5 pagineApplication FormNitty MeYaNessuna valutazione finora
- Experiment 1Documento3 pagineExperiment 1Nitty MeYaNessuna valutazione finora
- Your BibliographyDocumento1 paginaYour BibliographyNitty MeYaNessuna valutazione finora
- Heat SettingDocumento1 paginaHeat SettingNitty MeYaNessuna valutazione finora
- Experiment 4Documento2 pagineExperiment 4Nitty MeYaNessuna valutazione finora
- DSC PmmaDocumento1 paginaDSC PmmaNitty MeYaNessuna valutazione finora
- Components For Polyamides Products of PropeneDocumento58 pagineComponents For Polyamides Products of PropeneNitty MeYaNessuna valutazione finora
- Total Suspended & Dissolved SolidsDocumento22 pagineTotal Suspended & Dissolved SolidsNitty MeYaNessuna valutazione finora
- Appendix 1 IT Indemnity LetterDocumento1 paginaAppendix 1 IT Indemnity LetterNitty MeYaNessuna valutazione finora
- Important Contact ParticularsDocumento1 paginaImportant Contact ParticularsNitty MeYaNessuna valutazione finora
- DyesDocumento18 pagineDyesNitty MeYaNessuna valutazione finora
- Carbon Monoxide and EhtyleneDocumento58 pagineCarbon Monoxide and EhtyleneNitty MeYaNessuna valutazione finora
- Thermo SolutionDocumento9 pagineThermo SolutionNitty MeYaNessuna valutazione finora
- Density Determination by PycnometerDocumento5 pagineDensity Determination by PycnometerAlexandre Argondizo100% (1)
- Density ExperimentDocumento9 pagineDensity ExperimentNitty MeYaNessuna valutazione finora
- Density of A Liquid MixtureDocumento7 pagineDensity of A Liquid MixtureNitty MeYaNessuna valutazione finora
- Acetylene DienesDocumento16 pagineAcetylene DienesNitty MeYaNessuna valutazione finora
- ManualDocumento5 pagineManualNitty MeYaNessuna valutazione finora
- Density of A Liquid MixtureDocumento7 pagineDensity of A Liquid MixtureNitty MeYaNessuna valutazione finora
- Group 17Documento11 pagineGroup 17Nitty MeYaNessuna valutazione finora
- Chem 415 Experiment 1Documento6 pagineChem 415 Experiment 1ttussenoNessuna valutazione finora
- DensityDocumento5 pagineDensityNitty MeYaNessuna valutazione finora
- Organic Qualitative Analysis Aldehydes and KetonesDocumento4 pagineOrganic Qualitative Analysis Aldehydes and KetonesNitty MeYa50% (2)
- Chromium ComplexesDocumento3 pagineChromium ComplexesNitty MeYa100% (1)
- Unit 9 P-Block ElementsDocumento18 pagineUnit 9 P-Block ElementsfesinNessuna valutazione finora
- Alcohol, Aldehyde and KetonesDocumento12 pagineAlcohol, Aldehyde and KetonesFranky TeeNessuna valutazione finora
- Chromium ComplexesDocumento3 pagineChromium ComplexesNitty MeYa100% (1)
- Gas Law ExperimentDocumento3 pagineGas Law ExperimentNitty MeYaNessuna valutazione finora
- What Richter Scale PDFDocumento4 pagineWhat Richter Scale PDFquanta1983Nessuna valutazione finora
- Carbon Nanotube Antennas: Peter J. Burke Christopher Rutherglen Z. YuDocumento4 pagineCarbon Nanotube Antennas: Peter J. Burke Christopher Rutherglen Z. YuTapas DasNessuna valutazione finora
- Quick Installation Guide: RTH2300/RTH221Documento40 pagineQuick Installation Guide: RTH2300/RTH221IBJSC.comNessuna valutazione finora
- Cyclicvoltammetry Geetha1Documento35 pagineCyclicvoltammetry Geetha1Kashif RiazNessuna valutazione finora
- Ultrasonic TestDocumento5 pagineUltrasonic TestMufidAliBahtiarNessuna valutazione finora
