Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Solvent Miscibility and Polarity Chart
Caricato da
diptafaraCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Solvent Miscibility and Polarity Chart
Caricato da
diptafaraCopyright:
Formati disponibili
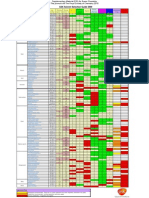
Acetic Acid Acetone Acetonitrile Benzene Butanol Carbon tetrachloride Chloroform Cyclohexane 1,2- Dichloroethane Dichloromethane Dimethyl formamide
Dimethylsulfoxide Dioxane Ethanol Ethyl acetate Ethyl ether Heptane Hexane Isopropyl alcohol Methanol Methyl-t-butyl ether Methyl ethyl ketone Pentane Tetrahydrafuran Toluene Water Xylene
Ethyl acetate Dimethylsulfoxide Isopropyl alcohol Dimethyl formamide Cyclohexane Tetrahydrafuran Methyl-t-butyl ether 1,2- Dichloroethane Dichloromethane Carbon tetrachloride Methyl ethyl ketone Acetonitrile Chloroform Acetic Acid Ethyl ether Methanol Benzene Heptane Acetone Xylene Pentane Hexane Dioxane Ethanol Toluene Butanol Water
Polarity Index1 Viscosity (cP) 6.2 1.26 5.1 0.32 5.8 0.37 2.7 0.65 4.0 0.73 1.6 0.97 4.1 0.57 0.2 1.00 3.5 0.79 3.1 0.44 6.4 0.92 7.2 2.00 4.8 1.54 5.2 1.20 4.4 0.45 2.8 0.32 0.0 0.39 0.0 0.33 3.9 2.30 5.1 0.60 2.5 0.27 4.7 0.45 0.0 0.23 4.0 0.55 2.4 0.59 9.0 1.00 2.5 0.61
1
UV (nm) Cutoff2 230 330 190 280 254 263 245 200 225 235 268 268 215 210 260 220 200 200 210 205 210 329 200 215 285 200 290
Solubility in Water (%) 100 100 100 0.18 0.43 0.08 0.815 0.01 0.81 1.6 100 100 100 100 8.7 6.89 0.0003 0.001 100 100 4.8 24 0.0004 100 0.051 100 0.018
Miscibile Immiscible
The polarity index is a measure of the relative polarity of a solvent and is useful for identifying suitable mobile phase solvents. The polarity index increases with polarity. For reverse phase chromatography eluent strength decreases as its polarity increases
2 UV
cutoff, the wavelength at which the solvent absorbance in a 1 cm path length cell is equal to 1 AU (absorbance unit) using water in the reference cell.
Solvent Polarity Chart
Relative Polarity Non-polar Formula R-H Ar-H R-O-R R-X R-COOR Group Alkanes Aromatics Ethers Alkyl halides Esters Aldehydes and ketones Amines Alcohols Solvents Petroleum ethers, hexanes, ligroin Toluene Diethyl ether Tricholoromethane, chloroform Ethyl acetate Acetone, MEK Pyridine, triethylamine MeOH, EtOH, IPA, Butanol Dimethyformamide Ethanoic Acid
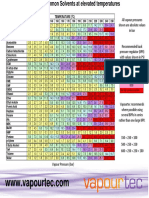
Solvent Miscibility and Viscosity Chart adapted from Paul Sadek The HPLC Solvent Guide Wiley-Interscience, 2002.
