Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Masquerade Syndromes
Caricato da
tony_chrisDescrizione originale:
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Masquerade Syndromes
Caricato da
tony_chrisCopyright:
Formati disponibili
Masquerade Syndromes
Quan Dong Nguyen, M.D.
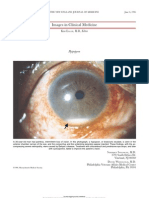
Masquerade syndromes are disorders that occur with intraocular inflammation and are often misdiagnosed as a chronic idiopathic uveitis. The term "Masquerade Syndrome" was first used in 1967 to describe a case of conjunctival carcinoma that manifested as chronic conjunctivitis [22]. Today, it is used to describe disorders that stimulate chronic uveitis. Because of the nature of the underlying diseases, which often have detrimental consequences, early diagnosis and prompt treatment are critical. We present two cases to illustrate malignancy masquerading as chronic uveitis. Case 1 RB was a 30-year-old caucasian female who presented in August , 1984, with a six-month history of blurry vision in the right eye (RE). Her visual acuity was 1/100 RE and 20/20 LE. Slit lamp examination revealed 2+ iritis, RE. Dilated funduscopic examination of RE revealed vitritis with large cells, suspicious for malignancy, and yellow discrete lesions at the RPE with prominent pigment in the central macula (Figure 1). Dilated examination of the left eye only showed few drusen.
Figure 1: Vitreous haze with subretinal lesions In September, 1984, the patient underwent pars plana vitrectomy, RE, which revealed atypical cells, suggestive of large cell lymphoma. Cranial magnetic resonance imaging (MRI) showed foci of hyperintensity in the corpus callosum, consistent with central nervous system (CNS) lymphoma. The patient was fully evaluated by an oncologist, and was treated with irradiation at the Massachusetts General Hospital (MGH). In October, 1994, the patient developed vitritis in LE. She was re-evaluated by her oncologist . The patient then received irradiation to the head and both eys. In September, 1995, her vision has returned to 20/20 RE, and 20/25 LE.
In August, 1987, the patient came back to the Infirmary, complaining of decreased vision in her RE. Her husband also stated that her personality had been "unusual" during the past several months. Her vision was 20/70 RE and 20/30 LE. There was 3+ vitritis in the RE. Repeated cranial MRI revealed recurrence of her large cell lymphoma. The patient was readmitted to the MGH Oncology Service for staging, and additional irradiation and intrathecal methotrexate. Case 2 RF was a 62-year-old caucasian man who had a history of intermittent uveitis and ocular hypertension in his right eye since 1990. Evaluations performed by his ophthalmologist had been non-revealing. The patient required continuous topical steroids and aqueous suppressor. In November, 1993, the patient underwent uncomplicated cataract extraction and implantation of posterior chamber intraocular lens in RE. Postoperatively, the patient developed significant inflammation and intraocular pressure elevation. In February, 1994, the patient underwent pars plana vitrectomy, RE. Again, postoperatively, he developed persistent inflamamtion and intermittent elevated ocular pressure. In June, 1995, the patient was referred I. At presentation, he had moderate panuveitis, RE, with an intraocular pressure of 28. Non-invasive uveitic laboratory evaluations were initiated. Patient was treated with diflunisal, 500 mg orally twice daily, and prednisolone, diclofenac, apraclonidine, and timolol, topically, RE. Two months later, the inflammation persisted. All evaluations were negative. Methotrexate therapy, 7.5 mg orally once weekly was initiated. During the next several months, the patient continued to have persistent iridocyclitis with elevated pressure. In April, 1996, we performed pars plana vitrectomy in the right eye. During the surgery, we detected a brownish lesion in the ciliary body, at the 12 oclock position, suggestive of a melanoma (Figure 2). Post-operatively, ultrasonography confirmed a lesion of moderate echo adherent to posterior iris, within the ciliary sulcus (Figure 3). A consultation was obtained from the Retina Service. The recommendation was observation of the lesion. During the next six months, the patient continued to have persistent uveitis and elevated intraocular pressure.
