Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Membrane Separation
Caricato da
Azzian AriffinTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Membrane Separation
Caricato da
Azzian AriffinCopyright:
Formati disponibili
INTRODUCTION
Membrane separation processes have very important role in separation industry. This Membrane Separation Unit has been designed to demonstrate the techniques of membrane separations which are becoming highly popular as they provide effective separation without the use of heating energy as in distillation processes. For the mass transfer at the membrane, two basic models can be distinguished: the solution-diffusion model and the hydrodynamic model. In real membranes, these two transport mechanisms certainly occur side by side, especially during the ultra filtration. Depending on the type of membrane, the selective separation of certain individual substances or substance mixtures is possible. Important technical applications include drinking water by reverse osmosis filtrations in the food industry, the recovery of organic vapors such as gasoline vapor recovery and the electrolysis for chlorine production. But also in wastewater treatment, the membrane technology is becoming increasingly important. The membrane separations are different from many of the other separation processes like solvent extraction, chromatographic methods involving partition, adsorption, ion exchange etc. which involve equilibrium between the substances getting separated and the separating phases. The onset of equilibrium in these processes restricts the extent of separation achievable and the equilibrium is shifted suitably to achieve the desired level of purity. Also, in some of these processes, a reversal of equilibrium is often necessary to regenerate the separating medium so that it can be reused. In these processes which are equilibrium governed, we always talk of how much separation is accomplished in terms of partition coefficients or distribution ratios. In contrast, membrane processes are rate governed processes wherein we talk not only of relative quantity of the substance separated but also about the rate at which the separation is taking place. Also, membrane processes are continuous separation processes and there is no need of membrane regeneration as is required in equilibrium governed processes. Because of some of its inherent advantages, the membrane processes are replacing some of the well known separation techniques for technological applications.
AIMS/OBJECTIVE In this experiment, we will be able to perform a characteristic study on 4 different types of membranes. This experiment requires approximately 100 gram of sodium chloride.
THEORY A membrane is a selective barrier that permits the separation of certain species in a fluid by combination of sieving and sorption diffusion mechanism. Separation is achieved by selectively passing (permeating) one or more components of a stream through the membrane while retarding the passage of one or more other components Membrane processes are characterized by the fact that a feed stream is divided into 2 streams: retentate and permeate. The retentate is that part of the feed that does not pass through the membrane, while the permeate is that part of the feed that does pass through the membrane. The optional "sweep" is a gas or liquid that is used to help remove the permeate. The component(s) of interest in membrane separation is known as the solute. The solute can be retained on the membrane and removed in the retentate or passed through the membrane in the permeate.
Membrane Information The membranes are manufactured under the PCI system. PCI Membranes manufactures an extensive range of tubular reverse osmosis, nanofiltration, ultrafiltration and open ultrafiltration membranes, which are widely used in the chemical and pharmaceutical process industries for food and beverage processing and for waste treatment. These membranes are ideally suited to processing liquids containing suspended solids, or where precipitation may occur during processing.
APPARATUS & MATERIALS Apparatus 1) TR 14 Membrane Test Unit apparatus. 2) 500 mL beakers. 3) Electronic balance. 4) Gloves Reagents 1) Sodium Chloride 2) Water
PROCEDURE General start-up procedure 1. All the valves were making sure initially closed. 2. A sodium chloride solution was prepared by adding 100g of sodium chloride into 20L of water. 3. The tank was filled up with the salt solution that prepared in step 2. The feed was maintained at room temperature. 4. The power for control panel was turn on. All sensors and indicators were check to functioning properly. 5. Thermostat was switch on and the thermo oil level was making sure above the coil inside thermostat. Thermostat was checked if connections were properly fitted. The temperature was adjusted at the thermostat to maintain feed temperature. 6. The unit is now ready for experiments.
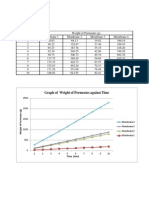
Experimental procedure 1. The experiment was started with membrane 1. Valves V2, V5, V7, V11 and V15 were opened. 2. To set the maximum working pressure at 20 bars, the plunger pump (P1) was switched on and valve V5 was slowly closed. The pressure value at pressure gauge was observed and the pressure regulator was adjusted to 20 bars. 3. Valve V5 was opened. Then, membrane maximum inlet pressure was set to 18 bars for Membrane 1 by adjusting the retentive control valve (V15). 4. The system was allowed to run for 5 minutes. Sample from permeate sampling port was collected and the sample was weighed using digital weighing balance. The weight of permeates was recorded every 1 minute for 10 minutes. 5. The step 1 to 5 was repeated for Membrane 2, 3 and 4. The respective sets of valves were opened and closed and the membrane maximum inlet pressure was adjusted for every membrane.
Membrane
Open Valves
Sampling Valves
Retentive control valve
Membrane maximum inlet pressure(bar) 18
V2, V5, V7, Open V19 and V15 V11 and V15 close V11 V2, V5, V8, Open V20 and V16 V12 and V16 close valve V12 V2, V5, V9, Open V21 and V17 V13 and V17 close valve V13 V2, V5, V10, Open V22 and V18 V14 and V18 close V14
12
10
8.5
6. The graph of permeate weight versus time was plotted.
General shut-down operation 1. The plunger pump was switch off. 2. Valve 2 was closed. 3. All liquid in the feed tank and product tank were drain by opening valves V3 and V4. 4. The entire pipes were flush with clean water. V3 and V4 were closed; the clean water was filled to the feed tank until 90% full. 5. The system was run with the clean water until the feed tank nearly empty for cleaning purposed.