Mobile phases, stationary phase, analyte and samples must be compatible
R-CO-R R-NH2 R-OH
Polar
R-COHN2 Amides R-COOH Carboxylic Acid H-O-H Water
Potrebbero piacerti anche
- Halogenated Benzenes, Toluenes and Phenols with Water: Solubility Data SeriesDa EverandHalogenated Benzenes, Toluenes and Phenols with Water: Solubility Data SeriesAri L. HorvathValutazione: 5 su 5 stelle5/5 (1)
- Advanced Pharmaceutical analysisDa EverandAdvanced Pharmaceutical analysisValutazione: 4.5 su 5 stelle4.5/5 (2)
- IKA Table SolventDocumento1 paginaIKA Table SolventjohnyeapNessuna valutazione finora
- Solvent SelectionDocumento3 pagineSolvent SelectionDesmond SeahNessuna valutazione finora
- LC Training Basic HPLC 2001 ADocumento124 pagineLC Training Basic HPLC 2001 Abile86Nessuna valutazione finora
- Alkaloids 3Documento5 pagineAlkaloids 3Nandya Nandiia100% (1)
- Solvent Polarity TableDocumento2 pagineSolvent Polarity Tableichsan hakim100% (1)
- Solvent Miscibility TableDocumento1 paginaSolvent Miscibility Tablewdsbarros100% (2)
- N Butyl AcetateDocumento3 pagineN Butyl AcetateslametNessuna valutazione finora
- Solvent Miscibility TableDocumento1 paginaSolvent Miscibility TableLa Ode Muhammad FitrawanNessuna valutazione finora
- Ganea MarianaDocumento6 pagineGanea MarianaK.D. PatelNessuna valutazione finora
- Vapour Pressure ChartDocumento1 paginaVapour Pressure Chartcraigorio616Nessuna valutazione finora
- Ethyl AcetateDocumento5 pagineEthyl Acetateslamet100% (2)
- Antoine Saturation Vapour PressureDocumento7 pagineAntoine Saturation Vapour PressuresdrtfgNessuna valutazione finora
- Antoine ConstantsDocumento1 paginaAntoine ConstantsYuriska AndiriNessuna valutazione finora
- Thin Layer ChromatographyDocumento4 pagineThin Layer Chromatographyministore kmcNessuna valutazione finora
- Artikel Tema 2 Praktikum Hayati Kelompok 1 FixinDocumento6 pagineArtikel Tema 2 Praktikum Hayati Kelompok 1 FixinFikri AnsyahNessuna valutazione finora
- CHEM 180: Christian MANAHAN Anna Esperanza LEGASPIDocumento19 pagineCHEM 180: Christian MANAHAN Anna Esperanza LEGASPIAnna LegaspiNessuna valutazione finora
- Determination of Hardness of Water (Step-By-Step Plan)Documento6 pagineDetermination of Hardness of Water (Step-By-Step Plan)ravenheart90Nessuna valutazione finora
- Petrochemicals Conversion FactorsDocumento8 paginePetrochemicals Conversion FactorsasdhjshfdsjauildgfyhNessuna valutazione finora
- Ace Top Hen OnDocumento6 pagineAce Top Hen OnQuynh NgaNessuna valutazione finora
- Application Nylon TubingDocumento1 paginaApplication Nylon TubinglayclbNessuna valutazione finora
- Ethylacetate 191005181836Documento27 pagineEthylacetate 191005181836Vedansh VedNessuna valutazione finora
- Miscibility ChartDocumento1 paginaMiscibility Chartmahamuninaresh1100% (1)
- C Aromatics: Hazchem Code EPA CodeDocumento3 pagineC Aromatics: Hazchem Code EPA CodeHector Flores MarcosNessuna valutazione finora
- Immiscible SolventsDocumento8 pagineImmiscible SolventsAgeng Wahyu PatrianitaNessuna valutazione finora
- 05 29 92Documento26 pagine05 29 92Eloi Martinez RabertNessuna valutazione finora
- Specific Gravity of Liquids TableDocumento6 pagineSpecific Gravity of Liquids TableibrahimNessuna valutazione finora
- Simultaneous Estimation of Residual Solvents (Ethanol, Acetone, Dichloromethane and Ethyl Acetate) in Dosage Form by GC-HS-FIDDocumento7 pagineSimultaneous Estimation of Residual Solvents (Ethanol, Acetone, Dichloromethane and Ethyl Acetate) in Dosage Form by GC-HS-FIDchiralicNessuna valutazione finora
- Binary Organic Azeotropes Useful For Solvent CleaningDocumento5 pagineBinary Organic Azeotropes Useful For Solvent Cleaningscribd3822Nessuna valutazione finora
- Quick HPLC Method ToDocumento10 pagineQuick HPLC Method ToSudhanshu DwivediNessuna valutazione finora
- Extraction Separation ProcessDocumento27 pagineExtraction Separation ProcessVictor Ali MentaNessuna valutazione finora