Figure 2: Superior melanotic mass
Figure 3: UTZ of Case 2 showing hyperechoic lesion in ciliary body In November, 1996, we performed a third vitrectomy and removal of the intraocular lens implant. Continued observation of the lesion was recommended by the retina consultant. During the next six months, the patient continued to have, although diminished, uveitis on methotrexate, diflunisal, and rimexolone drops four times daily. The intraocular pressure remained elevated in the right eye, despite therapy with topical betaxolol, dorzolamide, and apraclonidine, requiring the addition of latanoprost, which intermittently controlled the pressure. In July, 1997, retinal examination and ultrasound biomicroscopy showed that the ciliary body mass had enlarged, to approximately 7.0 x 6.0 x 4.2 mm, with extension into the limbus. Evaluation by the retinal oncologists confirmed the suspicion that the mass was probably a ciliary body melanoma. The patient elected to undergo proton beam irradiation and received a total of 70 Gy (gray) delivered in five fractions. One month after his proton beam treatment, the uveitis had dramatically improved, and the intraocular pressure had returned to normal. There are many disorders that can manifest as uveitis. Tables 1 and 2 list several non-malignant and malignant disorders, respectively, which can present as uveitis. In this paper, we will discuss malignant disorders in more details. Readers are encouraged to learn more about the nonmalignant disorders discussed elsewhere, which can also masquerade as uveitis. Table 1. Non-malignant ocular disoders masquerading as uveitis ________________________________________________________________________ Intraocular foreign body Retinal detachment Myopic degeneration Pigment dispersion syndrome Retinal degeneration
Multiple sclerosis Intraocular infections Drug reactions Post-vacination Table 2. Malignant ocular disorders masquerading as uveitis ________________________________________________________________________ Intraocular lymphomas Non-Hodgkins lymphoma of central nervous system Systemic non-Hodgkins lymphoma metastatic to eye Hodgkins lymphoma Carcinoma metastatic to eye Breast Kidney Lung Uveal Melanoma Iris Ciliary body Choroid Paraneoplastic syndromes Cancer-associated retinopathy Bilateral diffuse uveal melanocytic proliferation Childhood carcinoma Retinoblastoma Leukemia Medulloepithelioma
Juvenile xanthogranuloma Non-Hodgkins lymphoma of the Central Nervous System Non-Hodgkins lymphoma (NHL) of the central nervous system (CNS) is also known as Primary CNS Lymphoma, and can arise from the brain, spinal cord, leptomeninges, or the eye, and may spread throughout the CNS [4], as illustrated in case 1 above. The incidence of CNS lymphoma has increased over the last 10 years, from 2.7 to 7.5 cases per million [11]. Such increase may be secondary to the emergence of the AIDS and other causes of immunosuppression. The median age of patients is 50 to 60 years; male is slightly predominant over female [4,11]. Ocular involvement exists in 15 to 25% of cases, and can precede detectable disease in other parts of the CNS. A study from the National Eye Institute in 1993 revealed that about two-thirds of patients with ocular disease had undiagnosed CNS disease at the time of their diagnosis [24]. Intraocular Lymphoma Immunosuppression (e.g. being post transplantation, having AIDS) is the most common risk factor among patients with intraocular lymphoma. Typical symptoms are blurred vision and floaters; redness and pain rarely occurred. The vision is often better than the clinical symptoms. Slit lamp examination often shows mild anterior segment inflammation with aqueous cells and flare, and keratic precipitates [24]. In the vitreous, there are cells occuring in sheets, with subretinal yellow infiltrates through a hazy vitreous. Occasionally, there is hemorrhagic retinal vasculitis [5]. The diagnosis of intraocular lymphoma requires a thorough history and neurologic examination. MRI and lumbar pucture (LP) may be required. Cerebral spinal fluid (CSF) from LP should be delivered immediately to the cytology laboratory since lymphoma cells are fragile. If CSF shows no malignant cells, a completed vitrectomy can be performed to obtain vitreous specimen. Tissue culture medium enriched with 10% fetal calf serum can be added to collection chamber of vitrectomy machine to improve cell viability. Core vitreous should be obtained and taken immediately to cytology for processing 15]. Multiple vitreous specimens may be needed to detect malignant cells. In a study by Char and associates in 1988 [2], three out of 14 cases of intraocular lymphoma required more than one vitreous specimens to yield the diagnosis. Measurements of levels of various interleukins (IL) in the vitreous can also be helpful in making the diagnosis of intraocular lymphoma. Often, the ratio of IL-10 to IL-12 is elevated, in the context of atypia on vitreal biopsy. Recently, Whitcup and associates have reported elevated vitreous levels of IL-10 relative to levels of IL-6 in primary CNS lymphoma [25]. The use of corticosteroids can