Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Utd 0023
Caricato da
Hassan MurtazaTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Utd 0023
Caricato da
Hassan MurtazaCopyright:
Formati disponibili
Copyright 2005 UpToDate www.uptodate.
com (800) 998-6374 (781) 2374788
Pathophysiology and clinical features of atrial septal defects in adults-I
Susan E Wiegers, MD, FACC, FASE Martin G St John Sutton, MBBS, FRCP
UpToDate performs a continuous review of over 330 journals and other resources. Updates are added as important new information is published. The literature review for version 13.1 is current through December 2004; this topic was last changed on July 7, 2004.
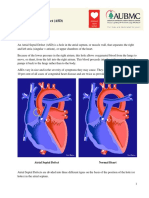
INTRODUCTION Atrial septal defect (ASD) is the most common congenital lesion in adults after bicuspid aortic valve. Although the defect is often asymptomatic until adulthood, potential complications of an undetected ASD include right ventricular failure, atrial arrhythmias, paradoxical embolization, cerebral abscess, and pulmonary hypertension that can become irreversible and lead to right-to-left shunting (Eisenmenger syndrome). The pathophysiology, anatomy, natural history, and clinical features of ASDs in adults will be reviewed here. The identification and assessment of ASDs and methods for treatment are discussed separately. (See "Identification and assessment of atrial septal defects in adults" and see "Management of atrial septal defects in adults-I"). Issues related to ASDs in children are also presented elsewhere. (See "Classification and clinical features of isolated atrial septal defects in children-I" and see "Management and outcome of isolated atrial septal defects in children"). PATHOPHYSIOLOGY Isolated communications in the atrial septum have several distinctive anatomic locations (see "Embryology" below). However, the pathophysiology of each lesion is similar. With a small ASD, left atrial pressure is slightly higher than right atrial pressure, resulting in the continuous flow of oxygenated blood from the left to the right atrium across the defect (show figure 1 and show echocardiogram 1). The pressure gradient between the two atria and the amount of shunt flow depend upon the size of the defect, and the relative distensibility of the right and left sides of the heart. Left-to-right shunting occurs primarily in late ventricular systole and early diastole, with some augmentation during atrial systole. Even when the right and left atrial pressures are equal, as occurs with a large defect, left-to-right shunting still occurs because of the greater compliance of the right compared to left ventricle. The shunt flow constitutes a "useless circuit" through the right atrium, right ventricle, pulmonary circulation, left atrium, and through the defect back to the right atrium. The pulmonary flow to systemic flow ratio can be as high as 8:1 but, in asymptomatic young adults, is more likely to be in the range of 2:1 to 5:1. The increased flow leads to right-sided dilatation evident on chest x-ray and echocardiographic imaging. The main pulmonary arteries dilate and the pulmonary vascularity increases. These changes may be evident on the chest x-ray, and large vessels in both the lower and upper lobes may be seen.
The right-sided volume overload is usually well tolerated for years. Eventual development of pulmonary arteriopathy signals the onset of progressive pulmonary hypertension and its sequela, Eisenmenger syndrome with right ventricular failure and right-to-left shunting of blood. The development of pulmonary hypertension is highly variable and depends not only on the size and duration of the shunt but on unknown individual factors. (See "Evaluation and medical management of Eisenmenger syndrome-I"). EMBRYOLOGY The septation of the atria begins as early as the fifth week of gestation. The septum primum arises from the superior portion of the common atrium and grows caudally to the endocardial cushions located between the atria and ventricles, eventually closing the orifice (ostium primum) between the atria (show figure 2). A second orifice (the ostium secundum) develops in the septum primum; this orifice is covered by another septum (the septum secundum) that arises on the right atrial side of the septum primum. The septum secundum grows caudally and covers the ostium secundum. However, the septum secundum does not completely divide the atria, but leaves an oval orifice (the foramen ovale) that is covered but not sealed on the left side by the flexible flap of the septum primum (show figure 2). In the fetus, the foramen ovale is held open by the pressure gradient between the right and left atria; the right atrial pressure is higher than that on the left and pushes the flexible septum primum aside. At birth, expansion of the lungs lowers right heart pressures at the same time that systemic vascular resistance rises, causing reversal of the atrial gradient. The septum primum is then held against the septum secundum and the interatrial shunt ceases. (See "Classification and clinical features of isolated atrial septal defects in children-I", section on Perinatal physiology). In approximately 70 percent of individuals, the primum and secundum septae fuse after birth creating an intact interatrial septum. However, in a significant proportion of the population, the septae do not fuse. If the foramen ovale is completely covered but not sealed, it is called a "probe patent" or simply "patent" foramen ovale (PFO), indicating that the foramen can be opened by a reversal of the interatrial pressure gradient or by an intracardiac catheter. Less commonly, an open communication persists between the atria after septation. Such a communication is called an atrial septal defect (ASD). CLASSIFICATION The various types of ASDs are classified according to their location and the nature of the embryologic defect: Isolated ASDs result from abnormal development of the septa that partition the common atrium of the developing heart into right and left chambers. Atrioventricular (AV) canal defects result primarily from maldevelopment of the partitioning of the AV canal by the endocardial cushions. Isolated ASDs include PFO, ASD at the fossa ovalis (secundum ASD), a defect superior to the fossa ovalis (superior sinus venosus type ASD, superior vena caval defect), a defect inferior to the fossa ovalis (inferior sinus venosus type ASD,
inferior vena caval defect), and coronary sinus defects (show figure 3). Primum ASDs are typically not isolated, but are associated with ventricular septal defects and/or AV valve malformations. AV canal defects are by definition not isolated and include complete forms, incomplete forms, and common atrium. Secundum ASD Defects in the area of the foramen ovalis are classified as secundum type ASD. This type of ASD can result from poor growth of the secundum septum or excessive absorption of the primum septum (show figure 3 and show echocardiogram 2). It is more common in females and accounts for 70 percent of all ASDs. Secundum ASDs may be associated with or continuous with other ASDs, such as a sinus venosus defect or an ostium primum defect. Mitral valve prolapse is present in up to 70 percent of patients with secundum ASD, perhaps related to a change in the left ventricular geometry associated with right ventricular volume overload. The rare combination of an ASD with mitral stenosis, the result of rheumatic valvulitis, is known as Lutembacher's syndrome. Although most cases of secundum ASD are isolated, some individuals have a family history of this defect with or without other congenital cardiac and extracardiac abnormalities. These syndromes typically present in childhood or adolescence and are discussed elsewhere. (See "Classification and clinical features of isolated atrial septal defects in children-I", section on Familial disorders). Primum ASD The primum type ASD develops if the primum septum does not fuse with the endocardial cushions, leaving a defect at the base of the interatrial septum that is usually large (show figure 3). Primum ASDs are usually not isolated, typically being associated with anomalies of the AV valves, particularly a cleft in the anterior mitral valve leaflet, and defects of the ventricular septum (called a complete endocardial cushion defect) or common AV canal. It has been suggested that both partial and complete AV canal defects are related to maldevelopment of the ventricular septum rather than a decrease in atrial septal tissue [1]. Sinus venosus ASD Sinus venosus defects account for about 10 percent of ASDs. These defects represent an abnormality in the insertion of the superior or inferior vena cava, which overrides the interatrial septum; the interatrial communication is then formed within the mouth of the overriding vein and is outside the area of the fossa ovalis (show figure 3) [2]. Sinus venosus ASDs are of two types, both of which may be associated with partial anomalous venous return [2]: Superior sinus venosus defects (sometimes called superior vena caval defects) are located in the atrial septum immediately below the orifice of the superior vena cava. The right upper lobe and middle lobe pulmonary veins often connect to the junction of the superior vena cava and right atrium, resulting in a partial anomalous pulmonary venous connection [3]. Inferior sinus venosus defects, also known as inferior vena caval defects, are less common. They are located in the atrial septum immediately above the orifice of the inferior vena cava. These defects are also often associated with partial
anomalous connection of the right pulmonary veins. Even if the pulmonary veins are in their usual anatomic location, either of the right pulmonary veins may have its flow directed into the right atrium depending upon the location of the venosus defect. The pulmonary veins may also be completely displaced and insert into either vena cava. The "scimitar sign" describes the opacity on the chest x-ray formed by the insertion of the pulmonary vein into the inferior vena cava. The hemodynamic effect of partial anomalous venous return depends upon the number of malplaced pulmonary veins and the proportion of right ventricular stroke volume that perfuses the lung drained by these veins. Coronary sinus ASD In coronary sinus ASDs, part or all of the common wall between the coronary sinus and the left atrium is absent. Many such patients also have a persistent left superior vena cava. Patent foramen ovale A patent foramen ovale (PFO), which can be detected in approximately 25 to 40 percent of normal adult hearts, is another form of interatrial communication associated with shunting of blood [4-7]. In an autopsy study of 965 normal hearts, the incidence of PFO progressively declined with age from 34 percent during the first three decades to 25 percent during the fourth through the eighth decades to 20 percent during the ninth and tenth decades [4]. However, the size of the PFO tended to increase with age from a mean of 3.4 mm in the first decade to 5.8 mm in the tenth decade. In almost all cases, the PFO was between 1 and 10 mm in size. The anatomic arrangement of the foramen and its valve permit right-to-left flow in utero, but no flow after birth. If left atrial pressure exceeds right atrial pressure and the flap valve remnant of septum primum of the foramen ovale is competent, interatrial shunting cannot occur. However, an elevation in right atrial pressure can cause right-to-left interatrial shunting through a PFO. Intermittent increases in right atrial pressure occur in normal individuals during early ventricular systole (show echocardiogram 3) and with the decreased intrathoracic pressure of inspiration [8]. A persistent, large fetal Eustachian valve can also direct inferior vena caval blood toward the midportion of the atrial septum and potentially into the left atrium and systemic circulation throughout the cardiac cycle. In addition, transient right-to-left shunting in patients with a PFO can be induced by the straining and release phases of the Valsalva maneuver. During the straining phase, the right atrial pressure rises disproportionately, and during release there is a sudden increase in systemic venous return into the right atrium. In one series of 148 patients with a PFO, 84 (57 percent) had right-to-left shunting at rest, and 136 (92 percent) had right-to-left shunting with straining or coughing [7]. The clinical importance of a PFO lies in its association with paradoxical embolism and stroke [5]. Paradoxical embolization occurs when an embolus arising in the systemic venous system or the right atrium crosses the PFO during right-to-left shunting and enters the systemic arterial circulation. A larger PFO (> or =4 mm) or one with a significant right-to-left shunt at rest are risk factors for adverse events [5]. (See "Atrial septal abnormalities (PFO, ASD, and ASA) and cerebral
emboli in adults-I", section on Paradoxical embolism). Left-to-right shunting does not occur with a PFO, provided that the septum primum of the foramen ovale remains competent (ie, fully covers the orifice of the foramen). In some patients, stretch on the margins of the foramen can cause the valve and foramen to become incompetent or patent; this is not truly an ASD since septal tissue is not missing. However, in most clinical studies, such patients are for practical purposes considered to have a small ASD. A PFO may be a familial trait. In a study of 62 patients with an ischemic stroke and 62 matched control siblings, the prevalence of a PFO in female siblings of patients with a PFO was 77 percent compared to 25 percent in female siblings of those without a PFO (odds ratio 9.8); there was no such association in men [9]. NATURAL HISTORY The natural history of ASDs has primarily been documented in reports prior to the age of enhanced diagnosis by echocardiography. Selection bias may have been present in these studies since it was the symptomatic patients who were brought to medical attention. Most children and adolescents with an ASD, particularly an isolated secundum ASD, are asymptomatic even in the presence of large shunts. This was illustrated in a review of 481 patients with a secundum ASD who were seen between 1957 and 1976 who underwent surgical correction before the age of 40; the defect was discovered on routine examination in 202 (42 percent) [10]. In comparison, patients who present in infancy, particularly with heart failure and/or failure to thrive, usually have associated cardiac defects [11]. (See "Classification and clinical features of isolated atrial septal defects in children-I", section on Presentation). Studies in which serial echocardiographic examinations were performed have shown that, among newborns with an ASD, the majority of defects close spontaneously in infancy, with the exception of those larger than 8 mm in diameter [12]. In comparison, spontaneous closure is uncommon in children, since most ASDs that will close spontaneously will have already done so. The natural history of secundum ASDs in children was illustrated in a series of 104 patients (average age 4.5 years at diagnosis) who had an isolated ASD >3 mm in size; serial echocardiograms were performed at an interval of more than six months between studies [13]. Spontaneous closure of the ASD occurred in only four patients, while ASD diameter increased in 65 percent; 30 percent of patients had a >50 percent increase in diameter and 12 percent had an increase to >20 mm. The only independent factor associated with growth was the initial size of the ASD. (See "Management and outcome of isolated atrial septal defects in children", section on Natural history). The increase in left-to-right shunting with age in many patients with uncorrected moderate to large ASDs increases the likelihood of developing symptoms as described in the next section. The increase in ASD size over time also has important implications for treatment, since there is the potential that ASD enlargement will be sufficient to preclude the use of percutaneous transcatheter closure with specific devices. (See "Management of atrial septal defects in adultsI", section on Percutaneous closure)
The continuation of this discussion of the pathophysiology and clinical features of atrial septal defects in adults is presented separately. (See "Pathophysiology and clinical features of atrial septal defects in adults-II").