Potrebbero piacerti anche
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Membrane Test UnitDocumento14 pagineMembrane Test UnitAzzian AriffinNessuna valutazione finora
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Manual Calculation: °F °F T 800 °F 100lbmole/hrDocumento1 paginaManual Calculation: °F °F T 800 °F 100lbmole/hrAzzian AriffinNessuna valutazione finora
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- No. Pages: Table of ContentDocumento18 pagineNo. Pages: Table of ContentAzzian AriffinNessuna valutazione finora
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Discussion: 2 Naoh, ODocumento2 pagineDiscussion: 2 Naoh, OAzzian AriffinNessuna valutazione finora
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Calibration CurveDocumento4 pagineCalibration CurveAzzian AriffinNessuna valutazione finora
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- Test 1Documento1 paginaTest 1Azzian AriffinNessuna valutazione finora
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Tutorial 2Documento1 paginaTutorial 2Azzian AriffinNessuna valutazione finora
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Result: Graph of Weight of Permeates Against TimeDocumento1 paginaResult: Graph of Weight of Permeates Against TimeAzzian AriffinNessuna valutazione finora
- AbstractDocumento15 pagineAbstractAzzian AriffinNessuna valutazione finora
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Art's Liquid-Vapor SeparatorDocumento13 pagineArt's Liquid-Vapor Separatorlutfi awnNessuna valutazione finora
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Arzt1998 PDFDocumento16 pagineArzt1998 PDFDeMargarettaNessuna valutazione finora
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- SPE-139837-MS - Integration Wireline Formation Testing and Well Testing Evaluation - An Example From The CaspianDocumento15 pagineSPE-139837-MS - Integration Wireline Formation Testing and Well Testing Evaluation - An Example From The CaspianSamanta MirandaNessuna valutazione finora
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Pantulan Cahaya Pada Cermin SatahDocumento4 paginePantulan Cahaya Pada Cermin SatahHEHENessuna valutazione finora
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- How To Opérate An Amine PlantDocumento4 pagineHow To Opérate An Amine PlantAry HernandezNessuna valutazione finora
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- TraversingDocumento14 pagineTraversingZaff CarrickNessuna valutazione finora
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Mechanics Level 2Documento32 pagineMechanics Level 2greycouncil100% (1)
- Corrosion - Electrochemical MethodsDocumento1 paginaCorrosion - Electrochemical MethodsjuegyiNessuna valutazione finora
- 9 Science Experiments About Light For KidsDocumento7 pagine9 Science Experiments About Light For KidsgaylebugayongNessuna valutazione finora
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Seismic Design of Retaining WallsDocumento23 pagineSeismic Design of Retaining WallsRutvik ShethNessuna valutazione finora
- Complete EDM Handbook - 14Documento12 pagineComplete EDM Handbook - 14soheil gazeranNessuna valutazione finora
- 278 Renu BajajDocumento2 pagine278 Renu BajajAbhinav ThakurNessuna valutazione finora
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- BIOLS102-UOB-Chapter 2Documento4 pagineBIOLS102-UOB-Chapter 2Noor JanahiNessuna valutazione finora
- Cloud Physics Review - Includes Cloud Physics and Cloud DynamicsDocumento5 pagineCloud Physics Review - Includes Cloud Physics and Cloud DynamicsJames WallaceNessuna valutazione finora
- Section A p3Documento8 pagineSection A p3Zunaidi JaafarNessuna valutazione finora
- 150.66-RP4 YcalDocumento92 pagine150.66-RP4 YcalJosé RamosNessuna valutazione finora
- Soil Dynamics and Machine FoundationsDocumento5 pagineSoil Dynamics and Machine FoundationsKarunyamayaNessuna valutazione finora
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Ultrafast Photodiode InGaAsDocumento2 pagineUltrafast Photodiode InGaAsJesus Eduardo Madronero RualesNessuna valutazione finora
- Deaerators: TEGO® AirexDocumento8 pagineDeaerators: TEGO® AirexjoeNessuna valutazione finora
- Acid Base ChemistryDocumento23 pagineAcid Base ChemistryJunegreg CualNessuna valutazione finora
- Tarea 2 FisicaDocumento5 pagineTarea 2 FisicaNestor UlloaNessuna valutazione finora
- What Is Von Mises StressDocumento3 pagineWhat Is Von Mises Stressdwarika2006Nessuna valutazione finora
- 3.design of BracketDocumento39 pagine3.design of BracketmahalakshmiNessuna valutazione finora
- Cengel - TabelasDocumento50 pagineCengel - TabelasBloommer1Nessuna valutazione finora
- Activity 3 - PH and BufferDocumento2 pagineActivity 3 - PH and BufferCHRISTIANA JADE DE CASTRONessuna valutazione finora
- Co-Ordination CompoundsDocumento11 pagineCo-Ordination CompoundsShashank AgarwalNessuna valutazione finora
- Chapter 5 French DB Short Questions PDFDocumento7 pagineChapter 5 French DB Short Questions PDFAman BhatiaNessuna valutazione finora
- Dunman High School Preliminary Exam Year 6: Paper 4 PracticalDocumento6 pagineDunman High School Preliminary Exam Year 6: Paper 4 PracticaloooNessuna valutazione finora
- ST-1 ITB NewDocumento19 pagineST-1 ITB NewES RouzaNessuna valutazione finora
- Reductor SHB50-FDocumento28 pagineReductor SHB50-FJaime Casas-corderoNessuna valutazione finora
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)