obscure the diagnosis, as the cells decrease in size and the viability of tumor cells diminishes. Histopathologically, lymphoma is characterized by large and pleomorphic cells with scanty cytoplasm, and hypersegmented nuclei with prominent nucleoli. Collections of lymphomatous cells can be found between Bruchs membrane and the retinal pigment epithelium. In B-cell lymphoma, malignant cells show B-cells markers with either a kappa or lambda light chain monoclonal response [15]. The differential diagnosis of intraocular lymphoma may include: sarcoidosis, syphilis, tuberculosis, acute retinal necrosis, and CMV retinitis [15]. Thus, diagnostic tests for these entities should be performed prior or in conjunction with those for intraocular lymphoma. Treatment of Non-Hodgkins Lymphoma of the Central Nervous System
Radiation is the first-line therapy as NHL-CNS is quite radiosensitive and responds well to 1,500 to 4,500 rads [15]. Some clinicians prefer to limit radiation therapy to the eye when there is only ocular involvements. However, there are many patients who have subclinical CNS involvement by the time intraocular lymphoma manifests. Thus, there are clinicians who will administer cranial irradiation in addition to orbital radiation, even in isolated ocular involvement [2,14,16]. Radiotherapy can also be combined with chemotherapy to achieve additional therapeutic effect. Often, placement of Omaya reservoir is required for intrathecal therapy. The use of chemotherapy before radiotherapy has been shown to decrease the incidence and degree of CNS toxicity [1]. Dahlborg and associates proposed the use of intra-arterial mannitol for blood-brain barrier disruption to enhance the effectiveness of chemotherapy. Recently, there has been report of intravitreal methotrexate as adjunct therapy [7]. Prognosis NHL-CNS demonstrates good initial response but recurrence is common. The five-year survival rate is less than 5% [15]. Study by Hochberg and Miller shows the overall median survival time from diagnosis is 13.5 months in primary CNS lymphoma [11]. Systemic Non-Hodgkins Lymphoma Metastatic to Eye Such metastasis can present as anterior or posterior uveitis, or hypopyon or hyphema in an uninflamed eye [10]. In NHL-CNS, malignant cells are located between the RPE and the Bruchs membrane. In systemic NHL with ocular metastasis, the cells infiltrate the choroid and may represent ocular melanoma [8]. Ocular involvement may be the initial presentation of the disease [8]. Lymphoid Hyperplasia of the Uvea Lymphoid hyperplasis of the uvea, also known as inflammatory pseudotumors of the uveal tract or intraocular pseudotumors, is characterized by infiltration of the uveal tract by welldifferentiated small lymphocytes. There can be anterior ubveitis, vitritis, choroidal infiltrates, and iris heterochromia [12,17,18]. Extraocular extension may present as conjunctival salmon-colored lesions, or orbital masses which may cause proptosis and diplopia [17]. The condition is painless unless severe glaucoma develops. The diagnosis is made by biopsy of extraocular extension or chorioretina. Lymphoid hyperplasia of the uvea often responds to corticosteroids and moderate dose of radiotherapy. The prognosis is favorable [12]. Uveal Melanoma The incidence of uveal melanoma is about six per million per year, 80% in the choroid, 12% in the ciliary body, and 8% in the iris [21]. Malignant melanoma involving ciliary body yields poor prognosis compared to other uveal melanomas. Primary choroidal melanomas can present as focal choroidal mass, which may mimic sarcoidosis, tuberculous granuloma, or posterior scleritis. Shields and colleagues reported that secondary intraocular pressure elevation was present in 17% of eyes with ciliary body melanomas [20]. The most common mechanism of elevated intraocular pressure was pigment dispersion and tumor invasion of the angle in ciliary body
melanomas. Uveitis, as described in the second case above, is rarely associated with ciliary body melanoma. Choroidal Metastases Study by Ferry and Font in 1974 showed that 50% of ocular metastases are recognized before the diagnosis of underlying malignant process [6]]. Renal and lung carcinomas are most likely to manifest with ocular metastases. Breast carcinomas also frequently metastasize to the eye; however, the primary lesion is known at the time of ocular lesion in greater than 90% of cases [15]. Childhood Malignancies Retinoblastoma is the most common intraocular malignant process in childhood. The initial presentation can be quite diverse, from tumor cells in the anterior chamber, mistaken as iritis, to leucokoria, strabismus, and uveitis of unknown etiology [3]. Cancer-Associated Retinopathy (CAR) First described in 1976 in three patients with oat cell carcinomas of the lung [19], this paraneoplastic syndrome has now been reported with a number of malignant conditions. Patients often present with loss of vision. The fundus can appear normal early in the course of the disease. However, there may be eventual vascular sheathing, disturbances of the RPE, and optic disc pallor. Retinal autoantibodies have