Pathophysiology and clinical features of atrial septal defects in adults-II
Susan E Wiegers, MD, FACC, FASE Martin G St John Sutton, MBBS, FRCP This topic represents a continuation of the discussion of the pathophysiology and clinical features of atrial septal defects in adults. (See "Pathophysiology and clinical features of atrial septal defects in adults-I"). CLINICAL MANIFESTATIONS It is estimated that most patients with an ASD with significant shunt flow (ie, pulmonary to systemic flow more than 2:1) will be symptomatic and require surgical correction by the age of 40 [1]. However, some patients do not become symptomatic until 60 years of age or older [2,3]. Initial symptoms associated with an ASD may be mild and ignored by the patient. As an example, in one series of 32 patients diagnosed by incidental findings on physical examination, chest x-ray, or echocardiography who were thought to be asymptomatic, exercise tolerance improved after closure of the ASD [4]. Atrial arrhythmias, exercise intolerance, fatigue, dyspnea, and overt heart failure are common manifestations of symptomatic ASDs. In the series cited above of 481 patients with a secundum ASD who were seen between 1957 and 1976 and who underwent surgery before the age of 40, more than one-half had symptoms of dyspnea and fatigue [1]. Atrial arrhythmias Atrial arrhythmias are a common manifestation of an ASD. In three series with a total of over 600 patients, atrial fibrillation or atrial flutter was present in almost 20 percent overall [3,5,6]. The risk of atrial arrhythmias increases with age and the pulmonary artery pressure [5,6]. In a report of 211 adults, the incidence of atrial fibrillation or atrial flutter prior to surgery was 1 percent for those aged 18 to 40, 30 percent for those aged 40 to 60, and 80 percent in those over the age of 60 [5]. The effect of surgical repair on these arrhythmias is discussed separately. (See "Management of atrial septal defects in adults-I", section on Atrial tachyarrhythmias). Patients with atrial fibrillation are at risk for embolic events, particularly if not appropriately anticoagulated [3,6]. (See "Anticoagulation to prevent embolization in atrial fibrillation-I"). Left ventricular dysfunction Left ventricular failure is uncommon, but can occur after many years in patients with an uncomplicated ASD. More subtle evidence of left ventricular dysfunction is a frequent finding. In a report of 12 adults with a secundum ASD, the mean cardiac index was significantly reduced compared to an age-matched group of normal subjects (3.6 versus 4.5 L/min per m2) [7].
The cause of this complication is unclear since the primary pathophysiologic change is right ventricular volume overload. Early studies suggested an associated intrinsic left ventricular abnormality. However, it is currently thought that reversible mechanical factors operating primarily on diastolic function are of primary importance [8]. In an echocardiographic study of 34 children (mean age 9 years) undergoing percutaneous device closure of an ASD, left ventricular enddiastolic volume (LVEDV) was diminished prior to the procedure as a result of leftward interventricular septal shift [9]. After ASD closure, LVEDV increased significantly (from 56.4 to 65.3 ml) as septal shift resolved, with a resulting increase in ejection fraction (from 54.9 to 62.1 percent). Systemic hypertension, if present, can exacerbate the hemodynamic changes due to an ASD. The development of left ventricular hypertrophy is associated with an increase in left ventricular stiffness. These changes tend to increase in left-to-right shunting, possibly resulting in the late development of pulmonary hypertension and right-sided heart failure. Stroke due to paradoxical embolization Patients with a PFO or, much less often, an ASD with a right-to-left shunt are at risk for stroke due to paradoxical embolization (stroke, transient ischemic attack, or peripheral emboli) [10-14]. Right-to-left shunting occurs in some patients at rest and in others during transient increases in right-sided pressure (eg, with a Valsalva maneuver or coughing) (see "Patent foramen ovale" above). In addition, Right-to-left shunting can be persistent in the presence of pulmonary hypertension (see "Pulmonary hypertension and Eisenmenger syndrome" below). The relative frequency of PFO and ASD was evaluated in a series of 103 patients (mean age 52 years) with a presumed paradoxical embolism [13]. A PFO alone was present in 81, an ASD alone in 12, and both a PFO and ASD in 10. PFO is also common in patients with cryptogenic stroke. In a review of 581 such patients who were under the age of 55, 216 (37 percent) had a PFO, 10 (1.7 percent) had an atrial septal aneurysm, and 51 (9 percent) had both [12]. The patients with PFO were younger and less likely to have traditional risk factors for stroke (hypertension, hypercholesterolemia, smoking) than those without PFO. Issues related to the causes, diagnosis, and management of paradoxical embolization are discussed in detail separately. Only about 10 to 20 percent of patients have a documented proximal deep vein thrombosis; other potential sources include thrombus forming at the edges of a PFO or in a concurrently present atrial septal aneurysm. (See "Atrial septal abnormalities (PFO, ASD, and ASA) and cerebral emboli in adults-I"). Migraine headache Migraine headache occurs with increased frequency in patients with PFO or, much less often, an ASD [12,14]. In a study of 581 young patients with a cryptogenic stroke, 267 (46 percent) had a PFO [12]. These patients were more likely to have a migraine (27 versus 14 percent without a PFO), especially when the PFO was associated with an atrial septal aneurysm (ASA) (adjusted odds ratio 2.71). It has been suggested that the migraines may result in at least some patients from movement across the interatrial defect of vasoactive substances such as serotonin that would normally be inactivated in the lungs. (See "Pathophysiology, clinical manifestations, and diagnosis of migraine in
adults", section on Right-to-left cardiac shunts). Pulmonary hypertension and Eisenmenger syndrome The normal pulmonary vasculature accommodates the increased volume of flow in patients with an ASD by recruitment of previously unperfused vessels. As a result, pulmonary artery pressures do not rise significantly unless the volume of pulmonary blood flow exceeds 2.5 times baseline. The development of pulmonary vascular injury is related to the degree and duration of right heart volume overload. (See "Pathophysiology of secondary pulmonary hypertension", section on Congenital heart disease and volume overload). Moderate to severe pulmonary hypertension is relatively uncommon in patients with an ASD, being present in less than 10 percent of adults at the time of diagnosis [3,15]. Patients with a sinus venosus defect have higher pulmonary artery pressures and resistances and develop pulmonary hypertension at an earlier age compared to patients with other forms of ASD. In a study of 169 patients, for example, pulmonary hypertension was present in 26 percent of those with a sinus venosus defect compared to 9 percent with an isolated secundum ASD; elevated pulmonary vascular resistance was present in 16 and 4 percent, respectively [16]. The development of severe irreversible pulmonary hypertension or Eisenmenger syndrome (irreversible pulmonary hypertension at near systemic levels and reversal of shunt flow to a predominantly right-to-left direction) is now uncommon because of surgical or percutaneous correction of the defect. However, there may be an appreciable lifetime incidence of severe pulmonary hypertension in unoperated ASDs, with some older estimates being in the range of 50 percent. Although Eisenmenger syndrome is much less common with ASDs than with ventricular septal defects, ASDs have been a common cause of the syndrome because of their greater prevalence [17]. The prognosis is relatively poor once Eisenmenger physiology has been established. (See "Evaluation and medical management of Eisenmenger syndrome-I"). The incidence of pulmonary hypertension and the efficacy of repair of the ASD were evaluated in a report of 179 consecutive adults over the age of 40 [3]. Among these patients, 26 percent had mild to moderate pulmonary hypertension (pulmonary artery systolic pressure 40 to 60 mmHg), 7 percent had severe pulmonary hypertension (pulmonary artery systolic pressure greater than 60 mmHg), and 2 percent had a marked elevation in pulmonary vascular resistance indicative of severe pulmonary vascular obstructive disease [3]. The patients who underwent surgical repair (at a mean age of 56 years) had a higher adjusted 10year survival when compared to those treated medically (95 versus 84 percent) and a much lower rate of functional deterioration (relative risk 0.21). (See "Management of atrial septal defects in adults-I"). Cyanosis Cyanosis in patients with ASD is usually associated with Eisenmenger syndrome in which there is shunting of unoxygenated blood from the right to the left atrium. In addition, transient reversal of the atrial pressure gradient and transient cyanosis can be induced by some respiratory maneuvers, such as forceful crying, Valsalva, and cough. In rare instances, right-to-left shunting across the ASD can lead to cyanosis without pulmonary hypertension being present. In these cases, some unusual anatomic feature is responsible for directing unoxygenated blood across the defect
to the left atrium in the absence of a reversed pressure gradient. Examples include prominent Eustachian valves, right atrial masses. and distortion of the usual anatomy by a dilated coronary sinus [18]. Pregnancy Women with an ASD may have problems during pregnancy, including arrhythmias, thromboembolism, and bleeding. However, there is no available evidence to suggest that pregnant patients should be managed differently from nonpregnant patients with respect to the indications for ASD closure [19]. (See "Pregnancy in women with congenital heart disease: Specific lesions-I", section on Left-to-right shunts). Scuba diving and altitude exposure Individuals with small ASDs who are treated medically may be at increased risk for complications when they experience high or low atmospheric pressure as with scuba diving or high-altitude climbing. Scuba diving in patients with an ASD or a PFO has been associated with increased risks of decompression illness and paradoxical emboli, while high-altitude exposure has been associated with risk of increased right-to-left shunting and oxygen desaturation. Among patients with an ASD (or other intracardiac shunt), scuba diving is generally contraindicated, while consultation with a cardiologist specializing in congenital defects is recommended before altitude exposure [10]. (See "Complications of diving", section on Cardiovascular disease, and see "High altitude and heart disease-I", section on Congenital heart disease). PHYSICAL FINDINGS The classic physical findings of an ASD are related to the degree and duration of the defect. The findings involve precordial palpation, heart sounds, and heart murmurs, and include the abnormalities associated with Eisenmenger syndrome. Precordial palpation Dilatation of the right atrium and right ventricle may initially be undetectable on physical examination. Eventually, large left-to-right shunts can lead to one or more of the following findings: An enlarged and hyperdynamic right ventricle can produce a right ventricular heave that is most pronounced along the left sternal border and in the subxiphoid area. Enlargement of the pulmonary artery may be associated with a palpable pulmonary artery impulse at the left upper sternal border. This may be more pronounced in patients with pulmonary hypertension. Heart sounds The characteristic finding in ASDs with large left-to-right shunts and normal pulmonary artery pressure is wide, fixed splitting of the second sound (S2), in contrast to the normal variation in splitting during the respiratory cycle. The second sound should be evaluated when the patient is sitting or standing because splitting may be relatively wide but not fixed in the supine position. (See "Auscultation of heart sounds-I"). Fixed splitting is thought to result from altered characteristics of the pulmonary vascular bed associated with increased pulmonary blood flow. In all individuals, the aortic and pulmonic closure sounds (A2 and P2) occur shortly after (but not instantly after) ventricular pressure falls below arterial pressure. The delay between the ventricular pressure drop and valve closure is referred to as the "hangout time," and is the delay due to pressure recoil from the arterial bed.
