Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Updated HPA Algorithm Swine Flu
Caricato da
Dr John CrippenCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Updated HPA Algorithm Swine Flu
Caricato da
Dr John CrippenCopyright:
Formati disponibili
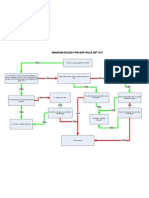
S3 WHO PANDEMIC ALERT PHASE 3: Algorithm for the management of returning
travellers and visitors from countries affected by swine influenza A/H1N1 presenting
with febrile respiratory illness: recognition, investigation and initial management
Updated on 27 April 2009. Please check HPA website for updates.
SCREENING & ASSESSMENT Infection Control &
Patients must fulfil a condition/test in boxes (1) and (2) Reporting
(1) CLINICAL As soon as the patient
Fever ≥ 38°C OR history of fever AND flu-like illness (two or more of the following symptoms: cough, rhinorrhea, mentions a febrile
limb/joint pain, headache.1) OR other severe/life-threatening illness suggestive of an infectious process. respiratory illness and
travel to an area of the
AND world affected by swine flu
A(H1N1), the following
(2) GEOGRAPHICAL precautions should be
taken before continuing
Onset of symptoms within seven days of visiting areas known to have cases of swine influenza A/H1N1: with the assessment.
- Mexico
- United States (California; Kansas; New York; Ohio; Texas) Primary Care/
Community:
Location:
At patient’s home if
possible; if not, away from
No Yes communal areas
Patient: facemask
Staff: facemask, plastic
apron and gloves
Unlikely to be swine Influenza A/H1N1. Inform local Health Protection Unit (HPU)
Treat and investigate as clinically indicated. immediately. Local HPU details at www.hpa.org.uk
Hospital:
Location:
HPU to contact HPA CfI Duty Doctor immediately.
Side room
In Northern Ireland inform CCDC, CCDC to inform
CDSC-NI tel: 028 9026 3765 Patient: facemask
Staff: facemask, plastic
apron and gloves
Nose and throat swabs should be taken and put into viral Take nose and throat swab for
media and sent to an appropriate HPA regional laboratory2 influenza testing.
for analysis.
If admitted to
hospital, inform
Start antivirals.3 hospital infection
Is the patient ill enough to require control and
Yes hospitalisation? occupational
health. Inform
If the patient’s illness is severe enough to warrant local laboratory of
No
hospital admission: sample status
- put patient under strict respiratory isolation and in a side
room
- healthcare staff to wear full personal protective equipment If the patient’s illness can be managed at home
(PPE) - Advise to self isolate until results of testing available
- keep number of staff caring for the patient to a minimum - Advise on respiratory and hand hygiene
Strict Respiratory
Isolation
Patient:
Strict respiratory isolation
in side room
FLU A NEGATIVE Staff:
Treat AND remove from strict respiratory isolation as FLU A POSITIVE Correctly fitted high
appropriate. Discharge if appropriate. Inform local HPU immediately. filtration mask (FFP34),
Follow-up until symptoms resolve if alternative diagnosis Local HPU inform CfI duty doctor immediately and gown, gloves and eye
is not established. discuss possible prophylaxis of contacts. protection
Consider HPA protocol for other undiagnosed serious HPU staff to use Avian Influenza Management System
illness.5 (AIMS) database to collect patient’s data, for the current
time.
Footnotes:
1 Vomiting and diarrhoea have been a feature of some of the confirmed US cases.
2 HPA regional laboratories can be found at http://www.hpa.org.uk/webw/HPAweb&Page&HPAwebAutoListName/Page/1153846674206?p=1153846674206.
3 Standard treatment dose of oseltamivir in adults (age >13 years old) is 75mg bd for 5 days. Standard treatment dose of zanamivir is 10mg bd for 5 days. (http://www.bnf.org/bnf/bnf/
current/119743.htm) Follow guidelines unless expert advice is to increase dose.
4 FFP3 standard masks, see HSE guidelines: http://www.hse.gov.uk/biosafety/diseases/avianflu.htm
5 Refer to HPA protocol for undiagnosed serious illness: a microbiological approach to investigation (http://www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/
1202115613395?p=1160495617061)
In case of uncertainty, discuss with local Health Protection Unit.