been identified in patients with CAR. Such finding, together with that of the disease responding to corticosteroid therapy, suggest that the retinal destruction may be partly immune-mediated [23]. Bilateral Diffuse Uveal Melanocytic Proliferation In 1966, Machemer first described bilateral diffuse melanocytic tumors in a patient with pancreatic carcinoma [13]. This condition can occurs in patients with carcinomas, but underlying processes are often occult. Histopathology reveals diffuse uveal tract infiltrates with benign, spindle-shaped melanocytic cells, which have foamy cytoplasm, but lack mitotic figures. Further study by Gass and Glazer in 1991 [9] showed the presence of multiple subtle round and oval subretinal red patches that show early hyperfluorescence on fluorescein angiography; multiple, slightly elevated, pigmented, and non-pigmented uveal melanocytic tumors with evidence of diffuse thickening of the uveal tract. In addition, there may be episcleral injecition, vitritis, and exudative retinal detachment. Summary There are many entities that can present as chronic intraocular inflammation. In the absence of a correct diagnosis, inappropriate therapy may be prescribed, which can be dangerous. Malignancy and other diseases should be considered in cases of chronic uveitis that do not respond to aggressive medical therapy, and in all patients with undiagnosed inflamamtory eye diseases. Direct treatment of the malignancy or underlying condition may be required to control the uveitis. References
1. Bleyer, Wa. Neurologic sequelae of methotrexate and ionizing radiation: a new classification. Cancer Treat Rep, 65 (suppl 1): 89-98, 1981. 2. Char DH, Ljung B-M, Miller T, et al. Primary intraocular lymphoma (ocular reticulum cell sarcoma): diagnosis and management. Ophthalmol, 95: 625-630, 1988. 3. Croxatto JO, Fernadez MR, Malbran ES. Retinoblastoma masquerading as ocular inflammation. Ophthalmologica, 1986: 48-53, 1983. 4. DeAngelis LM, Yahalom J, Thaler H, et al. Combined modality therapy for primary CNS lymphoma. J Clin Oncol, 10: 635-643, 1992. 5. De Smet MD, Nussenblatt RB, Davis JL, et al. Large cell lymphoma masquerading as a viral retinitis. Int Ophthalmol, 14: 413-417. 6. Ferry AP, Font RL. Carcinoma metastatic to the eye and orbit. A clinicopathologic study of 227 cases. Arch Ophthalmol, 92: 276-286, 1974. 7.. Fishburne BC, Wilson DJ, Rosenbaum JT, et al. Intravitreal methotrexate as an adjunctive treatment of intraocular lymphoma. Arch Ophthalmol, 115: 1152-1156, 1997. 8. Fredrick DR, Char DH, Ljung BM, et al. Solitary intraocular lymphoma as an initial presentation of widespread disease. Arch Ophthalmol, 107: 395-397. 9. Gass JDM, Glatzer RJ. Acquired pigmentation simulating Peutz-Jegherss syndrome: initial manidfestation of diffuse uveal melanocytic proliferation. Br J Ophthalmol, 75: 693-695, 1991. 10. Guzak SV. Lymphoma as a cause of hyphema. Arch Ophthalmol, 84: 229-231, 1970. 11. Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg, 65: 600607, 1986. 12. Jakobiec FA, Sacks E, Kronish JW, et al. Multiple static creamy choroidal infiltrates: an early sign of lymphoid neoplasia. Ophthalmol, 94: 397-406, 1987. 13. Machemer R. Zur Pathogenese des flchenhaften malignen Melanoms. Klin Monatsbl Augenheilkd, 148: 641-652, 1966 14. Margolis L, Fraser R, Lichter A, et al. The role of radiation therapy in the management of ocular reticulum cell sarcoma. Cancer, 45: 688-692, 1980. 15. Nussenblatt RB, Whitcup SM, Palestine AG. Uveitis: Fundamentals and Clinical Practice, Second Edition, Mosby, 1996: Chapter 29, 385-395. 16. Rouwen AJP, Wijermans PW, Boen-Tan TN, et al. Intraocular non-Hodgkins lymphoma treated with systemic and intrathecal chemotherapy and radiotherapy: a case report and review of the literature. Graefes Arch Clin Exp Ophthalmol, 227: 355-359, 1989. 17. Ryan SJ, Frank RN, Green WR. Bilateral inflammatory pseudotumors of the ciliary body. Am J Ophthalmol, 72: 586-591, 1971.
18. Ryan SJ, Zimmerman LE, King FM. Reactive lymphoid hyperplasia: an unusual form of intraocular pseudotumor. Trans Am Acad Ophthalmol Otolaryngol, 76: 652-671, 1971. 19. Sawyer RA, Selhorst JB, Zimmerman LE, et al. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol, 81: 606-613, 1976. 20. Shields CL, Shields JA, Shields MB, et al. Prevalence and mechanisms of secondary intraocular pressure elevation in eyes with intraocular tumors. Ophthalmol, 94: 839-846, 1987. 21. Spalton DJ, Hitchings RA, Hunter PA. Atlas of clinical ophthalmology. London: wolfe Publishing, Mosby-Yearbook, 1994: 9.11. 22. Theodore FH. Conjunctival carcinoma masquerading as chronic conjunctivitiss. Eye Ear Nose Throat Monthly, 46: 1419-1420, 1967. 23. Thirkill CE, Roth AM, Keltner JL. Cancer-associated retinopathy. Arch Ophthalmol, 105: 372375, 1987. 24. Whitcup SM, de Smet MD, Rubin BI, et al. Intraocular lymphoma: clinical and histopathologic diagnosis. Ophthalmol, 100: 1399-1406, 1993. 25. Whitcup SM, Stark-Vancs V, Wittes RE, et al. Association of interlukin 10 in the vitreous and cerebrospinal fluid and primary central nervous system lymphoma. Arch Ophthalmol, 115: 19571960, 1997.