In the systemic arterial circulation, the hangout time is short because of high aortic impedance and rapid pressure recoil; it does not vary with respiration. In contrast, the pulmonary hangout time is longer, because of the greater compliance of the pulmonary vascular bed, and is prolonged by inspiration, which increases pulmonary capacitance. As a result, P2 normally occurs after A2, and this separation ("splitting") of S2 increases with inspiration. With an ASD, the capacitance of the pulmonary bed is increased throughout the respiratory cycle without much respiratory variation. The increased and constant capacitance results in an increased and fixed hangout time, with wide splitting between the first and second components of the S2 and little respiratory variation. The intensities of the pulmonic and aortic components of S2 are equal in most patients with uncomplicated ASDs. Patients with pulmonary hypertension usually have an accentuated pulmonic component of S2. A similar finding is occasionally seen with normal pulmonary pressures, because of the proximity of the dilated pulmonary artery to the chest wall. The first heart sound (S1), which is heard best at the apex and lower left sternal border, is often split and the second component (tricuspid closure) is intensified in patients with an ASD. An explanation for this increase in intensity is that the large volume of diastolic blood flow from right atrium to right ventricle presses the tricuspid leaflets toward the right ventricular wall and the forceful right ventricular contraction causes the tricuspid leaflets to move abruptly cephalad during systole. Heart murmurs The shunt flow across the ASD has too low a velocity and produces too little turbulence to be audible, although it can be demonstrated by intracardiac phonocardiography. However, several other murmurs may be heard. (See "Auscultation of cardiac murmurs-I"). A midsystolic pulmonary flow or ejection murmur, resulting from the increased blood flow across the pulmonic valve, is classically present with moderate to large left-to-right shunts. This murmur is loudest over the second intercostal space and is usually not associated with a thrill. The presence of a thrill typically indicates a very large shunt or pulmonic stenosis. A murmur of mitral regurgitation may also be heard due to a cleft mitral valve in ostium primum defects and mitral valve prolapse in secundum defects. In the latter setting, an apical late or holosystolic murmur of mitral regurgitation radiating to the axilla may be heard. A diastolic rumble due to the increased flow across the tricuspid valve may be heard by a careful examiner but is usually quite subtle. A low-pitched diastolic murmur of pulmonic regurgitation may result from dilatation of the pulmonary artery. Pulmonary hypertension Right-to-left shunting due to pulmonary hypertension in the occasional patient with ASD may be associated with the following auscultatory findings: A right ventricular fourth heart sound
A midsystolic ejection click A midsystolic pulmonic murmur that is softer and shorter because the ejected stroke volume is less No tricuspid flow murmur Increased intensity of the pulmonic component of S2, but no fixed splitting A pulmonic insufficiency murmur, if present, is high-pitched A holosystolic murmur of tricuspid insufficiency may result from right ventricular and atrial enlargement Eisenmenger syndrome The development of Eisenmenger physiology is accompanied by signs of right ventricular failure (including elevated jugular venous pressure, hepatic congestion, and pedal edema), cyanosis, and clubbing, in addition to the auscultatory features of pulmonary hypertension described above. (See "Evaluation and medical management of Eisenmenger syndrome-I"). ELECTROCARDIOGRAM The electrocardiogram (ECG) may be normal with an uncomplicated ASD and small shunt. Most affected individuals have normal sinus rhythm, but atrial arrhythmias often occur beyond the third decade (especially atrial fibrillation but also atrial flutter and supraventricular tachycardia). P waves are typically normal with secundum ASDs. In comparison, sinus venosus ASDs are often associated with a leftward frontal plane P-wave axis (ie, negative in leads III and aVF and positive in lead aVL) [20]. This leftward shift is caused by an ectopic pacemaker resulting from an ASD located near the sinus node. First degree AV block can occur in any type of ASD, but is classically present in ostium primum defects in association with complete right bundle branch block and left anterior fascicular block. The rim of the ostium primum defect is in close spatial relationship to the His bundle accounting for abnormalities of impulse conduction through this area. The frontal plane QRS axis often ranges from +95 to +135 (right axis deviation) with a clockwise loop. Left axis deviation of the QRS axis with a counterclockwise frontal plane loop can occur with uncomplicated secundum ASD, although it usually suggests the presence of an AV canal defect. The QRS complex is often slightly prolonged and has a characteristic rSr' or rsR' pattern that is thought to result from disproportionate thickening of the right ventricular outflow tract, which is the last portion of the ventricle to depolarize. This pattern, which is often described as incomplete right bundle branch block, results from hypertrophy rather than a conduction disturbance. Patients with increasing pulmonary hypertension tend to lose the rSr' pattern in V1 and develop a tall monophasic R wave with a deeply inverted T wave. A notch on the R wave in the inferior leads (a pattern called "crochetage") has also been suggested as a sensitive and specific electrocardiographic sign of ASD. In one report, this finding was present in 73 percent of patients with ASD versus 7.4
percent of normals, 36 percent of ventricular septal defects, and 23 percent of patients with pulmonic stenosis [21]. In patients with ASD, it correlates with the size of the defect and the degree of left-to-right shunt. CHEST X-RAY The chest radiograph reflects the dilatation of the right atrium, ventricle, and pulmonary arteries. Left atrial enlargement may be seen if there is associated mitral regurgitation. Shunt vascularity is characterized by enlarged main pulmonary arteries and pulmonary vessels, without redistribution of flow to the apical vessels (show radiograph 1). In comparison to these classic findings, the radiographic appearance in patients diagnosed at a later age may be atypical. The atypical findings include normal vasculature, evidence of pulmonary venous hypertension, left atrial enlargement, and pulmonary edema [22]. In one study, atypical findings were more common in patients over the age of 50 (30 versus 6 percent in younger subjects) [22]. In patients with a sinus venosus defect, the insertion of the pulmonary vein into the inferior vena cava can be seen as an abnormal density on the chest x-ray called the "scimitar sign" [23]. The scimitar sign is a vertical, gently curved, rightsided paracardiac density that increases in width as it approaches the right cardiophrenic angle. ECHOCARDIOGRAPHY Echocardiography is the test of choice for the diagnosis of ASD. Transthoracic echocardiography is usually definitive in ostium secundum defects, while transesophageal echocardiography may aid in the sizing of defects, the diagnosis of sinus venosus defects, and the assessment of associated congenital anomalies or other abnormalities such as mitral valve prolapse. Shunt volume, shunt ratios, and pulmonary artery pressures can be measured with Doppler flow echocardiography. These issues are discussed in detail elsewhere. (See "Identification and assessment of atrial septal defects in adults").
Identification and assessment of atrial septal defects in adults
Susan E Wiegers, MD, FACC, FASE Martin G St John Sutton, MBBS, FRCP
UpToDate performs a continuous review of over 330 journals and other resources. Updates are added as important new information is published. The literature review for version 13.1 is current through December 2004; this topic was last changed on September 7, 2004.