Health Protection Agency www.hpa.org.uk
Page 1 of 1
Potrebbero piacerti anche
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Sense and MMRDocumento10 pagineSense and MMRDr John Crippen100% (2)
- Porky Pies: Posted Friday, 22 May 2009 at 13:59Documento9 paginePorky Pies: Posted Friday, 22 May 2009 at 13:59Dr John CrippenNessuna valutazione finora
- ST ND: TH THDocumento4 pagineST ND: TH THDr John CrippenNessuna valutazione finora
- Pharmacy OrlistatDocumento2 paginePharmacy OrlistatDr John CrippenNessuna valutazione finora
- Consultants With Merit AwardsDocumento73 pagineConsultants With Merit AwardsDr John CrippenNessuna valutazione finora
- Smoking Flowchart March09Documento1 paginaSmoking Flowchart March09Dr John CrippenNessuna valutazione finora
- Mad Wife Struck OffDocumento8 pagineMad Wife Struck OffDr John CrippenNessuna valutazione finora
- Salisbury Solicitor LetterDocumento4 pagineSalisbury Solicitor LetterDr John CrippenNessuna valutazione finora
- Jeni Barnett LBC MMR Show TranscriptDocumento22 pagineJeni Barnett LBC MMR Show TranscriptDr John CrippenNessuna valutazione finora
- Jeni Barnett LBC MMR Show TranscriptDocumento22 pagineJeni Barnett LBC MMR Show TranscriptDr John CrippenNessuna valutazione finora
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5795)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1091)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- Natural HistoryDocumento55 pagineNatural HistoryPalwasha KhanNessuna valutazione finora
- Aemses Sof Be LCP 2021 2022Documento16 pagineAemses Sof Be LCP 2021 2022ROMEO SANTILLANNessuna valutazione finora
- PCOS (Polycystic Ovarian Syndrome) Management Teaching PlanDocumento5 paginePCOS (Polycystic Ovarian Syndrome) Management Teaching PlanAudrey C. JunsayNessuna valutazione finora
- Global Action Plan EngDocumento28 pagineGlobal Action Plan EngBoni MagtibayNessuna valutazione finora
- CHN MCQ Set 2Documento15 pagineCHN MCQ Set 2Neenu RajputNessuna valutazione finora
- Format Job Safety Analysis (Jsa) Untuk KontraktorDocumento6 pagineFormat Job Safety Analysis (Jsa) Untuk Kontraktoraan khunaifiNessuna valutazione finora
- LNCT Group of Colleges, Bhopal: Subject: TopicDocumento8 pagineLNCT Group of Colleges, Bhopal: Subject: TopicmuskanNessuna valutazione finora
- Job Description of Staff Working in BHU PDFDocumento10 pagineJob Description of Staff Working in BHU PDFJamshaidzubairee100% (7)
- Tutorial Group 1Documento6 pagineTutorial Group 1Sanjeev NehruNessuna valutazione finora
- Providing EvidencesDocumento21 pagineProviding EvidencesLEILANIE ABADNessuna valutazione finora
- Preventing Early Pregnancy BriefDocumento8 paginePreventing Early Pregnancy BriefGansar Budi SantosoNessuna valutazione finora
- Community Nutrition Instructional DesignDocumento5 pagineCommunity Nutrition Instructional DesignChristine HernandezNessuna valutazione finora
- Health Declaration FormDocumento1 paginaHealth Declaration Formedward mirandilla miranda jr.Nessuna valutazione finora
- PRISMADocumento5 paginePRISMAGhany Hendra WijayaNessuna valutazione finora
- WHO Measles Outbreak Training Module 2Documento20 pagineWHO Measles Outbreak Training Module 2JalalNessuna valutazione finora
- How To Promote A USANA Business - RANK ADVANCEMENT TRAINING JULY 2020Documento20 pagineHow To Promote A USANA Business - RANK ADVANCEMENT TRAINING JULY 2020Mikaella SarmientoNessuna valutazione finora
- Andre FE (2008) - Vaccination Greatly Reduces Disease, Disability, Death and Inequity Worldwide PDFDocumento7 pagineAndre FE (2008) - Vaccination Greatly Reduces Disease, Disability, Death and Inequity Worldwide PDFHoa NắngNessuna valutazione finora
- Communicable Disease NursingDocumento11 pagineCommunicable Disease NursingMelvinFabraÜ100% (1)
- 1 3 19 668 PDFDocumento2 pagine1 3 19 668 PDFLetsShop BbsrNessuna valutazione finora
- SC EthiopiaDocumento58 pagineSC EthiopiasimbiroNessuna valutazione finora
- Cameroon: Maternal and Newborn Health DisparitiesDocumento8 pagineCameroon: Maternal and Newborn Health Disparitiescadesmas techNessuna valutazione finora
- Dan Sikap Remaja Putri Dalam Pencegahan Keputihan: Health Education Tentang Vulva Hygiene Mempengaruhi PengetahuanDocumento9 pagineDan Sikap Remaja Putri Dalam Pencegahan Keputihan: Health Education Tentang Vulva Hygiene Mempengaruhi PengetahuanRizka AprianiNessuna valutazione finora
- Anwari Et Al., 2020Documento11 pagineAnwari Et Al., 2020Balqis BaslemanNessuna valutazione finora
- Water Supply Site ReportDocumento5 pagineWater Supply Site Reportvikey maniaNessuna valutazione finora
- PDF 2420872588Documento1 paginaPDF 2420872588kariem noweerNessuna valutazione finora
- Contracted Pelvis by KABERA ReneDocumento14 pagineContracted Pelvis by KABERA ReneKABERA RENENessuna valutazione finora
- From Startup To Blue Chip: The Success Story of Gilead Sciences - InvestopediaDocumento1 paginaFrom Startup To Blue Chip: The Success Story of Gilead Sciences - InvestopediaExchangeAdminService100% (1)
- Mukinge College of Nursing PHN JAN 2018: Port HealthDocumento39 pagineMukinge College of Nursing PHN JAN 2018: Port HealthBa Mulenga100% (3)
- ZoomlionDocumento10 pagineZoomlionGloria MensNessuna valutazione finora
- Quarry Dust-Are You in Control?Documento29 pagineQuarry Dust-Are You in Control?Fritz LumasagNessuna valutazione finora