Masquerade Syndrome
Vicente Victor D Ocampo, JR., M.D. Matching Type 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. A. B. C. D. E. F. G. H. I. Cancer-Associated Retinopathy Bilateral Diffuse Melanocytic Proliferation Retinoblastoma Ocular metastases Masquerade Syndrome Uveal Melanoma Non-Hodgkins Lymphoma of the CNS Intraocular lymphoma Lymphoid hyperplasia of Uvea Systemic Non-Hodgkins Lymphoma metastatic to the eye 80% involve choroid Retinal autoantibodies Associated with Lung Carcinoma May appear as ocular melanoma Primary central nervous system lymphoma Intraocular pseudotumor Spindle-shaped melanocytic cells with no mitotic figures Most common intraocular malignancy in children Subretinal yellow infiltrates
J.
Suspected in chronic uveitis not responsive to aggressive medical therapy
Answer Key: 1. B 2. G 3. H 4. C 5. J 6. A 7. E 8. I 9. F 10. D
Potrebbero piacerti anche
- Harvard Medical School - High Impact Cancer Research Program BrochureDocumento16 pagineHarvard Medical School - High Impact Cancer Research Program BrochurePUTRI NOVI SITA DEWINessuna valutazione finora
- Professional Idiot A Memoir Stephen Steve o GloverDocumento117 pagineProfessional Idiot A Memoir Stephen Steve o Glover1bibbo100% (10)
- LymphomaDocumento20 pagineLymphomaChairul Adilla Ardy100% (1)
- 2016 EdBookDocumento931 pagine2016 EdBookvarun7189100% (1)
- Lecture Notes - Clinical Anesthesia - C. Gwinnutt Black Well, 2004) 1405115521Documento170 pagineLecture Notes - Clinical Anesthesia - C. Gwinnutt Black Well, 2004) 1405115521Niranjan KumarNessuna valutazione finora
- Ebook PDF Diagnostic Histopathology of Tumors 2 Volume Set 5th Edition PDFDocumento40 pagineEbook PDF Diagnostic Histopathology of Tumors 2 Volume Set 5th Edition PDFjoe.andes67297% (37)
- Cancer Awareness - 4 February 2023Documento6 pagineCancer Awareness - 4 February 2023Times MediaNessuna valutazione finora
- Uganda Dental ReportDocumento44 pagineUganda Dental Reportntambik21Nessuna valutazione finora
- NEET PG 2021 Medicine Dr. DilipDocumento224 pagineNEET PG 2021 Medicine Dr. DilipIntelli 6252Nessuna valutazione finora
- Rheumatoid Arthritis Associated Scleritis OPHTHALMOLOGY MCQSDocumento5 pagineRheumatoid Arthritis Associated Scleritis OPHTHALMOLOGY MCQSkishorechandraNessuna valutazione finora
- Ims Finals Complete Copy 1st Sem, Sy21-22Documento322 pagineIms Finals Complete Copy 1st Sem, Sy21-22hersey miayoNessuna valutazione finora
- PANDUAN PRAKTIK KLINIS (Total Knee ArthroplastyReplacement)Documento10 paginePANDUAN PRAKTIK KLINIS (Total Knee ArthroplastyReplacement)tony_chrisNessuna valutazione finora
- Ciliary Body Metastasis Masquerading As ScleritisDocumento13 pagineCiliary Body Metastasis Masquerading As ScleritisDurgeshRastogiNessuna valutazione finora
- Moorens Ulcer Diagnosis Management (Ulkus Mooren)Documento9 pagineMoorens Ulcer Diagnosis Management (Ulkus Mooren)rxxxpNessuna valutazione finora
- Issue Apr2012 Pdffiles CasereportDocumento5 pagineIssue Apr2012 Pdffiles CasereportyunjaealwaysNessuna valutazione finora
- Hypopyon in Acute Lymphoblastic Leukemia A Case ReportDocumento2 pagineHypopyon in Acute Lymphoblastic Leukemia A Case ReportInternational Journal of Innovative Science and Research TechnologyNessuna valutazione finora
- Tumor OrbitaDocumento4 pagineTumor OrbitaIda Bagus Deny PrayudiNessuna valutazione finora
- JAMA - A Child With A White Pupil (Retinoblastoma)Documento2 pagineJAMA - A Child With A White Pupil (Retinoblastoma)Rafael Carrillo-BaylonNessuna valutazione finora
- Topical Nepafenac 0.1% or 0.3% For The Treatment of Central Serous Chorioretinopathy: A Case Series of Chronic and Recurrent Disease and Review of The LiteratureDocumento6 pagineTopical Nepafenac 0.1% or 0.3% For The Treatment of Central Serous Chorioretinopathy: A Case Series of Chronic and Recurrent Disease and Review of The LiteratureBOHR International Journal of Current Research in Optometry and Ophthalmology (BIJCROO)Nessuna valutazione finora