INTRODUCTION Atrial septal defects (ASDs) are the most common congenital lesion in adults after bicuspid aortic valves. Although ASDs are often asymptomatic until adulthood, potential complications of an undetected lesion include irreversible pulmonary hypertension, right ventricular failure, atrial arrhythmias, paradoxical embolization and cerebral abscess. (See "Pathophysiology and clinical features of atrial septal defects in adults-I"). This topic will review the methods used for assessment of ASDs and estimation of the shunt flow volume and ratio. The pathophysiology, clinical features, and management of ASDs in adults are discussed separately. (See "Pathophysiology and clinical features of atrial septal defects in adults-I" and see "Management of
atrial septal defects in adults-I"). Issues related to ASDs in children are also presented elsewhere. (See "Classification and clinical features of isolated atrial septal defects in children-I" and see "Management and outcome of isolated atrial septal defects in children"). IDENTIFICATION OF THE DEFECT In most patients, echocardiography provides the desired information related to the presence of an ASD, the size of the defect, and associated abnormalities or complications. It is also possible to obtain hemodynamic information, to estimate the shunt flow through the defect and to determine pulmonary artery pressures noninvasively in most patients. Because of certain limitations of echocardiography and operator-dependence, previous "negative" studies should not be regarded as definitive. Other modalities may be helpful in selected patients. These include cardiac magnetic resonance imaging (CRM) and cardiac catheterization, which is now rarely performed for diagnostic purposes. Echocardiography Echocardiography is the test of choice for the diagnosis of ASD. Transthoracic echocardiography is usually definitive in ostium secundum defects, while transesophageal echocardiography may aid in the sizing of defects, the diagnosis of sinus venosus defects, and the assessment of associated congenital anomalies or other abnormalities such as mitral valve prolapse. Shunt volume, shunt ratios, and pulmonary artery pressures can be measured with Doppler flow echocardiography. M-mode The M-mode echocardiogram usually shows right ventricular enlargement in moderate and large ostium secundum ASDs, and paradoxical motion of the interventricular septum (show echocardiogram 1). However, these findings are nonspecific, since they can be seen with any form of right ventricular overload. Two-dimensional TTE The septal defect can be visualized with twodimensional transthoracic echocardiography (TTE). Clues to the presence of an ASD include abrupt discontinuity of the septum and slight thickening at its termination. Hypermobility of the septum, particularly in association with an abrupt discontinuity, is also suggestive. Two-dimensional TTE also may demonstrate enlargement of the right atrium, right ventricle, and pulmonary arteries, and show paradoxical motion of the ventricular septum. The standard view in which the area of the interatrial septum is best seen is the apical four chamber view where the interatrial septum is parallel to the ultrasound beam (show echocardiogram 2). However, an inaccurate diagnosis may result because the parallel angle of the echo beam to the atrial septum and the thinness of the fossa ovalis can cause artifactual echo dropout of the septum. The interatrial septum is perpendicular to the ultrasound beam in the subcostal view, which can afford complete visualization of the atrial septum. However, this image may be suboptimal, particularly in obese subjects. Off-axis apical, parasternal short axis and other non-standard views are frequently necessary to definitively investigate the interatrial septum. The diagnostic sensitivity of the subcostal approach was evaluated in a review of
154 patients (mean age 31 years) with a documented ASD (105 secundum, 32 primum, and 16 sinus venosus) in whom a satisfactory image of the atrial septum could be obtained [1]. The defect was successfully visualized in 89 percent of secundum ASDs, 100 percent of primum ASDs, and only 44 percent of sinus venosus ASDs. Contrast echocardiography, when performed, identified all defects missed by two-dimensional echocardiography. Since visualization of the atrial septum may be suboptimal in a transthoracic study, other clues to the presence of an intracardiac shunt should be assessed. Unexplained dilatation of the right atrium, right ventricle, and pulmonary arteries is suggestive of the presence of a shunt, as is paradoxical septal motion and diastolic flattening of the interventricular septum. The size of an ASD on two-dimensional TTE does not correlate well with shunt flow measured at catheterization. This is better achieved with color flow and pulsed Doppler. Contrast echo Contrast echocardiography involves the injection of microbubbles in agitated saline or another vehicle to confirm the presence of a right-to-left shunt. An adequate contrast injection causes opacification of the right atrium and ventricle. If there is a right-to-left interatrial shunt, early appearance of contrast can be seen in the left atrium. (See "Contrast echocardiography"). A right-to-left interatrial shunt can be detected by contrast echocardiography, usually with transesophageal echocardiography, in three settings: With reversal of flow through an uncomplicated ASD when right-sided pressure is transiently increased (eg, with a Valsalva maneuver or coughing) or briefly during the onset of left ventricular contraction (show echocardiogram 3 and show echocardiogram 4). With an ASD complicated by pulmonary hypertension, in which the left-to-right shunt is reversed. With a patent foramen ovale, in which the flow is, by definition, from right-toleft (show echocardiogram 5). Contrast echocardiography also may be used in the detection of a left-to-right shunt. Negative contrast in the right atrium is a helpful but insensitive sign for the diagnosis of left-to-right interatrial shunting [2,3]. In this setting, flow from the contrast-free left atrium produces areas in the right atrium in which contrast is not seen (show echocardiogram 6). In our laboratory, we routinely perform contrast injections in any patient with an unexplained dilatation of the right atrium or right ventricle. We use two injections, one at rest and one with Valsalva maneuver. Although no serious consequences have been reported, we do not perform these injections in pregnant patients or those with severe pulmonary hypertension. Doppler Color flow, pulsed, and continuous-wave Doppler all provide information in patients with an ASD. (See "Principles of Doppler echocardiography").
The addition of color flow Doppler imaging can confirm the presence of an ASD, estimate the defect size, and evaluate the efficacy of surgery. A shunt across an ASD typically shows turbulent flow in the direction of the shunt and minimal flow in the opposite direction (show echocardiogram 7 and show echocardiogram 8). The maximal width of the color flow jet across the interatrial septum in the view from which it is best visualized correlates fairly well with the size of the shunt at catheterization. A jet width greater than 15 mm can distinguish patients with a shunt ratio of greater than 2:1 (an indication for surgical closure of the ASD) from those with a lesser shunt [4]. (See "Management and outcome of isolated atrial septal defects in children" and see "Management of atrial septal defects in adultsI"). There are several limitations to color flow Doppler: It may not visualize shunt flow if the frame rate is too slow, since flow across the ASD may occur only during a portion of the cardiac cycle. Reducing the Nyquist limit (the upper limit of velocity that can be detected with a given Doppler pulse frequency) may allow the turbulent flow to be more easily seen. Ghosting of color across the interatrial septum sometimes gives the false impression of shunt flow. Sinus venosus defects, which are high up in the septum (show figure 1), may not be seen with TTE (only seen in 7 of 16 patients were identified in one report) [1]. A sinus venosus defect is one of the disorders that should be suspected in a patient with a left-to-right shunt who has no evidence for an ASD or a VSD. Contrast or transesophageal echocardiography (TEE) can identify almost all of these patients [1], while cardiac catheterization is less often performed. Estimation of jet width size does not take into account shunt flow due to associated anomalous pulmonary venous connections. Pulsed Doppler permits sampling of blood flow velocities from a specific region. It is particularly useful for assessing relatively low velocity flows and can therefore identify flow across the atrial septum and determine the pulmonary flow to systemic flow ratio. (See "Estimation of shunt flow ratio and volume" below and see "Principles of Doppler echocardiography"). In comparison, continuous-wave Doppler echocardiography is particularly useful for assessing high velocity flows. As a result, it can be used to evaluate the gradient across the atrial septum in patients with left atrial hypertension and restrictive ASDs and to assess obstruction to pulmonary venous return [5]. TEE Transesophageal echocardiography (TEE) is superior to TTE in its ability to image the interatrial septum and is often used when definitive results are not seen with TTE and in patients with chest wall deformities and lung disease in whom transthoracic echocardiographic windows may be poor. TEE has the following characteristics: It is extremely accurate for the diagnosis of all three types of septal defects (show echocardiogram 9). It may be particularly important for sinus venosus
defects, which are often missed with transthoracic echocardiography [1]. When performed with contrast or color flow Doppler, it can detect right-to-left shunting when there is transient reversal of flow through the ASD when rightsided pressure is increased (eg, with a Valsalva maneuver) or briefly during the onset of left ventricular contraction (show echocardiogram 3). It is much more sensitive than transthoracic echocardiography for the detection of left-to-right shunting as negative right atrial contrast (93 versus 58 percent in one report) [2]. It can detect flow through multiple ASDs (show echocardiogram 10) Estimation of defect size using the diameter of the Doppler color flow jet correlates with surgical findings. Transesophageal imaging also can be used to verify the absence of normal pulmonary venous connections; however, the level of anomalous venous connection to the superior or inferior vena cava is often out of the imaging planes and cannot be seen [6]. In this setting, MRI can be used to define the anatomy of the anomalous connections. Three-dimensional Three-dimensional echocardiography is an investigational technique [7-9]. It can facilitate precise sizing of ASDs and provide information that may be important for treatment (eg, transcatheter closure), such as the relationship of the ASD to atrial superior and inferior limbic band tissue and to the AV valves. Transcranial Doppler Transcranial contrast Doppler ultrasound is an alternative method for detecting right-to-left shunts due to a PFO [10-12]. With this technique, a probe is placed against the side of the skull just above the zygomatic arch (ie, over the middle cerebral artery). A contrast agent is injected and Doppler ultrasonography is performed at baseline and after a Valsalva maneuver [10]. (See "Contrast echocardiography"). Transcranial contrast Doppler appears to have comparable sensitivity and specificity to contrast TEE [13-15], and has the advantages of being noninvasive and of providing a quantitative assessment of the number of microbubbles reaching the brain. However, it gives no anatomic information regarding the cardiac site and nature of the shunt and may be prone to false positive results in patients with intrapulmonary shunts [14]. A 2004 assessment report from the American Academy of Neurology concluded that TEE was superior to transcranial Doppler because it provides direct anatomic information regarding the site and nature of the shunt and the possible presence of an atrial septal aneurysm [16]. (See "Atrial septal abnormalities (PFO, ASD, and ASA) and cerebral emboli in adults-I", section on Atrial septal aneurysm). Cardiac magnetic resonance imaging Data are more limited on the value of cardiac magnetic resonance (CMR), which can be used to determine defect size, estimate shunt flow, and detect anomalous venous connections [17-20]. In a study of 30 patients with ASD, defect size estimated from CRM was compared to defect size determined at catheterization and surgery [17]. Phase contrast cine MR