- Acid Base Indicators (PH Range and Preparation)Documento3 pagineAcid Base Indicators (PH Range and Preparation)Juan Camilo OrozcoNessuna valutazione finora
- List of Solvents PDFDocumento2 pagineList of Solvents PDFKeneth Rey SenyahanNessuna valutazione finora
- Extraho Means To Draw Out. A Good Extraction Procedure Should Bring All The Group of MaterialsDocumento14 pagineExtraho Means To Draw Out. A Good Extraction Procedure Should Bring All The Group of MaterialsRabiya BotanistNessuna valutazione finora
- Complexometric Titration With EDTADocumento8 pagineComplexometric Titration With EDTAManP13100% (1)
- Chemical Compatibility Chart: Group # Name Example Incompatible GroupsDocumento3 pagineChemical Compatibility Chart: Group # Name Example Incompatible GroupsPepe BadilloNessuna valutazione finora
- Analytical Guidelines 2011-10-17 IFRA Analytical Method - Determination of The Peroxide ValueDocumento5 pagineAnalytical Guidelines 2011-10-17 IFRA Analytical Method - Determination of The Peroxide ValueWimbo TrionoNessuna valutazione finora
- Lutein From Tagetes Erecta: SynonymsDocumento4 pagineLutein From Tagetes Erecta: SynonymsKrrliveNessuna valutazione finora
- Basic CombustiblesDocumento2 pagineBasic Combustiblesarvindgupta_2005Nessuna valutazione finora
- J1 PDFDocumento33 pagineJ1 PDFMSKNessuna valutazione finora
- EDTA MethodDocumento7 pagineEDTA MethodLalit PandeyNessuna valutazione finora
- Ester SynthesisDocumento7 pagineEster SynthesisNgô Ngọc Mai PhươngNessuna valutazione finora
- Physical Data of Compounds Used in Organic Chemistry Labs: IV.1 Concentrated Acids and BasesDocumento15 paginePhysical Data of Compounds Used in Organic Chemistry Labs: IV.1 Concentrated Acids and BasesAlodia Eunicia Orata CastilloNessuna valutazione finora
- Process Design and Economics Assignment Development of PFD and Process Concept DiagramDocumento9 pagineProcess Design and Economics Assignment Development of PFD and Process Concept Diagramshailaja chowdhuryNessuna valutazione finora
- Diethylether PDFDocumento2 pagineDiethylether PDFPrathamesh LagadNessuna valutazione finora
- Solubility of Acetaminophen in Organic Solvents PDFDocumento2 pagineSolubility of Acetaminophen in Organic Solvents PDFDyla Faradhyla75% (4)
- Tiamina, IPDocumento4 pagineTiamina, IPmagicianchemistNessuna valutazione finora
- Sample Preparation & Liquid Scintillation CountingDocumento38 pagineSample Preparation & Liquid Scintillation CountingAustine OsaweNessuna valutazione finora
- Complete Formulas PDFDocumento46 pagineComplete Formulas PDFramu_uppada67% (6)
- Compound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesDa EverandCompound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesNessuna valutazione finora
- Gel Electrophoresis of ProteinsDa EverandGel Electrophoresis of ProteinsMichael J DunnNessuna valutazione finora
- Application of IC-MS and IC-ICP-MS in Environmental ResearchDa EverandApplication of IC-MS and IC-ICP-MS in Environmental ResearchRajmund MichalskiNessuna valutazione finora
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresDa EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresValutazione: 5 su 5 stelle5/5 (1)
- Chemesthesis: Chemical Touch in Food and EatingDa EverandChemesthesis: Chemical Touch in Food and EatingShane T. McDonaldNessuna valutazione finora
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersDa EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNessuna valutazione finora
- Fourth International Conference on Non-Aqueous Solutions: Vienna 1974Da EverandFourth International Conference on Non-Aqueous Solutions: Vienna 1974V. GutmannNessuna valutazione finora
- Alcohols with Water: Solubility Data SeriesDa EverandAlcohols with Water: Solubility Data SeriesA. F. M. BartonNessuna valutazione finora
- Quantitative Biological and Clinical Mass Spectrometry: An IntroductionDa EverandQuantitative Biological and Clinical Mass Spectrometry: An IntroductionNessuna valutazione finora