- Leg Tuberculosis Presenting As Hypopion UveitisDocumento3 pagineLeg Tuberculosis Presenting As Hypopion UveitisIJAR JOURNALNessuna valutazione finora
- Alsberg 1 s2.0 S2451993621002279 MainDocumento5 pagineAlsberg 1 s2.0 S2451993621002279 MainDoug PulaNessuna valutazione finora
- A Review of Optic NeuritisDocumento5 pagineA Review of Optic NeuritissatrianiNessuna valutazione finora
- Dafpus 10 Sympathetic - Opthalmia-2Documento7 pagineDafpus 10 Sympathetic - Opthalmia-2Anonymous jh87ryNessuna valutazione finora
- Tacrolimus Optic NeuropathyDocumento7 pagineTacrolimus Optic NeuropathyVlady BordaNessuna valutazione finora
- Opth 9 1041Documento7 pagineOpth 9 1041petrarizkyNessuna valutazione finora
- Orbital Apex Syndrome Secondary To Herpes Zoster OphthalmicusDocumento4 pagineOrbital Apex Syndrome Secondary To Herpes Zoster OphthalmicusYosiita KartinaaNessuna valutazione finora
- Squamous Cell Carcinoma of The Conjunctiva - PMCDocumento5 pagineSquamous Cell Carcinoma of The Conjunctiva - PMCGung NandaNessuna valutazione finora
- EN + EosinofiliaDocumento3 pagineEN + EosinofiliaAna Flavia BaptistaNessuna valutazione finora
- Seasonal Monthly Variation Amongst Reported Cataract Surgeries in IndiaDocumento92 pagineSeasonal Monthly Variation Amongst Reported Cataract Surgeries in IndiafriendsofindiaNessuna valutazione finora
- American Journal of Ophthalmology Case Reports: Yael Sharon, David S. Chu TDocumento5 pagineAmerican Journal of Ophthalmology Case Reports: Yael Sharon, David S. Chu TDaviel Quin DavNessuna valutazione finora
- MainDocumento3 pagineMainomidazadmehr1375Nessuna valutazione finora
- Tubercular Posterior SchleritisDocumento6 pagineTubercular Posterior SchleritisInayatul muthmainnahNessuna valutazione finora
- Acute Multifocal Placoid Pigment EpitheliopathyDocumento8 pagineAcute Multifocal Placoid Pigment EpitheliopathyremotmNessuna valutazione finora
- 1 s2.0 S003962571630090X MainDocumento14 pagine1 s2.0 S003962571630090X MainBenjamin NgNessuna valutazione finora
- Capillaroscopy and Videocapillaroscopy Assessment of Skin.Documento16 pagineCapillaroscopy and Videocapillaroscopy Assessment of Skin.AlinaBNessuna valutazione finora
- The Risk of Complications of Uveitis in A District Hospital CohortDocumento6 pagineThe Risk of Complications of Uveitis in A District Hospital CohortSelfima PratiwiNessuna valutazione finora
- Bilateral Conjunctival Lymphoma of The Malt Type: About A CaseDocumento7 pagineBilateral Conjunctival Lymphoma of The Malt Type: About A CaseIJAR JOURNALNessuna valutazione finora
- Recurrent Optic Neuritis As A Clinical Manifestation in Multiple Sclerosis A Case Report - Anisa Feby ArifaniDocumento11 pagineRecurrent Optic Neuritis As A Clinical Manifestation in Multiple Sclerosis A Case Report - Anisa Feby Arifanidenny070111209Nessuna valutazione finora
- 58 Neeta EtalDocumento3 pagine58 Neeta EtaleditorijmrhsNessuna valutazione finora
- Imprimir 2Documento3 pagineImprimir 2Gino VitteriNessuna valutazione finora
- Iannetti 2010Documento4 pagineIannetti 2010FrancescFranquesaNessuna valutazione finora
- Uveitis 2Documento9 pagineUveitis 2srihandayaniakbarNessuna valutazione finora
- Journal Article 17 263 PDFDocumento5 pagineJournal Article 17 263 PDFwolfang2001Nessuna valutazione finora
- Primary Conjunctival Tuberculosis-2Documento3 paginePrimary Conjunctival Tuberculosis-2puutieNessuna valutazione finora
- Case ReportDocumento5 pagineCase ReportFerdina NidyasariNessuna valutazione finora
- Inflammatory Choroidal NeovascularizationDocumento7 pagineInflammatory Choroidal NeovascularizationMarj MendezNessuna valutazione finora
- Epithelioid Choroidal Melanoma in A Middle Aged Filipino A Case ReportDocumento4 pagineEpithelioid Choroidal Melanoma in A Middle Aged Filipino A Case ReportHerald Scholarly Open AccessNessuna valutazione finora
- Jurnal Pendahuluan MutaDocumento23 pagineJurnal Pendahuluan MutaMuta MimahNessuna valutazione finora