accurately estimated the size of all three types of atrial septal defects, while spin echo MR substantially overestimated the diameter and area of the defect. (See "Clinical utility of cardiovascular magnetic resonance imaging"). Radionuclide angiography First pass radionuclide angiography (RNA) has been used successfully to detect intracardiac shunting. Rapid bolus injection of a radionuclide with gamma camera imaging is used to detect early recirculation of blood from the left heart to the lungs. Radionuclide studies have also been used in the past for shunt quantification, but this technique has been superceded by other methods. In a comparative study of RNA and cine CMR in 16 patients, only eight patients had an adequate RNA study due to either poor bolus quality (five patients) or concurrent right-to-left shunting (three patients) [21]. In contrast, CMR was able to provide accurate shunt measurements in all patients. Cardiac catheterization Determination of the presence of an ASD by catheterization involves sampling of the oxygen content in the inferior vena cava, superior vena cava, right atrium, right ventricle, and pulmonary artery; a "stepup" in oxygen saturation is indicative of a shunt. (See "Swan-Ganz catheterization: Interpretation of tracings" section on Detection of left to right shunts). Cardiac catheterization, because it is invasive, is rarely indicated for diagnostic purposes in patients with an ASD. However, there a number of possible uses: The course of a catheter passed across the ASD may help determine the type of defect. Sinus venosus and primum ASDs are distinguished by high and low catheter positions, respectively, while in secundum ASDs, the catheter passes across the middle portion of the atrial septum. When pulmonary vascular disease is suspected, measurement of the pulmonary vascular resistance is important in determining whether closure of the defect is indicated. (See "Management and outcome of isolated atrial septal defects in children", section on Which ASDs should be closed?, and see "Management of atrial septal defects in adults-I", section on Fixed pulmonary hypertension). Catheterization can be helpful in patients suspected of having other associated anomalies such as partial anomalous pulmonary venous return. Cineangiography Cineangiography can be used to confirm the presence of an ASD with left-to-right shunting. Optimal direct visualization is achieved by an injection of contrast just outside the orifice of the right upper pulmonary vein in the cranially angulated left anterior oblique projection (also known as the fourchamber view). With this view, secundum ASDs are seen in the middle of the atrial septum, and sinus venous and AV canal defects are seen at the superior and inferior aspect of the atrial septum, respectively. ESTIMATION OF SHUNT FLOW RATIO AND VOLUME Operative closure of an atrial septal defect has traditionally been recommended when the ratio of pulmonary blood flow to systemic blood flow (Qp/Qs) is greater than 1.5:1 to 2:1. (See "Management and outcome of isolated atrial septal defects in children" and see "Management of atrial septal defects in adults-I"). Doppler echocardiography The Qp/Qs ratio is usually estimated with pulsed
Doppler echocardiography using the transthoracic approach. Compared to the gold standard of cardiac catheterization, pulsed Doppler echocardiography is noninvasive and easily permits serial monitoring. Thus, once an acceptable correlation between echocardiographic and catheterization laboratory estimates of shunt flow has been established for an individual laboratory, it is not unreasonable to forgo cardiac catheterization in young patients when possible associated anomalies have been ruled out by TEE. With pulsed Doppler echocardiography, the stroke volume through each valve is measured using the following formula: Stroke volume = cross sectional area x velocity time integral (VTI) of the Doppler flow signal Left-sided stroke volume is measured from the left ventricular outflow tract (LVOT) by assuming a circular geometry. The diameter of the LVOT is always measured in the parasternal long axis view. The maximum Doppler flow velocity apical to the aortic valve (VTI-LVOT) is taken in the apical four chamber view. The right-sided velocity time integral (VTI-PA) is measured in the pulmonary artery well before the bifurcation. The diameter of the pulmonary artery (PA) is then measured at the same level. Substitution into the stroke volume formula results in the following formula: Qp/Qs = PA diameter(2) x VTI-PA LVOT diameter(2) x VTI-LVOT The number two in parentheses (2) refers to the square of the LVOT or PA diameter. The equation requires that the diameters of the LVOT and PA be squared, making exact measurements of these diameters of prime importance in the calculation of the shunt ratio. Measurement of the PA diameter, taken in the parasternal short axis view at the base of the heart, can be problematic in some patients. Thus, this is the term in the equation that is most often responsible for inaccurate estimates of the shunt ratio. Nevertheless, this method correlates well with catheterization in patients with adequate transthoracic views [22]. Shunt flow volume can be estimated using the area of the defect (again assuming a circular geometry) and the velocity time integral of the shunt flow measured with the Doppler beam parallel to shunt direction [23]. Methods have also been developed to assess shunt volume using three-dimensional color Doppler echocardiography [24]. Serial monitoring Because ASDs often enlarge over time in children and adults, serial monitoring of Qp/Qs is warranted in asymptomatic patients who are managed medically. The recommended interval is yearly in children and every two to three years in adults. Cardiac catheterization A formal "shunt run" at cardiac catheterization measures the oxygen content in the blood at multiple sites, and the Fick equation is then used to calculate the ratio of pulmonary to systemic flow (show table 1). (See "Swan-Ganz catheterization: Interpretation of tracings" section on Detection of left to right shunts).
The sequence of steps in this determination is as follows: The "shunt run" is performed, measuring oxygen saturation in the inferior vena cava (IVC), superior vena cava (SVC), right atrium (RA), right ventricle (RV), and pulmonary artery (PA). Accurate catheter position during this procedure is essential. Mixed venous oxygen saturation (MvO2) can then be calculated as follows: MvO2 = [(3 x SVC oxygen saturation) + (IVC oxygen saturation)] / 4 The SVC oxygen saturation is multiplied by three because SVC flow is approximately three times IVC flow. Systemic arterial oxygen saturation (SaO2) is measured by arterial blood gas analysis or by pulse oximetry. (See "Pulse oximetry"). Oxygen (O2) consumption is determined (this assessment should be based upon direct measurement, rather than assumed from available nomograms). This measurement requires substantial patient cooperation. (See "Oxygen delivery and consumption"). The patient's blood hemoglobin concentration ([Hgb]) is obtained from a complete blood count or blood gas analysis. The systemic blood flow (Qs) is calculated from the Fick equation (show table 1): Qs = (O2 consumption) (13.4 x [Hgb] x [SaO2 - MvO2]) The pulmonary blood flow (Qp) is also calculated from the Fick equation (show table 1): Qp = (O2 consumption) (13.4 x [Hgb] x [pulmonary venous O2 saturation - PA O2 saturation]) This calculation requires a value for the pulmonary venous O2 saturation, which is not usually measured directly (although a catheter can be inserted across the ASD and into the pulmonary veins). If the SaO2 is above 95 percent, it may be used as an approximation of the pulmonary venous measurement. If the SaO2 is less than 95 percent, a right-to-left shunt may be present and must be excluded by contrast echocardiography or other means. An SaO2 of less than 95 percent not due to a right-to-left shunt (eg, due to chronic lung disease) should be used as the accurate estimate of the pulmonary venous O2 saturation. If a right-to-left shunt is present, an assumed value of 98 percent for the pulmonary venous O2 saturation is used. With the results of the Qp and Qs determinations, the shunt flow ratio and volume can be determined. The shunt ratio is Qp/Qs. If there is no evidence of an associated right-to-left shunt, the volume of the left-to-right shunt is determined as Qp - Qs. More complex calculations are required to determine shunt volume in the presence of bidirectional shunting.
Management of atrial septal defects in adults-I
Susan E Wiegers, MD, FACC, FASE Martin G St John Sutton, MBBS, FRCP
UpToDate performs a continuous review of over 330 journals and other resources. Updates are added as important new information is published. The literature review for version 13.1 is current through December 2004; this topic was last changed on October 29, 2004.
INTRODUCTION Atrial septal defect (ASD) is the most common congenital lesion in adults after bicuspid aortic valve. Although the defect is often asymptomatic until adulthood, potential complications of an undetected ASD include right ventricular failure, atrial arrhythmias, paradoxical embolization, cerebral abscess, and pulmonary hypertension that can become irreversible and lead to right-to-left shunting (Eisenmenger syndrome). This topic will review the role of medical and surgical therapy and percutaneous repair in adults with ASDs and the clinical issues related to a perforated atrial septal aneurysm, which can have a similar presentation to that of an ASD. The pathophysiology, anatomy, natural history, and clinical features of ASDs in adults and the identification and assessment of ASDs are discussed separately. (See "Pathophysiology and clinical features of atrial septal defects in adults-I" and see "Identification and assessment of atrial septal defects in adults"). Issues related to ASDs in children are also presented elsewhere. (See "Classification and clinical features of isolated atrial septal defects in children-I" and see "Management and outcome of isolated atrial septal defects in children"). NATURAL HISTORY Most ASDs less than 8 mm in diameter close spontaneously in infants. However, spontaneous closure is unusual in children and adults. The natural history of secundum ASDs in children was illustrated in a series of 104 patients (average age 4.5 years at diagnosis) who had an isolated ASD >3 mm in size; serial echocardiograms were performed at an interval of more than six months between studies [1]. Spontaneous closure of the ASD occurred in only four patients (4 percent), while ASD diameter increased in 65 percent; 30 percent of patients had more than a >50 percent increase in diameter and 12 percent had an increase to >20 mm. (See "Management and outcome of isolated atrial septal defects in children", section on Natural history). The increase in left-to-right shunting with age in many patients with uncorrected moderate to large ASDs increases the likelihood of developing symptoms. It is estimated that most patients with an ASD with significant shunt flow (ie, pulmonary to systemic flow more than 2:1) will be symptomatic and require closure of the defect by the age of 40. However, many patients become symptomatic and require closure at older ages [2-8]. (See "Defect size and Qp/Qs" below). Increasing defect size also has important implications for treatment, since there is the potential that ASD enlargement will be sufficient to preclude the use of percutaneous transcatheter closure with certain devices. (See "Percutaneous closure" below).
INDICATIONS FOR DEFECT CLOSURE There are two main indications for closure of an ASD: the development of symptoms, and a high rate of shunt flow. Symptoms Exercise intolerance, fatigue, dyspnea, overt heart failure, and paradoxical embolization are manifestations of symptomatic ASDs that warrant defect closure. In a series of 481 patients with a secundum ASD who were seen between 1957 and 1976 and who underwent surgery before the age of 40, more than one-half had symptoms of dyspnea and fatigue [9]. (See "Pathophysiology and clinical features of atrial septal defects in adults-I"). Atrial tachyarrhythmias, particularly atrial fibrillation and atrial flutter occur in approximately 20 percent of patients and are often the presenting symptom. However, these arrhythmias alone are probably not an indication for defect closure since the incidence may not be reduced after surgery. (See "Atrial tachyarrhythmias" below). Migraine headache occurs with increased frequency in patients with a PFO or, less often, an ASD and may result in at least some patients from movement across the interatrial defect of vasoactive substances such as serotonin that would normally be inactivated in the lungs. (See "Pathophysiology, clinical manifestations, and diagnosis of migraine in adults", section on Right-to-left shunt). Closure of the defect has been reported to prevent or reduce the frequency of subsequent migraines. However, the data are conflicting and it is premature to recommend percutaneous closure of a PFO or ASD solely as a treatment for migraine. (See "Acute and preventive treatment of migraine headache in adults-I", section on Right-to-left cardiac shunt). Paradoxical embolization Patients with a PFO or, much less often, an ASD with a right-to-left shunt are at risk for stroke due to paradoxical embolization (stroke, transient ischemic attack, or peripheral emboli). Right-to-left shunting occurs in some patients at rest and in others during transient increases in rightsided pressure (eg, with a Valsalva maneuver or coughing). In addition, Right-toleft shunting can be persistent in the presence of pulmonary hypertension. The relative frequency of PFO and ASD was evaluated in a series of 103 patients (mean age 52 years) with a presumed paradoxical embolism [10]. A PFO alone was present in 81, an ASD alone in 12, and both a PFO and ASD in 10. PFO is also common in patients with cryptogenic stroke. In a review of 581 such patients who were under the age of 55, 216 (37 percent) had a PFO, 10 (1.7 percent) had an atrial septal aneurysm, and 51 (9 percent) had both [11]. The patients with PFO were younger and less likely to have traditional risk factors for stroke (hypertension, hypercholesterolemia, smoking) than those without PFO. Issues related to the causes, diagnosis, and management of paradoxical embolization are discussed in detail separately. Only about 10 to 20 percent of patients have a documented proximal deep vein thrombosis; other potential sources include thrombus forming at the edges of a PFO or in a concurrently present atrial septal aneurysm. The role of defect closure compared to medical therapy for the prevention of recurrent stroke is not well defined [12,13]. (See "Atrial septal abnormalities (PFO, ASD, and ASA) and cerebral emboli in adults-I", section on Prevention of recurrent stroke).