- FRN Seminar Notes PDFDocumento183 pagineFRN Seminar Notes PDFnamdaeyoung100% (3)
- Aquatic Adaptations - Poonam SinghDocumento46 pagineAquatic Adaptations - Poonam Singhaksahu01234Nessuna valutazione finora
- TsunamiDocumento13 pagineTsunamiVageesha Shantha Veerabhadra SwamyNessuna valutazione finora
- Bec198 2Documento6 pagineBec198 2Tine AbellanosaNessuna valutazione finora
- Sewa AlatDocumento12 pagineSewa Alatcitra puspita sari100% (1)
- Piping PresentationDocumento144 paginePiping PresentationSUNIL TVNessuna valutazione finora
- Tds ManDocumento2 pagineTds MandchyNessuna valutazione finora
- Globular Protein Gelation: Walraj Gosal, Simon B. Ross-Murphy"Documento7 pagineGlobular Protein Gelation: Walraj Gosal, Simon B. Ross-Murphy"Joel PeñaNessuna valutazione finora
- Kpa C Kpa C Kpa C: Fig. 9 Comparison Between Bearing Capacity Values DeterminedDocumento5 pagineKpa C Kpa C Kpa C: Fig. 9 Comparison Between Bearing Capacity Values DeterminedfacedoneNessuna valutazione finora
- Equilibrium and Spontaneity: Universitas Negeri SemarangDocumento19 pagineEquilibrium and Spontaneity: Universitas Negeri SemarangMuhammad Sultan Al-hafizhNessuna valutazione finora
- CATALOGO TECNICO - HW TRIPLE INV - PUBL-6991 (1203) YHJ (K) E 12 To 24 ZJMEXCORX - MOTOREXDocumento6 pagineCATALOGO TECNICO - HW TRIPLE INV - PUBL-6991 (1203) YHJ (K) E 12 To 24 ZJMEXCORX - MOTOREXElvis Nilton Mires RojasNessuna valutazione finora
- Solved Problems in Mechanics 2016Documento28 pagineSolved Problems in Mechanics 2016Debbie TonogNessuna valutazione finora
- Polymer Additives...Documento37 paginePolymer Additives...Enaye MajiriNessuna valutazione finora
- Heat Transfer IntroductionDocumento13 pagineHeat Transfer IntroductionKhaled Mosharraf MukutNessuna valutazione finora
- Surface Tension in Food Analysis - 17FET113Documento15 pagineSurface Tension in Food Analysis - 17FET113Sagar BadnakheNessuna valutazione finora
- Microsoft Word - No-14 6084 Red Insulating VarnishDocumento3 pagineMicrosoft Word - No-14 6084 Red Insulating VarnishA PadmanathanNessuna valutazione finora
- NGV 2 2007Documento78 pagineNGV 2 2007eko handoyoNessuna valutazione finora
- Inverted T BeamsDocumento234 pagineInverted T BeamsAnna GNessuna valutazione finora
- Form 2208 - Page 1: Surface Pump Installation DataDocumento3 pagineForm 2208 - Page 1: Surface Pump Installation DataErich ThomasNessuna valutazione finora
- Smardt Ac Catalogue TD-0081Documento47 pagineSmardt Ac Catalogue TD-0081DavidNessuna valutazione finora
- Electromagnetic Flow MeterDocumento4 pagineElectromagnetic Flow MeterDavi RebouçasNessuna valutazione finora
- Applied Thermal Engineering: D. Meresse, S. Harmand, M. Siroux, M. Watremez, L. DubarDocumento9 pagineApplied Thermal Engineering: D. Meresse, S. Harmand, M. Siroux, M. Watremez, L. DubarYemane TesfayeNessuna valutazione finora
- Journal Pre-Proof: Journal of Environmental Chemical EngineeringDocumento48 pagineJournal Pre-Proof: Journal of Environmental Chemical EngineeringAKASHNessuna valutazione finora
- Water Purification Using Solar Still: Submitted by Mohamed Shakeeb Ahmed 4MH11ME069Documento18 pagineWater Purification Using Solar Still: Submitted by Mohamed Shakeeb Ahmed 4MH11ME069yatharth sharmaNessuna valutazione finora