- Approach To Optic Neuritis - An UpdateDocumento11 pagineApproach To Optic Neuritis - An UpdateAlexandros GidarakosNessuna valutazione finora
- Herpes Zoster OphthalmicusDocumento12 pagineHerpes Zoster OphthalmicusGio Vano NaihonamNessuna valutazione finora
- Penetrating Keratoplasty in Active Acanthamoeba KeratitisDocumento5 paginePenetrating Keratoplasty in Active Acanthamoeba Keratitisdrvishalkulkarni2007Nessuna valutazione finora
- 0410RT Feature BennettDocumento4 pagine0410RT Feature BennettRaissaNessuna valutazione finora
- Case Report: Tuberculous Scleritis A Challenging DiagnosticDocumento10 pagineCase Report: Tuberculous Scleritis A Challenging DiagnosticIJAR JOURNALNessuna valutazione finora
- Jurding Mata EndophthalamitisDocumento24 pagineJurding Mata EndophthalamitisBenk Setsuna F. SeieiNessuna valutazione finora
- Tubercular ScleritisDocumento5 pagineTubercular Scleritismutya yulindaNessuna valutazione finora
- Challenging Treatment of Bilateral Multiple Infection Panuveitis in HIV/AIDS PatientsDocumento5 pagineChallenging Treatment of Bilateral Multiple Infection Panuveitis in HIV/AIDS PatientsRosyid PrasetyoNessuna valutazione finora
- Articulo 2 OftalmoDocumento6 pagineArticulo 2 OftalmoJhonathan Andres Garcia FiallosNessuna valutazione finora
- Recurrent Sebaceous Gland Carcinoma of Upper Eyelid - A Case ReportDocumento2 pagineRecurrent Sebaceous Gland Carcinoma of Upper Eyelid - A Case ReportInternational Journal of Innovative Science and Research TechnologyNessuna valutazione finora
- Someunusualparaneoplasticsyndromes: Diagnosis in OncologyDocumento6 pagineSomeunusualparaneoplasticsyndromes: Diagnosis in OncologyMohamed Nuri ShembeshNessuna valutazione finora
- Neuroretinitis Syphilis in Human Immunodeficiency Virus-Infected PatientDocumento6 pagineNeuroretinitis Syphilis in Human Immunodeficiency Virus-Infected PatientAnindya AgrasidiNessuna valutazione finora
- Journal Article 17 276Documento3 pagineJournal Article 17 276Lia BirinkNessuna valutazione finora
- CSCR MedscapeDocumento13 pagineCSCR MedscapeFelix Valerian HalimNessuna valutazione finora
- Orbital Pathology: W. Müller-Forell, S. PitzDocumento38 pagineOrbital Pathology: W. Müller-Forell, S. PitzArif Rahman WardhaniNessuna valutazione finora
- Conjunctivitis As A Manifestation of Wegener 'S GranulomatosisDocumento5 pagineConjunctivitis As A Manifestation of Wegener 'S GranulomatosisAsd DsaNessuna valutazione finora
- Reti No Blast OmaDocumento55 pagineReti No Blast OmaIndranil GhoshNessuna valutazione finora
- White Dot Syndromes Harvey S. Uy, M.DDocumento11 pagineWhite Dot Syndromes Harvey S. Uy, M.DShimaa MersalNessuna valutazione finora
- A Case of Choroidal Neovascularization Secondary To Unilateral Retinal Pigment Epithelium DysgenesisDocumento4 pagineA Case of Choroidal Neovascularization Secondary To Unilateral Retinal Pigment Epithelium DysgenesisdedypurnamaNessuna valutazione finora
- Uveitis Associated With Multiple Sclerosis: Complications and Visual PrognosisDocumento4 pagineUveitis Associated With Multiple Sclerosis: Complications and Visual PrognosisnovywardanaNessuna valutazione finora
- Squamous Cell Carcinoma of The Conjunctiva: A Rare Case ReportDocumento4 pagineSquamous Cell Carcinoma of The Conjunctiva: A Rare Case ReportGita GelloyNessuna valutazione finora
- PIIS0008418219302121Documento4 paginePIIS0008418219302121J MNessuna valutazione finora
- Choroidal NeovascularizationDa EverandChoroidal NeovascularizationJay ChhablaniNessuna valutazione finora
- AFib 2Documento31 pagineAFib 2tony_chrisNessuna valutazione finora
- International Food Safety News 2000 9, 7/8 Proquest Agriculture JournalsDocumento1 paginaInternational Food Safety News 2000 9, 7/8 Proquest Agriculture Journalstony_chrisNessuna valutazione finora
- Role of Oral Anti Diabetic DrugsDocumento27 pagineRole of Oral Anti Diabetic DrugsjaidelindNessuna valutazione finora
- The New England Journal of MedicineDocumento2 pagineThe New England Journal of Medicinetony_chrisNessuna valutazione finora