Defect size and Qp/Qs Larger ASDs impose a greater hemodynamic burden on the right ventricle than smaller ASDs, and therefore present a more compelling rationale for repair. It has been suggested that ASDs should be closed in the presence of echocardiographic evidence of right ventricular volume overload, although there are no data to support this view [14]. A more generally accepted clinical standard is to repair ASDs with a significant measured shunt, as defined by the pulmonary-to-systemic flow ratio (Qp/Qs). In the absence of pulmonary hypertension, Qp/Qs is closely correlated with the size of the ASD [15]. Although the gold standard for Qp/Qs measurement is cardiac catheterization, Doppler echocardiography is most often used once the existence of an acceptable correlation between echocardiographic and catheterization laboratory estimates of shunt flow has been established for an individual laboratory. (See "Identification and assessment of atrial septal defects in adults", section on Estimation of shunt flow ratio and volume). There are no systematic data that identify a threshold value for Qp/Qs for repair of an ASD. A Qp/Qs >2:1 is a well-established indication [16], but many authors have advocated lower thresholds of 1.7:1 [2] or 1.5:1 [17]. The American Heart Association recommended a threshold Qp/Qs > or =1.5:1, but these guidelines specifically excluded patients over the age of 21 years [18]. The Canadian Cardiac Society recommended a threshold Qp/Qs >2:1, or >1.5:1 in the presence of reversible pulmonary hypertension [19]. Spontaneous closure of ASDs is unusual in children and adults and defect size and shunt flow can increase over time [1]. (See "Pathophysiology and clinical features of atrial septal defects in adults-I", section on Natural history). As a result, asymptomatic patients who do not meet Qp/Qs criteria for closure of the defect may do so at a later time. For this reason, serial measurements of Qp/Qs by echocardiography are typically performed every two to three years. (See "Identification and assessment of atrial septal defects in adults", section on Doppler echocardiography). Fixed pulmonary hypertension Severe fixed pulmonary hypertension, defined as a pulmonary vascular resistance greater than 1200 dyne sec cm(-5) m2 (15 Wood units) (normal <120 dyne sec cm(-5) m2 [1.5 Wood units]) identifies a group of patients with prohibitive mortality, regardless of the mode of therapy. It has been suggested that, in this setting, maintenance of interatrial communication provides a mechanism to decompress the right ventricle and is actually advantageous. The outcomes in such patients were evaluated in a large retrospective study in which 6 percent of 702 patients with an isolated ASD (secundum or sinus venosus type) had pulmonary vascular obstructive disease [20]. Among these 40 patients (mean age 46 years, 34 of whom were women), 26 underwent surgery and 14 were treated medically. The patients with severe pulmonary hypertension did poorly: at a mean follow-up of 12 years, all four surgically treated patients died; six of nine medically treated patients died; and the remaining three patients had progression of symptoms. Survival after surgery was much better (19 of 22) in patients with less severe pulmonary hypertension. However, the development of percutaneous closure techniques as well as
improvements in the medical management of patients with pulmonary vascular disease have reduced the risks associated with ASD repair in this setting. (See "Pulmonary hypertension" below). The syndrome of pulmonary hypertension and right-to-left shunting (Eisenmenger syndrome) presents a number of other clinical concerns, including thrombosis, bleeding, increased risk of maternal mortality during pregnancy, and consideration for lung or heart-lung transplantation. These issues are discussed separately. (See "Evaluation and medical management of Eisenmenger syndrome-I"). Pregnancy Women with an ASD may have problems during pregnancy, including arrhythmias, thromboembolism, and bleeding. However, there is no available evidence to suggest that pregnant patients should be managed differently from nonpregnant patients with respect to the indications for ASD closure [14]. (See "Pregnancy in women with congenital heart disease: Specific lesions-I", section on Left-to-right shunts). Scuba diving and altitude exposure Individuals with small ASDs who are treated medically may be at increased risk for complications when they experience high or low atmospheric pressure as with scuba diving or high-altitude climbing. Scuba diving in patients with an ASD has been associated with increased risks of decompression illness and paradoxical emboli, while high-altitude exposure has been associated with risk of increased right-to-left shunting and oxygen desaturation. Among patients with an ASD (or other intracardiac shunt), scuba diving is generally contraindicated, while consultation with a cardiologist specializing in congenital defects is recommended before altitude exposure [21]. (See "Complications of diving", section on Cardiovascular disease, and see "High altitude and heart disease-I", section on Congenital heart disease). EFFICACY OF SURGICAL REPAIR The benefit of ASD repair is influenced by the type of ASD, the patient's age, the size of the defect, and the presence or absence of pulmonary hypertension. The following discussion is organized in part according to the different types of ASD. (See "Pathophysiology and clinical features of atrial septal defects in adults-I", section on Classification). Ostium secundum ASD Ostium secundum ASDs account for approximately 70 percent of ASDs. Surgical and medical therapy for the management of secundum ASD in adults were compared in a randomized trial of 473 patients over age 40 (mean age 51) [2]. After a median follow-up of 7.3 years, the following results were observed: The composite primary end point (death, pulmonary embolism, major arrhythmias, stroke, recurrent pulmonary infection, functional class deterioration, or heart failure) occurred more frequently with medical than surgical therapy (21 versus 11 percent, hazard ratio 2.0). This difference was almost entirely due to a higher incidence of recurrent pneumonia among the medically treated patients. The overall mortality rate was not statistically different with medical versus surgical therapy (5.8 versus 4.3 percent), but there was a nonsignificant trend toward a higher sudden death rate with medical treatment (2.9 versus 0.9 percent). On multivariate analysis (adjusted for age at entry, mean pulmonary artery
systolic pressure >35 mmHg, previous atrial tachyarrhythmia, and cardiac index <3.5 L/m2), there was a significantly higher mortality with medical management (hazard ratio 4.1). Surgical management also appeared to be beneficial in an earlier retrospective study of 179 patients diagnosed with ASD after the age of 40 years, 91 percent of whom had ostium secundum defects [3]. On multivariate analysis, surgical closure was associated with a significant reduction in 10-year mortality after adjusting for baseline characteristics (5 versus 16 percent with medical therapy, adjusted relative risk 0.31). In addition, there was a much lower likelihood of functional deterioration compared with the medical group (11 versus 34 percent, adjusted relative risk 0.21) and a higher likelihood of improved functional status (32 versus 3 percent). Improvement in functional status after surgery was most common (69 percent) in patients with New York Heart Association (NYHA) functional class III or IV (show table 1). Symptomatic relief and better than predicted survival compared to medical therapy have also been described in patients who undergo surgery at 60 years of age or older [6]. Ostium primum ASD Ostium primum ASDs are typically larger than ostium secundum ASDs. However, the long-term outcome for both groups of patients appears to be similar when adjusted for defect size. The generally good outcome after surgery was illustrated in a review of 33 adults with ostium primum defects who underwent surgical closure at a mean age of 42 [5]. After a mean follow-up of 5.3 years, 85 percent were alive, asymptomatic, and in functional NYHA class I (show table 1). All deaths occurred late after surgery and none appeared related to the ASD or its repair. The presence of increasing age, symptoms, and the presence of atrial arrhythmias, mitral regurgitation, or moderately elevated pulmonary vascular resistance did not predict mortality or surgical complications. Ostium primum defects are frequently associated with a cleft anterior mitral valve leaflet or tricuspid mitral valve. A cleft leaflet, even if not severely regurgitant at the time of ASD closure, should be repaired to avoid the need for a second operation. Thus, complete echocardiographic assessment prior to surgery is mandatory in ostium primum defects to assess the mitral valve and detect this commonly associated abnormality. Ostium primum ASDs may also be associated with a more extensive defect involving the ventricular septum (atrioventricular septal defect). Sinus venosus ASD The clinical course of patients with a sinus venosus ASD is thought to be similar to that of patients with other types of isolated ASD. Most studies of the surgical management of patients with ASD have not reported (and perhaps not looked for) differences among patients with a sinus venosus ASD compared to those with secundum or primum ASDs [3,4]. A possible difference in natural history was suggested in a report of 169 patients: 31 had a sinus venosus ASD and 138 had a secundum ASD [22]. The patients with a sinus venosus defect were more likely to have pulmonary hypertension (26 versus 9 percent) and elevated pulmonary vascular resistance (16 versus 4 percent). In addition, elevated pulmonary vascular resistance occurred at a younger age in those with a sinus venosus ASD.
These observations suggest that patients with sinus venosus ASDs should be monitored more closely for the development of pulmonary hypertension and may require closure of the defect at a younger age. Perioperative mortality A report from the Mayo Clinic of 123 patients who underwent ASD repair between 1956 and 1960 noted a perioperative mortality rate of 3.3 percent [7]. However, most subsequent studies described no perioperative mortality [2,3,5,8,23] or a rate of about 1 percent in adults [4,24]. An exception may occur in patients > or =60 years of age who, in one series, had a 6 percent (4 of 66) perioperative mortality [6]. All four patients required additional operative procedures. Long-term outcomes Most patients do well over the long-term after successful surgical closure [2-8]. As noted above, they also appear to do better than with continued medical therapy [2,3,6]. Younger patients may have better long-term outcomes than older patients. This was illustrated in the report cited above of 123 patients of all ages who underwent surgery at the Mayo Clinic and were followed for at least 27 years [7]. The 27-year survival rates for patients in the younger two quartiles at the time of surgery (< or =11 years and 12 to 24 years) were not different from age-matched controls (97 and 93 percent). In contrast, 27-year survival was significantly less than controls for patients in the third quartile (25 to 41 years; 84 versus 91 percent) or fourth quartile (>41 years; 40 versus 59 percent). Atrial tachyarrhythmias Atrial tachyarrhythmias, primarily atrial fibrillation and atrial flutter, occur in approximately 20 percent of patients with an ASD and are often the presenting symptom [2,3,17,25]. The risk of these arrhythmias increases with patient age (especially over age 40) and with higher pulmonary artery pressures [17,25]. In a report of 211 adults, for example, the incidence of atrial fibrillation (AF) or atrial flutter prior to surgery was 1 percent for those aged 18 to 40, 30 percent for those aged 40 to 60, and 80 percent in those over the age of 60 [25]. The natural history of these arrhythmias after surgical closure was evaluated in a report of 213 symptomatic adults [17]. Atrial fibrillation or atrial flutter was present in 40 patients preoperatively and resolved in 16 (40 percent) at a mean follow-up of 3.8 years. New onset of these arrhythmias after surgery occurred in 7.5 percent of patients over age 40 but in none of 106 younger patients. With respect to the comparative effects of surgical repair or medical therapy, some studies found no difference in the rate of AF or atrial flutter in the two groups [3,26]. In a report of 179 patients, for example, new onset AF or atrial flutter developed with equal frequency during follow-up (15 and 17 percent with surgery and medical therapy, respectively) [3]. In another series, surgery reduced the incidence of atrial flutter in all age groups but not AF [25]. The lack of benefit of surgery in reducing the incidence of AF is presumably related to irreversible factors that predispose to the arrhythmia. The Cox maze procedure, performed at the time of ASD closure, decreases the long-term incidence of AF in selected patients [25,27,28]. This procedure involves the placement of incisions in the atria to interrupt the macroreentrant circuits that sustain atrial flutter and fibrillation. The use of radiofrequency ablation to create linear endocardial lesions
is also effective in preventing recurrences of AF, but has not been reported in patients with ASD. (See "Nonpharmacologic strategies to prevent recurrent atrial fibrillation-I"). Patients with atrial fibrillation are at risk for embolic events, particularly if not appropriately anticoagulated [3,17]. (See "Anticoagulation to prevent embolization in atrial fibrillation-I"). SURGICAL APPROACH Technique The traditional surgical approach for ASD repair has been a median sternotomy [23], although a right anterolateral submammary subpectoral approach to produce a cosmetic incision has been preferred in females [29,30]. However, there has been increased use of minimally invasive approaches. These include right parasternal or upper hemisternotomy [31], a right submammary bikini line incision in women [23], limited median sternotomy in men [23], and a mini-median or transxiphoid sternotomy in children and young adults [23,32]. An anterolateral approach should not be used in prepubescent girls to avoid possible damage to the breast bud [23]. (See "Minimally invasive cardiac surgery-I"). Pericardial or Dacron patches are used, while primary closure of the defect is not recommended [23]. Some surgeons advise cannulation of the superior vena cava rather than the right atrium to reduce the incidence of postoperative arrhythmias [33]. A cleft mitral valve is common in patients with ostium primum ASDs. Such clefts should typically be repaired at the time of surgery to avoid the need for a second operation. Other associated congenital anomalies also may require correction, especially in the setting of ostium primum ASDs. Thus, complete echocardiographic assessment prior to surgery is mandatory in ostium primum defects. (See "Ostium primum ASD" above). Intraoperative TEE demonstrates the adequacy of repair and detects residual flow across the surgical site (show echocardiogram 1). TEE also excludes the presence of tricuspid or mitral regurgitation, either of which can occur if tension from repair on the valvular annulus distorts the geometry of leaflet coaptation. Postoperative management There are a number of postoperative considerations in patients who have undergone ASD repair. Transthoracic echocardiography should be performed to evaluate the adequacy of the repair and document the expected postoperative decrease in right atrial and right ventricular size and pulmonary pressures (show echocardiogram 1). Beta blockers, if not contraindicated, should be given to reduce the risk of postoperative AF. The benefit is seen when therapy is begun prior to or immediately after surgery and is independent of the agent or dose used. The optimal duration of therapy is uncertain, but beta blockers are often continued until the first postoperative visit. (See "Arrhythmias after cardiac surgery: Atrial fibrillation and atrial flutter-I", section on Prophylactic therapy). Anticoagulation is often recommended for several months after surgery in adults undergoing ASD closure because of concern about postoperative AF. Long-
term periodic follow-up is indicated because of the late occurrence of AF and atrial flutter and stroke, particularly adults over age 40 [3,17]. Patients with preoperative atrial tachyarrhythmias are at particular risk. (See "Anticoagulation to prevent embolization in atrial fibrillation-I"). Sinus node dysfunction and prolonged atrioventricular conduction have been reported both before and after surgery. Surgery can damage the AV node or its blood supply, and some patients require a pacemaker in the postoperative period. In addition, there may be a small increase in the lifetime risk for the development of sinus and AV nodal dysfunction. The continuation of this discussion of the management of atrial septal defects in adults is presented separately. (See "Management of atrial septal defects in adults-II").
Management of atrial septal defects in adults-II
Susan E Wiegers, MD, FACC, FASE Martin G St John Sutton, MBBS, FRCP This topic represents a continuation of the discussion of the management of atrial septal defects in adults. (See "Management of atrial septal defects in adults-I"). PERCUTANEOUS CLOSURE Percutaneous device closure is an alternative to surgical closure in patients with ostium secundum ASDs that have appropriate anatomic characteristics. The United States Food and Drug Administration's Center for Devices and Radiological Health has approved the Amplatzer Septal Occluder for patients who meet the FDA criteria for closure (show figure 1) [1]. Several other devices have been developed and are used for closure of VSDs, including the CardioSEAL device [2]. No device has approval currently for PFO closure. Antiplatelet therapy (aspirin and clopidogrel) is given to all patients receiving a percutaneous closure device for at least six months to protect against thrombus formation [3-5]. (See "Complications" below). The following discussion will review the efficacy and complications associated with percutaneous device closure of a secundum ASD. The characteristics of the Amplatzer and CardioSEAL devices and other devices that are still investigational are discussed separately. (See "Devices for percutaneous closure of a secundum atrial septal defect"). Anatomic requirements Nonoperative device closure is only applicable to secundum defects. The ideal lesion for percutaneous closure is considered to be a defect less than 30 mm in diameter with a rim of tissue around the defect of at least 5 mm to prevent obstruction of the coronary sinus, right pulmonary veins, vena cavae, or atrioventricular valves. Approximately half to two-thirds of ASDs in adults meet these criteria [6,7]. More than one device can be inserted to close multiple ASDs [2,8]. Echocardiographic monitoring The use of transesophageal or intracardiac echocardiography can facilitate the deployment of percutaneous ASD closure
devices. Measurement of the size and location of the ASD by transesophageal echocardiography (TEE) can help select the appropriate device. In addition, TEE can be used to guide the procedure in real time, an approach that may eliminate the need for fluoroscopy [9,10]. Two or even three dimensional TEE is particularly helpful when multiple devices are inserted to close multiple ASDs [8]. The development of intracardiac echocardiography (ICE) has provided a modality for evaluating cardiac anatomy that may be particularly well-suited for use in the management of ASDs [11-15]. ICE can provide guidance for device placement and facilitates proper device selection by providing an accurate assessment of the size and shape (typically elliptical) of the defect [13]. ICE has the following advantages compared to TEE. It does not require endotracheal intubation or general anesthesia [12,15]; it permits continuous monitoring during the procedure; and it may shorten the duration of the procedure [15]. In one series, 94 patients underwent ICE during percutaneous closure of an ASD or PFO [11]. All devices were deployed successfully. During the procedure, ICE identified a previously unrecognized anatomical diagnosis in 32 patients (an additional ASD or PFO, a redundant atrial septum, or an atrial septal aneurysm). Complications occurred in only four patients: AF in three and supraventricular tachycardia in one. Two of the arrhythmias resolved spontaneously and two required cardioversion with no recurrence. Outcomes Clinical studies have described favorable outcomes in the great majority of patients treated with percutaneous closure [2,16-20]. In a series of 100 patients, for example, the Amplatzer device was successfully implanted in 93 with a procedure time ranging from 30 to 180 minutes [16]. The total ASD occlusion rate at three months was 99 percent. An initial experience with the CardioSEAL device involved 50 patients (median age 9.7 years); 22 percent had multiple ASDs and 38 percent had a deficient rim of atrial tissue (<4 mm) [19]. After a mean follow-up of 9.9 months, all patients were asymptomatic. A small residual shunt was present in 46 percent of patients without evidence of hemodynamic consequences. Percutaneous ASD closure improves functional capacity, even in apparently asymptomatic patients. This was illustrated in a study of 32 patients who underwent ASD closure at a mean age of 43 years [20]. The mean baseline Qp/Qs was 2.0. At six months, cardiopulmonary exercise testing demonstrated a significant improvement in peak oxygen uptake with exercise compared to testing obtained before closure (25.6 versus 21.9 mL/kg per min). There was a significant correlation between the magnitude of the functional benefit and the baseline Qp/Qs, but even those patients with Qp/Qs <2.0 had a significant functional improvement. (See "Functional exercise testing: Ventilatory gas analysis"). Pulmonary hypertension As noted above, severe fixed pulmonary hypertension is generally considered to be a contraindication to surgical repair of an ASD. However, the development of percutaneous closure techniques as well as improvements in the medical management of patients with pulmonary vascular disease have reduced the risks associated with ASD repair in this setting. It has been suggested that any patient with a net left-to-right shunt, a pulmonary