- Cranial NervesDocumento8 pagineCranial Nervestony_chrisNessuna valutazione finora
- Uveitis Information Group Factsheet: Punctate Inner Choroidopathy or (PIC)Documento4 pagineUveitis Information Group Factsheet: Punctate Inner Choroidopathy or (PIC)tony_chrisNessuna valutazione finora
- Cranial NervesDocumento8 pagineCranial Nervestony_chrisNessuna valutazione finora
- Anatomy: Upper AirwayDocumento34 pagineAnatomy: Upper Airwaytony_chrisNessuna valutazione finora
- Intragam P AU PI 11.00 (Supplied) 2Documento8 pagineIntragam P AU PI 11.00 (Supplied) 2tony_chrisNessuna valutazione finora
- William TDocumento7 pagineWilliam Ttony_chrisNessuna valutazione finora
- London, 14 December 2005 Doc. Ref.: EMEA/CHMP/405911/2005: Pre-Authorisation Evaluation of Medicines For Human UseDocumento7 pagineLondon, 14 December 2005 Doc. Ref.: EMEA/CHMP/405911/2005: Pre-Authorisation Evaluation of Medicines For Human Usetony_chrisNessuna valutazione finora
- Figure 1 LBPDocumento1 paginaFigure 1 LBPtony_chrisNessuna valutazione finora
- Nej M 199606063342305Documento1 paginaNej M 199606063342305tony_chrisNessuna valutazione finora
- Jadwal Jaga Co-Ass Anestesi: Hari Minggu I Minggu II Minggu III Minggu IVDocumento2 pagineJadwal Jaga Co-Ass Anestesi: Hari Minggu I Minggu II Minggu III Minggu IVtony_chrisNessuna valutazione finora
- Coping With The Physical Impact ofDocumento52 pagineCoping With The Physical Impact oftanugirotraNessuna valutazione finora
- Round Cell Tumors FinalDocumento86 pagineRound Cell Tumors FinalKush Pathak100% (2)
- Complete Small Med Surgery 2Documento65 pagineComplete Small Med Surgery 2MelanieNessuna valutazione finora
- CA Stomach FinalDocumento83 pagineCA Stomach Finalrajmv7Nessuna valutazione finora
- TSEBTDocumento17 pagineTSEBTcornejo1Nessuna valutazione finora
- Endoscopic Management of Duodeno-Ileal Fistula Secondary To Diffuse B-Cell LymphomaDocumento3 pagineEndoscopic Management of Duodeno-Ileal Fistula Secondary To Diffuse B-Cell LymphomaMaghfirah MahmuddinNessuna valutazione finora
- Rodent OncologyDocumento24 pagineRodent Oncology黃皓Nessuna valutazione finora
- 5 4a Childhood Malignancy Part 1 DR Melanie Victoria G DarDocumento10 pagine5 4a Childhood Malignancy Part 1 DR Melanie Victoria G DarSamatha SamathaNessuna valutazione finora
- Retroperitoneal MassDocumento22 pagineRetroperitoneal Massapi-434838777Nessuna valutazione finora
- Pathology AIIMSDocumento26 paginePathology AIIMSvkNessuna valutazione finora
- Oncology Work Plan For Students 2022-2023Documento34 pagineOncology Work Plan For Students 2022-2023Ahmed SaeedNessuna valutazione finora
- Advances in Immunology Vol. 111. Frederick W. Alt (Eds.)Documento213 pagineAdvances in Immunology Vol. 111. Frederick W. Alt (Eds.)myjesa07Nessuna valutazione finora
- CancerDocumento4 pagineCancerJan Jamison ZuluetaNessuna valutazione finora
- Assig# 3 Pathology - Docx (Ali Aasam Khan CU-1774-2020)Documento21 pagineAssig# 3 Pathology - Docx (Ali Aasam Khan CU-1774-2020)Ali Aasam KhanNessuna valutazione finora
- Defining The Stages of Aggressive Non-Hodgkin's LymphomaDocumento3 pagineDefining The Stages of Aggressive Non-Hodgkin's LymphomaomarbenhagiNessuna valutazione finora
- 2021 How I Treat Adult T-Cell Leukemia-LymphomaDocumento12 pagine2021 How I Treat Adult T-Cell Leukemia-Lymphomamateusmalacarne96Nessuna valutazione finora
- Ncma 219 Finals CompleteDocumento70 pagineNcma 219 Finals CompleteKENSEY MOORE EBROLENessuna valutazione finora
- Nls Diagnostic of Nasopharynx CancerDocumento5 pagineNls Diagnostic of Nasopharynx Cancerapi-186101394Nessuna valutazione finora
- Cpho023 02 07Documento9 pagineCpho023 02 07Kyung-Nam KohNessuna valutazione finora
- 1.13 Hyper-CVAD-MA Version 2.1Documento5 pagine1.13 Hyper-CVAD-MA Version 2.1Alina CrissNessuna valutazione finora
- Grupp Et Al - 2013 - Chimeric Antigen Receptor-Modified T Cells For AcuDocumento16 pagineGrupp Et Al - 2013 - Chimeric Antigen Receptor-Modified T Cells For AcuChae SonNessuna valutazione finora