vascular resistance less than 800 to 960 dyne sec cm(-5) m2 (10 to 12 Wood units), and a resting systemic arterial oxygen saturation > or =90 percent will benefit from ASD closure [21]. The potential efficacy of percutaneous closure was demonstrated in a report of 29 patients with a secundum ASD and a baseline peak pulmonary artery pressure >40 mmHg (mean 65 mmHg) in whom an Amplatzer device was implanted [22]. Complete ASD occlusion was achieved in 28 patients (97 percent). Immediately after the procedure, the mean peak pulmonary artery pressure decreased to 54 mmHg; at a mean of 21 months, it decreased further to 31 mmHg. There were no procedural complications. Functional status was improved after the procedure and was maintained at a mean of 21 months. Six of twelve patients who had atrial fibrillation at baseline recovered sinus rhythm by discharge. Comparison to surgery The limited data comparing surgical and percutaneous closure of a secundum ASD suggest that the rate of procedural success is comparable or possibly better with surgery, but that the rate of complications and length of hospital stay may be reduced with the percutaneous approach. It is likely that percutaneous closure will replace surgical closure in many patients, especially the elderly and others with significant operative risks and those with pulmonary hypertension. The best comparative data come from a study of 442 children undergoing percutaneous closure with the Amplatzer device (median age 9.8 years) and 154 undergoing surgery (median age 4.1 years) [23]. The rate of procedural success was significantly higher with surgery (100 versus 96 percent), while the percutaneous closure group had significant reductions in complication rate (7 versus 24 percent) and mean hospital stay (1.0 versus 3.4 days). There were no deaths in either group. Similar short-term differences were noted in another report comparing 61 patients who underwent surgical closure (median age 20 years) with 61 who underwent closure with an Amplatzer device (median age 12 years) [24]. The first study also described long-term outcomes [23]. At 12 month follow-up of patients treated with the Amplatzer device, a residual shunt was present in 1.5 percent; one patient required a second procedure to correct a residual shunt at 24 month follow-up. Complications Complications associated with transcatheter closure of an ASD include device embolization or malposition and arrhythmias (usually atrial but can include sudden death) [16,18,23,25]. The type and frequency of complications was evaluated in a report of 417 patients (mean age 27 years) who underwent secundum ASD closure with the Amplatzer or CardioSEAL/STARFlex device [18]. The following frequency of complications was noted: Sudden death in one patient 1.5 years after ASD closure. Device embolization or malposition requiring surgery in 2.4 percent and managed percutaneously in 1.0 percent. In another series of 124 patients, 6.5 percent required surgery for device malposition or embolization [25]. Atrial fibrillation or supraventricular tachycardia in 2.4 percent. Other complications (heart block, pericardial effusion, iliac vein dissection,
groin hematoma) in 2.2 percent. Device arm fractures, which are an occasional complication with the CardioSEAL device (14 percent in one series), are much less common with the STARFlex modification [2]. Another report summarized 10 studies of 1355 patients who underwent percutaneous PFO closure after a first embolic event [26]. Major complications with percutaneous closure (including death, hemorrhage requiring transfusion, cardiac tamponade, need for surgical intervention, and fatal pulmonary emboli) occurred in 1.5 percent. Minor complications (bleeding not requiring transfusion, atrial arrhythmias, device embolization, device thrombosis, air embolism, and others) occurred in 7.9 percent. Transient atrioventricular block has also been described, with a frequency ranging from 1 to 6 percent [16,27]. Atrioventricular block may be more common with larger devices and smaller patients. This problem appears to resolve or improve spontaneously in most cases. Although rare, sudden death can occur after percutaneous device implantation. The frequency with which this might occur was addressed in a review of three databases involving 777 patients who received a device for an ASD or VSD [28]. At a median follow-up of 25 months, sudden death occurred in nine patients (1.2 percent); three had undergone repair of an ASD alone. Patients who experienced sudden death were more likely to have had multiple devices inserted and to have a history of serious tachyarrhythmias, severe valve regurgitation, one or more cardiac surgeries, pulmonary hypertension, and ventricular dysfunction. Antiplatelet therapy (aspirin and clopidogrel) is given to all patients receiving a percutaneous closure device for at least six months to protect against thrombus formation [3-5]. In a review of 407 consecutive patients with an ASD and 593 consecutive patients with a PFO who were treated with a variety of devices, thrombus was detected by TEE at four weeks in 14 patients (1.4 percent) and at six months in six patients (0.6 percent) [3]. Thrombus formation was significantly more common with the CardioSEAL and STARFlex devices than with the Amplatzer device (5.9 versus 0 percent). Once antagonism of heparin in the first hours after implantation was stopped, thrombus occurred in only one of 183 patients (0.5 percent) treated with aspirin and clopidogrel. The thrombus resolved in 17 of 20 patients after anticoagulation with heparin and/or warfarin; in three patients the thrombus was removed surgically. Four of the 20 patients suffered embolic events (strokes in three and a transient ischemic attack in one). ENDOCARDITIS PROPHYLAXIS The 1997 expert committee of the American Heart Association concluded that an isolated ASD or a repaired ASD were associated with a negligible risk of endocarditis and that antibiotic prophylaxis was not recommended unless there was concurrent mitral regurgitation [29]. (See "Antimicrobial prophylaxis for bacterial endocarditis"). ATRIAL SEPTAL ANEURYSM An atrial septal aneurysm is a congenital localized outpouching that can involve the entire atrial septum or be limited to the region of the fossa ovalis. The prevalence of atrial septal aneurysm during routine
echocardiography is about 5 percent. Although most of these aneurysms are asymptomatic, they can be associated with systemic and cerebral emboli. The relative frequency with which atrial septal aneurysm is associated with paradoxical embolization was addressed in a review of 581 patients with cryptogenic stroke who were under the age of 55: 10 (1.7 percent) had an atrial septal aneurysm, and 51 (9 percent) had both and atrial septal aneurysm and an ASD [30]. (See "Atrial septal abnormalities (PFO, ASD, and ASA) and cerebral emboli in adults-I", section on Atrial septal aneurysm). A perforated aneurysm may be associated with a significant left-to-right shunt and present like an ASD. Perforated aneurysms can be closed surgically or percutaneously, depending upon their morphology. In a retrospective review of 50 patients, perforated aneurysms were classified as follows [31]: Type Type Type Type A - aneurysm with persistent foramen ovale B - aneurysm with single ASD C - aneurysm with two perforations D - aneurysm with multiple perforations
Transcatheter device closure using an Amplatzer or CardioSEAL device was possible in all eighteen patients with a type A aneurysm. In the nine patients with a type B aneurysm, five had successful device closure, while four required surgery. Device closure was achieved in all 10 patients with a type C aneurysm, but four had a residual shunt. All thirteen patients with a type D aneurysm underwent surgery.
Potrebbero piacerti anche
- Australia and Tasmania FactsDocumento4 pagineAustralia and Tasmania FactsHassan MurtazaNessuna valutazione finora
- ArgentinaDocumento3 pagineArgentinaHassan MurtazaNessuna valutazione finora
- Sample Chapter On Communication Skills and Ethics From Revision Notes For MRCP PacesDocumento70 pagineSample Chapter On Communication Skills and Ethics From Revision Notes For MRCP PacesHassan MurtazaNessuna valutazione finora
- Asthma 1Documento4 pagineAsthma 1Hassan MurtazaNessuna valutazione finora
- KRL General Hospital: Career OpportunitiesDocumento2 pagineKRL General Hospital: Career OpportunitiesHassan MurtazaNessuna valutazione finora
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5782)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (72)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (265)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (119)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- Embryonic Heart Development and Congenital Heart DefectsDocumento182 pagineEmbryonic Heart Development and Congenital Heart DefectsDaNy Chiriac0% (1)
- Cia CivDocumento6 pagineCia CivCamila Fda. Muñoz G.Nessuna valutazione finora
- Embryology of Cardiovascular System 1: DR - Navajyothi, Mbbs MDDocumento59 pagineEmbryology of Cardiovascular System 1: DR - Navajyothi, Mbbs MDcheckmateNessuna valutazione finora
- 2018 Fetal Echocardiography 1st Ed PDFDocumento233 pagine2018 Fetal Echocardiography 1st Ed PDFRaúl Santiváñez del Aguila100% (2)
- Congenital Heart Disease Surgery TransDocumento9 pagineCongenital Heart Disease Surgery TransPuguinGamingNessuna valutazione finora
- Cardiac Anatomy and Physiology ReviewDocumento144 pagineCardiac Anatomy and Physiology ReviewNb + XB = AVNessuna valutazione finora
- 38 Endocardial Cushion DefectsDocumento12 pagine38 Endocardial Cushion DefectsVictor PazNessuna valutazione finora
- Atrial Septal DefectDocumento7 pagineAtrial Septal DefectRose WidantiNessuna valutazione finora
- Embryology04 CardiovascularSystemDocumento48 pagineEmbryology04 CardiovascularSystemAlexandre NascimentoNessuna valutazione finora
- Echocardiographic Evaluation of Patent Foramen Ovale Prior To Device ClosureDocumento12 pagineEchocardiographic Evaluation of Patent Foramen Ovale Prior To Device ClosureIris AszalosNessuna valutazione finora
- Acr 2016 Dxit Exam Sets - WebDocumento129 pagineAcr 2016 Dxit Exam Sets - WebElios NaousNessuna valutazione finora
- Echocardiographic Atlas of Adult Congenital Heart Disease: Hakimeh Sadeghian Zahra Savand-RoomiDocumento486 pagineEchocardiographic Atlas of Adult Congenital Heart Disease: Hakimeh Sadeghian Zahra Savand-RoomiGeorgiana-Gratiela MalaescuNessuna valutazione finora
- Atrial Septal DefectDocumento38 pagineAtrial Septal DefectJelita SihombingNessuna valutazione finora
- Embryonic Development of The Cardiovascular and Respiratory System During The First TrimesterDocumento34 pagineEmbryonic Development of The Cardiovascular and Respiratory System During The First TrimesterDennis SukadanaNessuna valutazione finora
- Internal Medicine Orals E&a Academia (Exclusive)Documento209 pagineInternal Medicine Orals E&a Academia (Exclusive)Shreya SinghNessuna valutazione finora
- Embryology of Spinal CordDocumento5 pagineEmbryology of Spinal CordSteven MatualiNessuna valutazione finora
- Chapter 24 - Alterations of Cardiovascular Function in Children - Nursing Test BanksDocumento19 pagineChapter 24 - Alterations of Cardiovascular Function in Children - Nursing Test BanksNeLNessuna valutazione finora
- Nev 2006Documento47 pagineNev 2006Oscar Condori CahuataNessuna valutazione finora
- Atrioventricular Canal Defect: Seoul National University Hospital Department of Thoracic & Cardiovascular SurgeryDocumento22 pagineAtrioventricular Canal Defect: Seoul National University Hospital Department of Thoracic & Cardiovascular SurgeryMohan MadhavanNessuna valutazione finora
- Penatalaksanaan Mouth Preparation Pada Anak Dengan Kelainan Jantung Bawaan Tipe Atrial Septal Defect Di Bawah Anestesi UmumDocumento5 paginePenatalaksanaan Mouth Preparation Pada Anak Dengan Kelainan Jantung Bawaan Tipe Atrial Septal Defect Di Bawah Anestesi Umumnonaviona_nonavionaNessuna valutazione finora
- Echo Assessment in ASD, VSDDocumento46 pagineEcho Assessment in ASD, VSDJumadi SyhuraNessuna valutazione finora
- Quiz - Development of Human Cardiovascular SystemDocumento7 pagineQuiz - Development of Human Cardiovascular Systemlucky mbaselaNessuna valutazione finora